Abstract
Background
Poly-ADP-ribose polymerases (PARPs) are key mediators of cellular stress response. They are intimately linked to cellular metabolism through the consumption of NAD+. PARP1/ARTD1 in the nucleus is the major NAD+ consuming activity and plays a key role in maintaining genomic integrity.
Scope of review
In this review, we discuss how different organelles are linked through NAD+ metabolism and how PARP1 activation in the nucleus can impact the function of distant organelles. We discuss how differentiated cells tame PARP1 function by upregulating an endogenous inhibitor, the histone variant macroH2A1.1.
Major conclusions
The presence of macroH2A1.1, particularly in differentiated cells, raises the threshold for the activation of PARP1 with consequences for DNA repair, gene transcription, and NAD+ homeostasis.
Keywords: PARP1, NAD+, Epigenetics, MacroH2A, Metabolism, Homeostasis
Highlights
-
•
Beyond DNA repair, PARP1 is essential for metabolic homeostasis.
-
•
Epigenetic mechanisms prevent metabolic disorders through PARP1 taming.
-
•
Beyond cancer, the development of PARP1 inhibitors offers diverse clinical potential.
1. PARP1 is a key mediator of nuclear poly-ADP-ribose signaling
The covalent linkage of adenosine diphosphate (ADP)-ribose units to effector molecules such as proteins, nucleic acids, or small molecules is a reversible, evolutionarily conserved chemical modification found in all kingdoms of life (reviewed in [1]). The ADP-ribosylation of proteins, a post-translational modification, has been implicated in many physiological processes, including gene transcription, protein degradation, cell proliferation and differentiation, DNA damage and repair, aging, inflammation, cell death, host–virus interactions, and metabolism [[2], [3], [4], [5], [6]]. The reaction is catalyzed by ADP-ribosyltransferases (ARTs) that include most members of the poly-ADP-ribose polymerase (PARP) family of proteins as well as some members of the sirtuin family [7,8]. Although PARPs and sirtuins differ substantially in their protein structure, they both use NAD+ as a substrate (Figure 1). Unlike PARPs, the majority of sirtuins use NAD+ for deacetylation and not ADP-ribosylation. This study focuses on PARPs. The function of sirtuins and their crosstalk with PARPs was previously reviewed elsewhere [[9], [10], [11]].
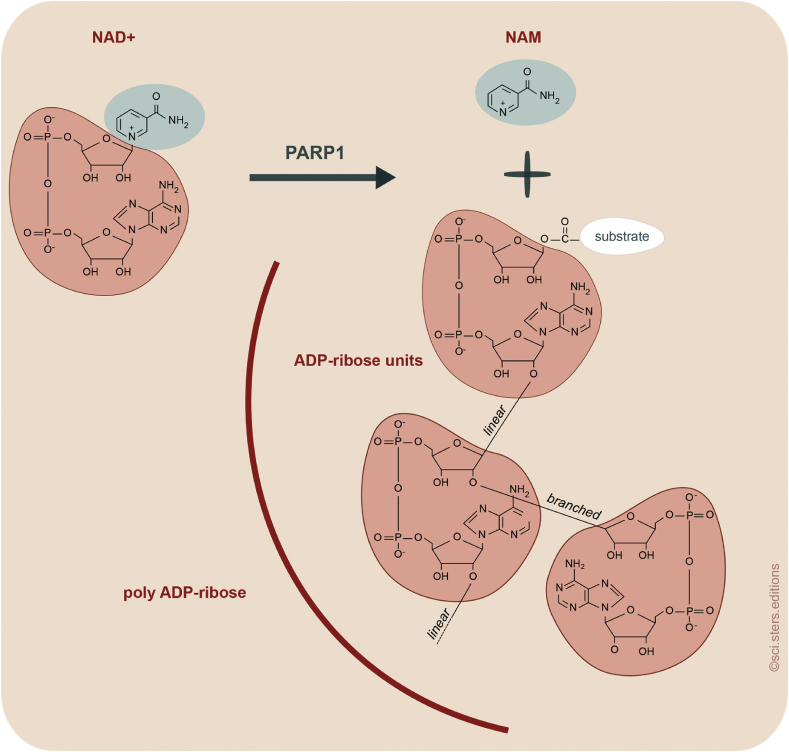
Figure 1.
PARPs catalyze poly-ADP-ribosylation. Several members of the PARP family are able to generate linear and branched ADP-ribose chains. PARP1 provides the major PARP activity in the nucleus. It transfers the ADP-ribose moiety of NAD+ onto substrates that can be other ADP-ribose units (poly-ADP-ribosylation) or proteins. Nicotinamide is the side product of this reaction.
1.1. The family of PARP enzymes
The PARP family of ARTs, also known as ARTD [12], catalyzes the modification of proteins through the addition of one or more ADP-ribose groups (Figure 1). In humans, 17 members of this family of enzymes have been identified and are classified according to their activity [13]: PARP1, 2, 5a, and 5b catalyze the polymerization of ADP-ribose units (PARylation) into linear or branched chains through α(1–2)O-glycosidic bonds of neighboring ribose moieties. All other PARPs with the exception of PARP9 and PARP13 catalyze the addition of single ADP-ribose units onto target proteins through a process that is generally referred to as mono-ADP-ribosylation or MARylation. To date, no enzymatic activity has been detected for PARP9 and PARP13 [7]. In addition to their conserved catalytic ART domain, PARPs contain additional structural domains that confer distinct localizations and functions or tightly regulate their spatiotemporal activity [14]. Based on their domain architecture, PARPs can be sub-divided into four groups: DNA-dependent PARPs 1–3, ankyrin repeat containing tankyrases PARP5a and b, Cys-Cys-Cys-His zinc finger containing PARP7, PARP12, and PARP13, and macrodomain containing PARPs 9, 14, and 15. The most abundant and best-characterized family member is PARP1, a nuclear enzyme with diverse cellular functions ranging from DNA repair, transcriptional control, genome stability, and cell death (reviewed in [15]).
1.2. Activation of PARP1 by DNA damage
The PARP1 protein consists of multiple functional domains: an N-terminal DNA binding domain composed of three zinc finger motifs (Zn1-3), an auto-modification domain, and the C-terminal ART domain. The catalytic ART domain is further composed of a conserved tryptophan, arginine, and glycine-rich WRG domain and a regulatory helical domain (HD). The HD inhibits substrate binding to the catalytic ART domain [16]. The zinc fingers Zn1 and Zn2 are sequence-independent sensors of DNA strand breaks that recruit PARP1 to a variety of DNA damage structures, including double strand breaks (DSB) and nicked or gapped single strand breaks (reviewed in [17]). The binding of Zn1 and Zn2 to DNA creates a domain–domain interaction surface that directs the structural rearrangement of the remaining enzyme into its activated conformation, ultimately resulting in a destabilization of the auto-inhibitory HD domain and allowing NAD+ binding to the catalytic site [18]. The fully activated PARP1 subsequently modifies itself and target proteins with long, branched PAR chains that can span up to 200 ADP-ribose moieties in length [19]. This creates an interaction scaffold for PAR binding DNA repair proteins that are recruited to the sites of PARP1 activity. These include important DNA repair factors such as XRCC1, APLF, CHFR, MRE11, and ATM that can be attracted to PAR synthesis sites through their linear, low-complexity PAR binding motifs (summarized in [20]). It has also been proposed that high local PAR concentrations can induce a compartmentalization at the DNA break through liquid–liquid phase separation, attracting positively charged intrinsically disordered proteins such as the FET family of RNA binding proteins, which are required for DNA repair [21]. “Reader” proteins containing macrodomains, PAR binding zinc finger (PBZ) domains, or tryptophan containing WWE domains specifically recognize mono- or poly-ADP-ribosylation through the direct engagement of the PAR polymer in their binding pockets (reviewed in [22]). In addition to the highly specific interaction of PAR reader modules with PAR nucleic acid, the high density of negative charges in the PAR chains leads to chromatin expansion and can dissociate DNA from histone octamers and release PARP1 from DNA damage sites, which downregulates the activity of the PARP1 enzyme [23]. Several studies clearly showed that the compact chromatin structure is additionally relaxed by the recruitment of specific chromatin remodeling enzymes such as ALC1/CHD1L or CHD2 that promote chromatin expansion [24,25] and increase accessibility of DNA for repair proteins [26,27]. PAR-activated signaling is finally terminated by the PAR hydrolyzing enzymes PARG and ARH3 [28,29]. These so-called “erasers” create locally high concentrations of ADP-ribose that can be converted into ATP by the pyrophosphatase NUDIX5 [30]. This nuclear ATP source has been proposed to fuel the DNA repair machinery, chromatin remodeling, and hormone-induced transcriptional regulation.
1.3. Regulation of PARP1 and its role in transcription
PARP1 is an important mediator of extra- and intracellular stress signals. The cellular outcome depends on the level of PARP1 activation and can range from activation of the DNA damage repair machinery to initiation of cell death [31,32]. In addition to the allosteric activation by DNA breaks, PARP1 activity is subjected to regulatory mechanisms through post-translational modifications and protein–protein interactions. Methylation by Set7/9 [33], phosphorylation by ERK1/2 [34], or its interaction with phosphorylated ERK2 [35] increase PARP1 activity. In contrast, the interaction of PARP1 with HPF1 promotes the PARylation of histones, reduces its auto-modification activity, and generally shifts the specificity of PARP1 from glutamate, aspartate, and lysine residues to serines as main PAR acceptor sites [36,37]. As further discussed in Section 4, histone variant macroH2A1.1 dampens PARP1 activity by binding its auto-modified form [38,39].
An additional important function of PARP1 is its role as a transcriptional regulator. Two general mechanisms have been proposed for the function of PARP1: (1) by regulation of the local and higher order chromatin structure through direct interaction with nucleosomes or PARylation of histones and chromatin-associated factors, and (2) as a co-regulator through interactions with the transcriptional machinery or sequence specific transcription factors [6,40]. While most studies show a requirement of PARP1 catalytic activity for its transcriptional function [41,42], others reported transcriptional modulation independent of PARP activity [43].
In sum, PARP1 is an abundant nuclear enzyme whose stress-induced activation during transcription or DNA damage leads to dramatic alterations in nuclear structure and function and is closely intertwined with cellular metabolism through the consumption of NAD+.
2. NAD+ metabolism
Nicotinamide adenine dinucleotide (NAD+) is an essential cofactor involved in most reductive-oxidative metabolic pathways including glycolysis and the citric acid cycle (reviewed in [44]). NAD+ and its reduced form NADH function as acceptor and donor molecules of two electrons and one proton. In the mitochondria, this function allows the coupling of the citric acid cycle to the electron transport chain, which is essential for the efficient conversion of nutrients into energy. The membrane potential generated by the electron transport chain drives oxidative phosphorylation generating adenosine triphosphate (ATP).
In addition to its function in redox reactions, NAD+ serves as an ADP-ribose donor for enzymes regulating important biological processes such as transcriptional regulation, calcium signaling, and DNA repair (reviewed in [45]). These enzymes include PARPs, the deacetylase family of sirtuins, and synthetases generating the second messenger cyclic ADP-ribose. In this section, we briefly discuss the synthesis of NAD+ and the relevance of its subcellular compartmentalization for inter-organelle communication (Figure 2). Those interested in the nutritional aspects of NAD+ and the possibilities for pharmacological intervention should refer to two previous reviews [44,45].
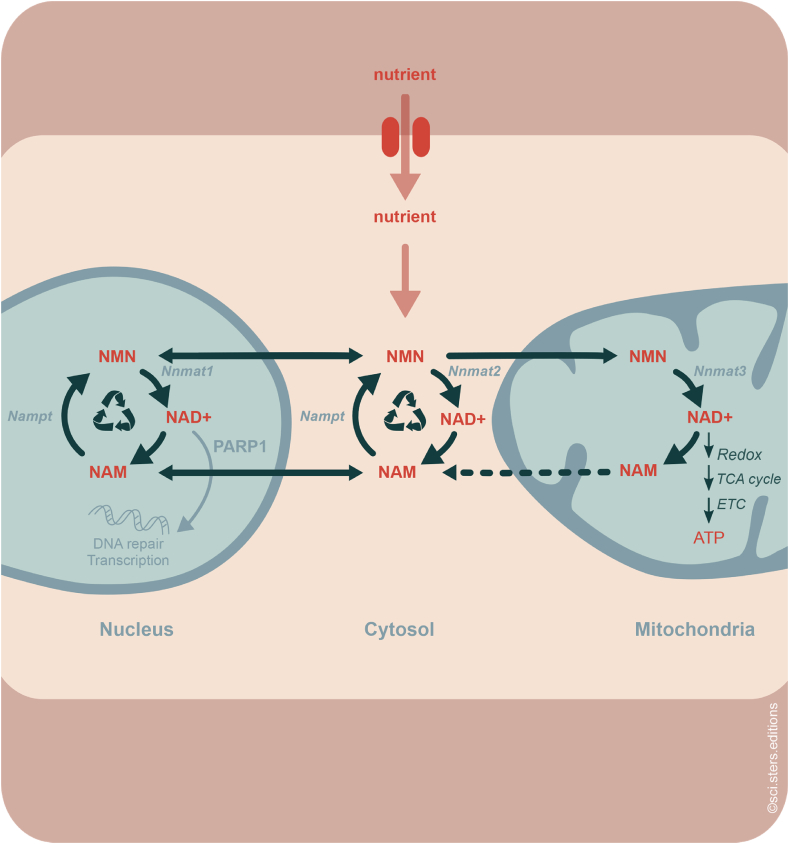
Figure 2.
NAD+metabolism connects cellular sub-compartments. Most cellular NAD+ is regenerated through the salvage pathway from NAM and to a smaller extent through de novo biosynthesis from dietary sources such as NR (nutrient). The rate-limiting reaction from NAM to NMN is catalyzed by NAMPT enzyme in the nucleocytosol. Three different NMNAT enzymes catalyze the compartmentalized regeneration of NAD+ from the same pool of NMN. The NAD+/NADH pair has key redox functions in the tricarboxylic acid (TCA) cycle and the electron transport chain to drive ATP generation.
2.1. Regeneration of the NAD+ pool
Steady-state cellular NAD+ levels are between 200 and 500 μM [44]. Most cells replenish their NAD+ pool primarily through a salvage pathway and to a lesser extent through de novo synthesis from dietary sources. Dietary sources are nicotinamide (NAM) and nicotinic acid and are commonly referred to as vitamin B3 and their ribosides and the amino acid tryptophan [45]. The NAD+ salvage pathway allows the recycling of NAM that is the product generated by NAD+ consuming enzymes. The rate-limiting step is the conversion of NAM into nicotinamide mononucleotide (NMN) by nicotinamide phosphoribosyltransferase (NAMPT) [46]. Three nicotinamide mononucleotide adenylyltransferases (NMNAT1-3) catalyze the conversion of NMN to NAD+. The recycling of one molecule of NAD+ from NAM comes at the cost of two ATP molecules.
2.2. Compartmentalization of NAD+ metabolism and synthesis
Most metabolic pathways involving NAD+/NADH-dependent redox reactions occur in mitochondria and cytosol [45]. In addition to mitochondria and cytoplasm, a substantial amount of NAD+ is consumed in the nucleus. Nuclear NAD+ consuming enzymes include PARP1, PARP2, and the sirtuins SIRT1 and SIRT2. Hence, the regeneration of NAD+ through the salvage pathway is compartmentalized (Figure 2). While the NAMPT enzyme is located in the cytosol and nucleus, the three NMNAT enzymes have a distinctly subcellular distribution. NMNAT1 is nuclear, NMNAT2 is located in the cytoplasm and at the external membrane of the Golgi, and NMNAT3 localizes inside mitochondria [47]. Interestingly, PARP1 has been shown to directly interact with NMNAT1 and recruit it to specific promoter sites where the locally synthesized NAD+ “feeds” the PARP1 catalysis-dependent transcriptional modulation of target genes [48]. Importantly, the NMNATs of all cell compartments compete for the same pool of NMN produced by NAMPT thereby placing the intermediate in a central position of linking NAD+ metabolism of different compartments. This is illustrated by the observation that glucose-induced upregulation of cytosolic NMNAT2 in adipogenic cells leads to reduced availability of NAD+ in the nucleus with consequences for PARP1-mediated gene regulation and cell differentiation [49].
In conclusion, reactions catalyzed by PARPs, sirtuins and cyclic ADP-ribose synthetases consume NAD+ while redox reactions do not change the overall pool of the NAD+/NADH pair. Separate NAD+ pools exist in compartments and organelles. They are dynamic and communicate through common precursors of the salvage pathway.
3. The impact of acute PARP1 activation on metabolism
More than 50 years ago, NMN was shown to induce a nucleotide polymer when added to liver nuclear extract [50]. This nucleotide polymer was later shown to be PAR. This was the first indication that the NAD+ salvage pathway, PARP activity, and the generation of PAR were linked to each other. In eukaryotic cells, PARP1 accounts for the largest amount of PARP activity in cells and is the major NAD+ consuming entity in the nucleus [51]. Acute activation of PARP1 through hormone stimulation or DNA damage can lead to the depletion of 50%–80% of total cellular NAD+ [52,53]. PARP1 activation is usually transient and NAD+ levels are quickly recovered, within 4–5 h in cases of hormone stimulation [52]. However, prolonged activation of PARP1 in cases of persistent stress can impair the energetic balance of cells [44], leading to severe ATP depletion and triggering apoptosis [54].
Conversely, inhibition of PARP1 can increase energy balance and improve mitochondrial function. We recently summarized the current knowledge about PARP1 loss-of-function studies in mice [55]. Although the outcomes were variable and strongly diet and genetic context dependent, most of these studies suggested that PARP inhibition is metabolically favorable. Pharmacologic PARP inhibitors or genetic inhibition of PARP1 protect mice from diabetes when induced by administration of the β-cell toxin streptozotocin [56,57]. Similarly, both PARP inhibitors and PARP1 knock-out reduce disease parameters in obesity-challenged mice. This includes reduced diabetes-associated kidney damage in the genetic Lbdr (db/db) model [58] and protection against obesity in mice on high-fat diets [59,60].
In several cases, the favorable effect of PARP1 inhibition was linked to an increased availability of NAD+ for other reactions. Treatment of cell cultures with PARP inhibitors increased the cellular NAD+ levels [52,59,61]. Interestingly, increased NAD+ availability frequently leads to higher mitochondrial activity. The Auwerx Lab showed an indirect effect on respiration exerted by increased NAD+-dependent SIRT1 activity in the nucleus that is mediated by the upregulation of genes promoting respiration [59,62]. Studying differentiated muscle cells, we further found a direct effect of inhibited PARP1 mediated by increased availability of the NAD+ precursor NMN in the cytosol and increased regeneration of NAD+ in the mitochondria [39,55].
Taken together, PARP1 activity in the nucleus can affect the metabolism of distant compartments, notably mitochondrial respiration, through the consumption of NAD+ and the depletion of precursors from common pools. The inhibition of PARP1 is emerging as a potential therapeutic strategy to modulate NAD+ levels and shift NAD+ usage to other reactions and compartments.
4. Taming of the PARP1 stress response by the histone variant macroH2A1.1 in differentiated cells
Terminally differentiated and proliferating cells have different requirements for DNA repair and thus for DNA damage-induced PARP1 signaling. While proliferating cells require fast repair until the next entry into the S-phase, this is much less critical in non-cycling cells that most likely acquire less DNA damage and might be able to tolerate it for a prolonged time. As such, in retrospect, it is not surprising that metazoan species have evolved an inhibitory mechanism dampening but not abrogating PARP1 signaling in terminally differentiated states. In the context of chromatin, one of these endogenous inhibitors is the histone variant macroH2A1.1.
4.1. MacroH2A histone variants
The exchange of a replication-coupled histone for a histone variant is one of the most drastic changes that can occur on the level of the nucleosome and endow the immediate chromatin environment with new structural and biophysical properties [63]. In particular, for H2A and H3, many histone variants exist. Among these, three macroH2A histone variants stick out because they have a unique tripartite structure consisting of a histone-fold domain followed by a C-terminal unstructured linker connecting with a globular macrodomain [64]. The linker protrudes from the compact structure of the nucleosome near the dyad axis and places the macrodomain at an accessible position outside the chromatin fiber. MacroH2A proteins contribute to epigenetic regulation in development, differentiation, and somatic cell reprogramming and cancer. Those interested in these aspects of macroH2As should refer to two recent reviews in which we provided a synthesis of the current knowledge [63,65]. As a resource to the field, we published an overview of loss-of-function studies conducted in mice highlighting differences in genetic context and experimental approaches [55]. The molecular mechanism by which macroH2As mediate their epigenetic function is yet unknown but is likely related to a major role in regulating higher order chromatin structures including heterochromatin [[66], [67], [68]].
4.2. MacroH2A1.1 macrodomain binds and inhibits ADP-ribosylated PARP1
Two genes and one alternative splicing event produce three macroH2A proteins that differ in their macrodomains [69]. Macrodomains are ancient globular folds that have a binding pocket for ADP-ribose [70]. The capacity to bind ADP-ribose is limited to the splice variant macroH2A1.1 [68,71]. MacroH2A1.1 differs in only 26 amino acids from the alternative splice variant macroH2A1.2 [72]. These amino acids form part of the binding pocket required for ADP-ribose binding [71]. The expression of both variants is tissue specific [72] and macroH2A1.1 is highly expressed in terminally differentiated cells such as enterocytes and myotubes [39,73]. The binding mode of ADP-ribose allows macroH2A1.1 to also bind the terminal part of PAR chains and proteins post-translationally modified by ADP-ribosylation [74]. The latter has been shown for auto-modified PARP1. Although this has not been formally tested, the ADP-ribose bound structure of the macrodomain suggests that it would be compatible with binding mono-ADP-ribosylated proteins. All macroH2As co-purify with PARP1 in the context of purified mono-nucleosomes; however, only macroH2A1.1 containing nucleosomes show an inhibition of the associated PARP1 activity [38]. Using in vitro assays, we found that the macrodomain of macroH2A1.1 is sufficient for inhibiting PARP1 [39]. In both cases, the inhibitory capacity is strictly dependent on the integrity of the binding pocket and its capacity to bind ADP-ribose [38,39]. As such, the PARP inhibitory function is strictly limited to the splice isoform macroH2A1.1 (Figure 3). We and others were unable to confirm an earlier report suggesting that the macrodomains of the other macroH2A proteins would be able to inhibit PARP1 [75].
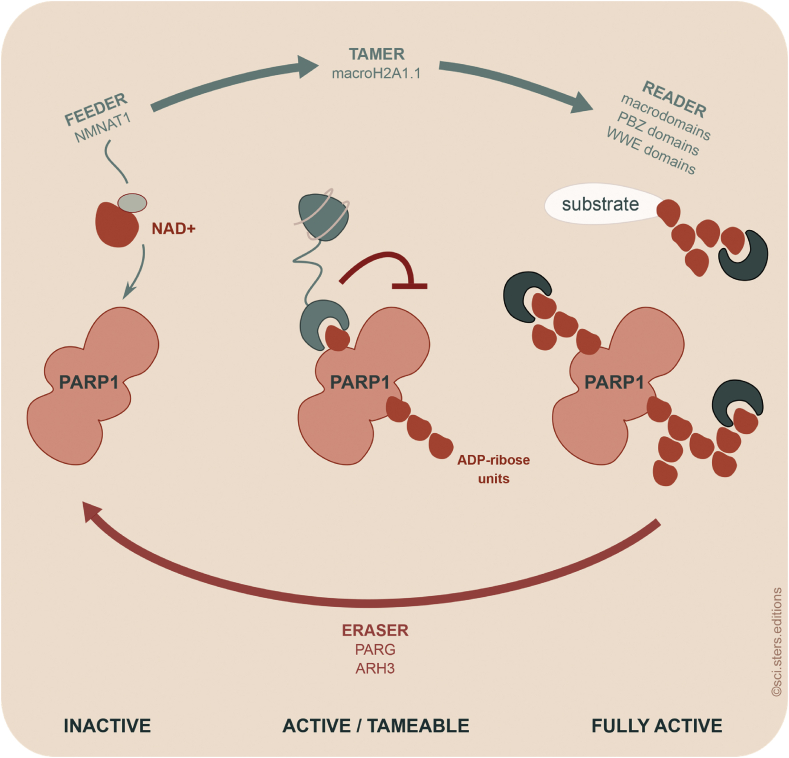
Figure 3.
MacroH2A1.1 is an endogenous inhibitor of PARP1. NMNAT1 acts as feeder providing NAD+ to PARP1. Activation of PARP1 is accompanied by auto-modification. Binding of the “tamer” macroH2A1.1 leads to its inhibition. The taming can be overcome by PARP1 hyperactivation and hypermodification. Proteins containing poly-ADP-ribose (PAR) interaction domains including macrodomains can act as readers of the ADP-ribose units. PARG and ARH3 are the erasers that remove PAR chains.
At present, it is unclear how exactly auto-modification and macroH2A1.1 binding modulate the activity of PARP1. But as increased auto-modification correlates with increased activation, it is possible that auto-modification stabilizes the enzyme in its active conformation. We can further envision two possible scenarios for how the binding of macroH2A1.1 ADP-ribosylated to PARP1 interferes with its activity: (i) The close binding of a single macrodomain to mono-ADP-ribosylated PARP1 or a form of PARP1 modified with a short PAR chain directly affects the conformation and activity of the enzyme. (ii) The binding of the macrodomains to all ends of growing PAR chains deprives PARP1 of access to its target site, which does not allow sufficient auto-modification to stabilize the active state. A major difference between these two hypothetical mechanisms is that one-to-one binding would suffice for the first mechanism while the second would require an excess of macroH2A1.1 macrodomains. Our observation that close to equimolar levels of macroH2A1.1 were sufficient to inhibit PARP1 in myotubes argues for the first and more direct mechanism [39] (Figure 3). In cases where macroH2A1.1 is present at lower levels than PARP1 or in situations where the PARP1-activating stimulus is too strong, endogenous inhibition is not expected to occur. However, macroH2A1.1 would still be able to recruit active and auto-modified forms of PARP1 with longer, multiple, and branched PAR chains to genomic loci (Figure 3). This has the potential to explain how macroH2A1.1 cooperates with PARP1 in gene regulation [38,42] as well as in DNA repair. Two examples of the latter are the promotion of cell survival in response to ionizing radiation [76] and the repair of oxidative damage [77]. Taking all these studies together, the relative ratio of macroH2A1.1 and PARP1 decides whether macroH2A1.1 acts as inhibitor or cooperative partner of PARP1. Inhibition is limited to situations in which the level of macroH2A1.1 is equal or exceeds the level of PARP1.
4.3. MacroH2A1.1 couples differentiated state and NAD+ metabolism
We studied the PARP inhibitory function of macroH2A1.1 during the terminal differentiation of myoblasts to myotubes. Our study suggested that this endogenous inhibition operates in terminally differentiated myotubes but not in proliferating myoblasts [39]. We found that macroH2A1.1 is barely expressed in fast proliferating cells [78] but rapidly upregulated through a switch in alternative splicing during myogenic differentiation, reaching levels matching those of PARP1 [39]. Inhibition of PARP1 by macroH2A1.1 leads to decreased NAD+ consumption in the nucleus and sustained levels of the precursor NMN. This in turn leads to increased pools of mitochondrial NAD+ and improved oxidative phosphorylation. The upregulation of macroH2A1.1 allows differentiated muscle cells to avoid unnecessary NAD+ consumption in the nucleus, prioritizing its use in the mitochondria for ATP production and cell survival. Several lines of observation suggest that this mechanism is general and not limited to muscle tissue. First, macroH2A1.1 is also upregulated during differentiation of other cell types including embryonic stem cells, colon cancer cells, and adipocytes [73,78,79]. Second, the overexpression of macroH2A1.1 but not macroH2A1.2 in liver cells prevented the accumulation of fat most likely by promoting its oxidation in mitochondria [80,81].
Taken together, differentiating cells have evolved an elegant mechanism coupling new energetic requirements of terminal differentiation to a change in chromatin composition, setting a higher threshold for PARP1 activation and nuclear NAD+ consumption. High levels of macroH2A1.1 inhibit PARP1, but the exact molecular mechanism of this taming remains to be elucidated.
5. Conclusion and outlook
Organisms have co-evolved mechanisms governing genome stability, transcriptional regulation, and metabolic homeostasis. It is not surprising that these mechanisms are interconnected. Shared metabolites are able to communicate states between distant organelles. It is now well accepted that NAD+ metabolism plays a major role in communication between the nucleus and mitochondria [44,45]. While the nucleus is responsible for all reactions using genomic DNA as a template, mitochondria are the cells’ major energy source. As such, it is not surprising that cells have evolved mechanisms to coordinate the activities of both organelles. Although mitochondria are a clearly separate metabolic compartment, they are connected to the nucleocytosolic compartment through reliance on NMN as a common precursor of NAD+. However, it is still unclear how exactly NMN enters the mitochondria. Another important question is whether alternative pathways and resources exist that can replenish the mitochondrial NAD+ pool. Novel fluorescence-based methods that enable measuring NAD+ levels in different compartments in situ will be very helpful to answer these questions [82]. Future work will need to address the question, which metabolites other than NMN act as signaling molecules between distant compartments?
A challenge for the PARP field is the development of methods that enable the detection of low or basal enzymatic activity. The measurement of PARP-1 activity in cells relies on the detection of its product, the instable PAR polymer that transiently accumulates after PARP-1 hyperactivation. Under steady-state conditions, the PAR polymer cannot be detected due to its rapid turnover. While it has been convincingly shown that acute activation of PARP1 by DNA damage or other stresses leads to NAD+ depletion, much less is known about the level of basal NAD+ consumption. Our study on myotubes suggested that even in non-cycling differentiated cells without induction of DNA damage by exogenous agents, basal PARP1 activity exists and if not blunted by macroH2A1.1 can consume a substantial amount of NAD+ [39]. Compensated by the salvage pathway, this was reflected by a depletion of the precursor NMN. The exact consequences of auto-MAR and PARylation for PARP activity remain to be studied and will provide a better understanding of the molecular basis of PARP1 inhibition by macroH2A1.1 binding. In this line, it is interesting to note that the linker of macroH2A1 itself can be ADP-ribosylated on serine 146 [83]. This leads us to wonder if the linker modification would conversely affect macroH2A1.1 capacity to bind and inhibit PARP1. Interestingly, the linker sequences differ between macroH2A1 and macroH2A2.
The involvement of macroH2A histone variants in DNA repair is complex and future research will need to dissect which functions are based on the interplay between macroH2A1.1 and PARP1 and which functions are contributions by other macroH2A isoforms and independent of PARP1. PARP1 activation is an early event in the DNA repair process and its partial inhibition by macroH2A1.1 reduces PARP1-dependent chromatin expansion [68], avoids DNA damage-induced NAD+ depletion, and alters the dynamics of PAR by delaying its production and increasing its half-life [77]. MacroH2A1.1 is recruited to PAR [74,84], which can lead to the large-scale reorganization of the chromatin [74] or its local incorporation into the chromatin surrounding the damaged site [76]. As a consequence of these functions, macroH2A1.1 promotes cell survival in response to ionizing radiation [76] and the repair of oxidative damage [77]. In mice, the genetic ablation of macroH2A1.1 led to a reduction of hematopoietic stem cells after high-dose irradiation [85]. Functions more related to later time points of the DNA damage repair process have been reported for macroH2A1.2. These include a role in the necessary re-condensation of chromatin in cooperation with the histone K9 methyltransferase PRDM2 [86]. At fragile sites and telomeres lengthened through the alternative ALT pathway, the presence of macroH2A contributes to a protective environment and promotes DNA repair through homologous recombination [87,88]. It remains to be tested whether the same domains of macroH2A mediate both the PARP1-independent functions in DNA repair as well as the function in regulating heterochromatin architecture [66,68].
For the histone variant community, it will be of great interest to understand to which extent the function of macroH2A1.1 as an endogenous inhibitor of PARP1 is relevant in different tissues and cell types. Physiological studies in knock-out animals can be expected to provide important information. More molecular questions are how the PARP1-related function intersects with the role of macroH2A variants to regulate nuclear organization and three-dimensional chromatin architecture [66,68]. Furthermore, it will be of great interest to understand when the two functions were acquired during evolution. Interestingly, two other histone variants have been related to metabolic activity. H2A.X is best known for its role in DNA damage signaling [89] but also regulates mitochondrial shape, activity, and biogenesis most likely through sustaining the expression of the key transcriptional regulator PCG-1 alpha [90]. Overexpression of the macroH2A1.2 isoform in mice had a systemic effect on metabolism and reduced fat mass [91]. Future research will need to examine the contributions made by different histone variants and to which extent they are mediated through gene regulation or direct effects on metabolic reactions.
Funding
Research in the Ladurner and Buschbeck labs was supported by the following grants: the Deutsche Forschungsgemeinschaft SFB 646 and SFB 1064 collaborative research centers (to AGL); the Deutsche Forschungsgemeinschaft CIPSM and SyNergy research excellence clusters (to AGL); the Bavarian BioSysNet Program (to AGL); the ERA-NET Neuron project Food4Thought funded by the Bundesministerium für Bildung und Forschung (to AGL); the Marie Skłodowska Curie Training network “ChroMe” H2020-MSCA-ITN-2015-675,610 (to MB and AGL); MINECO RTI2018-094005-B-I00 (to MB); MINECO-ISCIII PIE16/00011 (to MB); the Deutsche José Carreras Leukaemie Stiftung DJCLS 14R/2018 (to MB); AGAUR 2017-SGR-305 (to MB); and Fundació La Marató de TV3 257/C/2019 (to MB). Research at the IJC is supported by the La Caixa Foundation, the Fundació Internacional Josep Carreras, Celgene Spain, and the CERCA Program/Generalitat de Catalunya.
Author contributions
The literature revision introduction and discussion of the PhD thesis by SHB served as the basis for this review. MB wrote all of the sections related to NAD+ metabolism including the section on macroH2A1.1. GK and AGL wrote the section on PARP1. The figures were designed by sci.sters.editions (https://sci-sters-editions.com). All the authors edited the full manuscript and approved the final version.
Contributor Information
Andreas G. Ladurner, Email: andreas.ladurner@bmc.med.lmu.de.
Marcus Buschbeck, Email: mbuschbeck@carrerasresearch.org.
Conflict of interest
AGL is a founder and shareholder of Eisbach Bio GmbH. SHB is co-founder of sci.sters.editions. The authors declare no other conflicts of interest.
References
- 1.Palazzo L., Mikoč A., Ahel I. ADP-ribosylation: new facets of an ancient modification. FEBS Journal. 2017;284:2932–2946. doi: 10.1111/febs.14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraus W.L., Lis J.T. PARP goes transcription. Cell. 2003;113:677–683. doi: 10.1016/s0092-8674(03)00433-1. [DOI] [PubMed] [Google Scholar]
- 3.Gibson B.A., Kraus W.L. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nature Reviews Molecular Cell Biology. 2012;13:411–424. doi: 10.1038/nrm3376. [DOI] [PubMed] [Google Scholar]
- 4.Vyas S., Chesarone-Cataldo M., Todorova T., Huang Y.-H., Chang P. A systematic analysis of the PARP protein family identifies new functions critical for cell physiology. Nature Communications. 2013;4:2020. doi: 10.1038/ncomms3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniels C.M., Ong S.-E., Leung A.K.L. Perspective, Molecular Cell. 2015;58:911–924. doi: 10.1016/j.molcel.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupte R., Liu Z., Kraus W.L. PARPs and ADP-ribosylation: recent advances linking molecular functions to biological outcomes. Genes & Development. 2017;31:101–126. doi: 10.1101/gad.291518.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vyas S., Matic I., Uchima L., Rood J., Zaja R., Hay R.T. Family-wide analysis of poly(ADP-ribose) polymerase activity. Nature Communications. 2014;5:882. doi: 10.1038/ncomms5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liszt G., Ford E., Kurtev M., Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. Journal of Biological Chemistry. 2005;280:21313–21320. doi: 10.1074/jbc.M413296200. [DOI] [PubMed] [Google Scholar]
- 9.Haigis M.C., Sinclair D.A. Mammalian sirtuins: biological insights and disease relevance. Annual Review of Pathology: Mechanisms of Disease. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sebastián C., Satterstrom F.K., Haigis M.C., Mostoslavsky R. From sirtuin biology to human diseases: an update. Journal of Biological Chemistry. 2012;287:42444–42452. doi: 10.1074/jbc.R112.402768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantó C., Sauve A.A., Bai P. Molecular aspects of medicine. Molecular Aspects of Medicine. 2013;34:1168–1201. doi: 10.1016/j.mam.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hottiger M.O., Hassa P.O., Lüscher B., Schüler H., Koch-Nolte F. Toward a unified nomenclature for mammalian ADP-ribosyltransferases. Trends in Biochemical Sciences. 2010;35:208–219. doi: 10.1016/j.tibs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Amé J.-C., Spenlehauer C., de Murcia G. The PARP superfamily. BioEssays. 2004;26:882–893. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- 14.Langelier M.-F., Eisemann T., Riccio A.A., Pascal J.M. ScienceDirectPARP family enzymes: regulation and catalysis of the poly(ADP-ribose) posttranslational modification. Current Opinion in Structural Biology. 2018;53:187–198. doi: 10.1016/j.sbi.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Amours D., Desnoyers S., D'Silva I., Poirier G.G. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochemical Journal. 1999;342:249–268. [PMC free article] [PubMed] [Google Scholar]
- 16.Langelier M.F., Planck J.L., Roy S., Pascal J.M. Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1. Science. 2012;336:728–732. doi: 10.1126/science.1216338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhuri A.R., Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nature Reviews Molecular Cell Biology. 2017;18:610–621. doi: 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langelier M.-F., Zandarashvili L., Aguiar P.M., Black Ben E., Pascal J.M. Analog reveals PARP-1 substrate-blocking mechanism and allosteric communication from catalytic center to DNA-binding domains. Nature Communications. 2018;9:844. doi: 10.1038/s41467-018-03234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiehlbauch C.C., Aboul-Ela N., Jacobson E.L., Ringer D.P., Jacobson M.K. High resolution fractionation and characterization of ADP-ribose polymers. Analytical Biochemistry. 1993;208:26–34. doi: 10.1006/abio.1993.1004. [DOI] [PubMed] [Google Scholar]
- 20.Liu C., Vyas A., Kassab M.A., Singh A.K., Yu X. The role of poly ADP-ribosylation in the first wave of DNA damage response. Nucleic Acids Research. 2017;45:8129–8141. doi: 10.1093/nar/gkx565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altmeyer M., Neelsen K.J., Teloni F., Pozdnyakova I., Pellegrino S., Grøfte M. Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose) Nature Communications. 2015;6:8088. doi: 10.1038/ncomms9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teloni F., Altmeyer M. Readers of poly(ADP-ribose): designed to be fit for purpose. Nucleic Acids Research. 2016;44:993–1006. doi: 10.1093/nar/gkv1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortusewicz O., Amé J.-C., Schreiber V., Leonhardt H. Feedback-regulated poly(ADP-ribosyl)ation by PARP-1 is required for rapid response to DNA damage in living cells. Nucleic Acids Research. 2007;35:7665–7675. doi: 10.1093/nar/gkm933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahel D., Horejsí Z., Wiechens N., Polo S.E., Garcia-Wilson E., Ahel I. Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science. 2009;325:1240–1243. doi: 10.1126/science.1177321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sellou H., Lebeaupin T., Chapuis C., Smith R., Hegele A., Singh H.R. The poly(ADP-ribose)-dependent chromatin remodeler Alc1 induces local chromatin relaxation upon DNA damage. Molecular Biology of the Cell. 2016;27:3791–3799. doi: 10.1091/mbc.E16-05-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottschalk A.J., Timinszky G., Kong S.E., Jin J., Cai Y., Swanson S.K. Poly(ADP-ribosyl)ation directs recruitment and activation of an ATP-dependent chromatin remodeler. Proceedings of the National Academy of Sciences. 2009;106:13770–13774. doi: 10.1073/pnas.0906920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luijsterburg M.S., de Krijger I., Wiegant W.W., Shah R.G., Smeenk G., de Groot A.J.L. PARP1 links CHD2-mediated chromatin expansion and H3.3 deposition to DNA repair by non- homologous end-joining. Molecular Cell. 2016;61:547–562. doi: 10.1016/j.molcel.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barkauskaite E., Jankevicius G., Ladurner A.G., Ahel I., Timinszky G. The recognition and removal of cellular poly(ADP-ribose) signals. FEBS Journal. 2013;280:3491–3507. doi: 10.1111/febs.12358. [DOI] [PubMed] [Google Scholar]
- 29.Fontana P., Bonfiglio J.J., Palazzo L., Bartlett E., Matic I., Ahel I. Serine ADP-ribosylation reversal by the hydrolase ARH3. Elife. 2017;6 doi: 10.7554/eLife.28533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright R.H.G., Lioutas A., Le Dily F., Soronellas D., Pohl A., Bonet J. ADP-ribose-derived nuclear ATP synthesis by NUDIX5 is required for chromatin remodeling. Science. 2016;352:1221–1225. doi: 10.1126/science.aad9335. [DOI] [PubMed] [Google Scholar]
- 31.Aredia F., Scovassi A.I. Biochemical pharmacology. Biochemical Pharmacology. 2014;92:157–163. doi: 10.1016/j.bcp.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Luo X., Kraus W.L. On PAR with PARP: cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes & Development. 2012;26:417–432. doi: 10.1101/gad.183509.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kassner I., Andersson A., Fey M., Tomas M., Ferrando-May E., Hottiger M.O. SET7/9-dependent methylation of ARTD1 at K508 stimulates poly-ADP-ribose formation after oxidative stress. Open Biol. 2013;3:120173. doi: 10.1098/rsob.120173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kauppinen T.M., Chan W.Y., Suh S.W., Wiggins A.K., Huang E.J., Swanson R.A. Direct phosphorylation and regulation of poly(ADP-ribose) polymerase-1 by extracellular signal-regulated kinases 1/2. Proc. Natl. Acad. Sci. U.S.a. 2006;103:7136–7141. doi: 10.1073/pnas.0508606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen-Armon M., Visochek L., Rozensal D., Kalal A., Geistrikh I., Klein R. DNA-independent PARP-1 activation by phosphorylated ERK2 increases Elk1 activity: a link to histone acetylation. Molecular Cell. 2007;25:297–308. doi: 10.1016/j.molcel.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Gibbs-Seymour I., Fontana Pietro, Rack J.G.M., Ahel I. HPF1/C4orf27 is a PARP-1-interacting protein that regulates PARP-1 ADP-ribosylation activity. Molecular Cell. 2016;62:432–442. doi: 10.1016/j.molcel.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonfiglio J.J., Fontana Pietro, Zhang Q., Colby T., Gibbs-Seymour I., Atanassov I. Serine ADP-ribosylation depends on HPF1. Molecular Cell. 2017;65:932–940.e6. doi: 10.1016/j.molcel.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouararhni K., Hadj-Slimane R., Ait-Si-Ali S., Robin P., Mietton F., Harel-Bellan A. The histone variant mH2A1.1 interferes with transcription by down-regulating PARP-1 enzymatic activity. Genes & Development. 2006;20:3324–3336. doi: 10.1101/gad.396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Posavec Marjanović M., Hurtado-Bagès S., Lassi M., Valero V., Malinverni R., Delage H. MacroH2A1.1 regulates mitochondrial respiration by limiting nuclear NAD+ consumption. Nature Structural & Molecular Biology. 2017:1–14. doi: 10.1038/nsmb.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marjanović M.P., Crawford K., Ahel I. PARP, transcription and chromatin modeling. Seminars in Cell & Developmental Biology. 2016:1–12. doi: 10.1016/j.semcdb.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Kim M.Y., Mauro S., Gévry N., Lis J.T., Kraus W.L. NAD+-Dependent modulation of chromatin structure and transcription by nucleosome binding properties of PARP-1. Cell. 2004;119:803–814. doi: 10.1016/j.cell.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Chen H., Ruiz P.D., Novikov L., Casill A.D., Park J.W., Gamble M.J. MacroH2A1.1 and PARP-1 cooperate to regulate transcription by promoting CBP-mediated H2B acetylation. Nature Structural & Molecular Biology. 2014;21:981–989. doi: 10.1038/nsmb.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Z., Kraus W.L. Catalytic-independent functions of PARP-1 determine Sox2 pioneer activity at intractable genomic loci. Molecular Cell. 2017;65:589–603. doi: 10.1016/j.molcel.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cantó C., Menzies K.J., Auwerx J. Metabolism and the controlof energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metabolism. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strømland Ø., Niere M., Nikiforov A.A., VanLinden M.R., Heiland I., Ziegler M. Keeping the balance in NAD metabolism. Biochm. Soc. Trans. 2019;47:119–130. doi: 10.1042/BST20180417. [DOI] [PubMed] [Google Scholar]
- 46.Verdin E. NAD⁺ in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–1213. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]
- 47.Berger F., Lau C., Dahlmann M., Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. Journal of Biological Chemistry. 2005;280:36334–36341. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- 48.Zhang T., Berrocal J.G., Yao J., DuMond M.E., Krishnakumar R., Ruhl D.D. Regulation of poly(ADP-ribose) polymerase-1-dependent gene expression through promoter-directed recruitment of a nuclear NAD +Synthase. Journal of Biological Chemistry. 2012;287:12405–12416. doi: 10.1074/jbc.M111.304469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryu K.W., Nandu T., Kim J., Challa S., DeBerardinis R.J., Kraus W.L. Metabolic regulation of transcription through compartmentalized NAD +biosynthesis. Science. 2018;360 doi: 10.1126/science.aan5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chambon P., Weill J.D., Mandel P. Nicotinamide mononucleotide activation of new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochemical and Biophysical Research Communications. 1963;11:39–43. doi: 10.1016/0006-291x(63)90024-x. [DOI] [PubMed] [Google Scholar]
- 51.Altmeyer M., Hottiger M.O. Poly(ADP-ribose) polymerase 1 at the crossroad of metabolic stress and inflammation in aging. Aging. 2009;1:458–469. doi: 10.18632/aging.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright R.H.G., Castellano G., Bonet J., Le Dily F., Font-Mateu J., Ballare C. CDK2-dependent activation of PARP-1 is required for hormonal gene regulation in breast cancer cells. Genes & Development. 2012;26:1972–1983. doi: 10.1101/gad.193193.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fouquerel E., Goellner E.M., Yu Z., Gagné J.-P., de Moura M.B., Feinstein T. ARTD1/PARP1 negatively regulates glycolysis by inhibiting hexokinase 1 independent of NAD. Cell Reports. 2014;8:1819–1831. doi: 10.1016/j.celrep.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fouquerel E., Sobol R.W. ARTD1 (PARP1) activation and NAD+ in DNA repair and cell death. DNA Repair. 2014;23:27–32. doi: 10.1016/j.dnarep.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hurtado-Bagès S., Guberovic I., Buschbeck M. The MacroH2A1.1 – PARP1 Axis at the intersection between stress response and metabolism. Frontiers in Genetics. 2018;9:17860. doi: 10.3389/fgene.2018.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yonemura Y., Takashima T., Miwa K., Miyazaki I., Yamamoto H., Okamoto H. Amelioration of diabetes mellitus in partially depancreatized rats by poly(ADP-ribose) synthetase inhibitors. Evidence of islet B-cell regeneration. Diabetes. 1984;33:401–404. doi: 10.2337/diab.33.4.401. [DOI] [PubMed] [Google Scholar]
- 57.Burkart V., Wang Z.Q., Radons J., Heller B., Herceg Z., Stingl L. Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic beta-cell destruction and diabetes development induced by streptozocin. Nature Medicine. 1999;5:314–319. doi: 10.1038/6535. [DOI] [PubMed] [Google Scholar]
- 58.Szabo C., Biser A., Benko R., Bottinger E., Susztak K. Poly(ADP-Ribose) polymerase inhibitors ameliorate nephropathy of type 2 diabetic leprdb/db mice. Diabetes. 2006;55:3004–3012. doi: 10.2337/db06-0147. [DOI] [PubMed] [Google Scholar]
- 59.Pirinen E., Cantó C., Jo Y.S., Morato L., Zhang H., Menzies K.J. Pharmacological inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metabolism. 2014;19:1034–1041. doi: 10.1016/j.cmet.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lehmann M., Pirinen E., Mirsaidi A., Kunze F.A., Richards P.J., Auwerx J. ARTD1-induced poly-ADP-ribose formation enhances PPARγ ligand binding and co-factor exchange. Nucleic Acids Research. 2014;43:129–142. doi: 10.1093/nar/gku1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oláh G., Szczesny B., Brunyánszki A., López-García I.A., Gerö D., Radák Z. Differentiation-associated downregulation of poly(ADP-ribose) polymerase-1 expression in myoblasts serves to increase their resistance to oxidative stress. PloS One. 2015;10 doi: 10.1371/journal.pone.0134227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai P., Cantó C., Oudart H., Brunyánszki A., Cen Y., Thomas C. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metabolism. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buschbeck M., Hake S.B. Variants of core histones and their roles in cell fate decisions, development and cancer. Nature Reviews Molecular Cell Biology. 2017:1–16. doi: 10.1038/nrm.2016.166. [DOI] [PubMed] [Google Scholar]
- 64.Chakravarthy S., Gundimella S.K.Y., Caron C., Perche P.-Y., Pehrson J.R., Khochbin S. Structural characterization of the histone variant macroH2A. Molecular and Cellular Biology. 2005;25:7616–7624. doi: 10.1128/MCB.25.17.7616-7624.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corujo D., Buschbeck M. Post-translational modifications of H2A histone variants and their role in cancer. Cancers. 2018;10:59. doi: 10.3390/cancers10030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Douet J., Corujo D., Malinverni R., Renauld J., Sansoni V., Posavec Marjanović M. MacroH2A histone variants maintain nuclear organization and heterochromatin architecture. Journal of Cell Science. 2017;130:1570–1582. doi: 10.1242/jcs.199216. [DOI] [PubMed] [Google Scholar]
- 67.Fu Y., Lv P., Yan G., Fan H., Cheng L., Zhang F. MacroH2A1 associates with nuclear lamina and maintains chromatin architecture in mouse liver cells. Scientific Reports. 2015:1–12. doi: 10.1038/srep17186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kozlowski M., Corujo D., Hothorn M., Guberovic I., Mandemaker I.K., Blessing C. MacroH2A histone variants limit chromatin plasticity through two distinct mechanisms. EMBO Reports. 2018;19 doi: 10.15252/embr.201744445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Posavec M., Timinszky G., Buschbeck M. Macro domains as metabolite sensors on chromatin. Cellular and Molecular Life Sciences. 2013;70:1509–1524. doi: 10.1007/s00018-013-1294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karras G.I., Kustatscher G., Buhecha H.R., Allen M.D., Pugieux C., Sait F. The macro domain is an ADP-ribose binding module. The EMBO Journal. 2005;24:1911–1920. doi: 10.1038/sj.emboj.7600664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kustatscher G., Hothorn M., Pugieux C., Scheffzek K., Ladurner A.G. Splicing regulates NAD metabolite binding to histone macroH2A. Nature Structural & Molecular Biology. 2005;12:624–625. doi: 10.1038/nsmb956. [DOI] [PubMed] [Google Scholar]
- 72.Pehrson J.R., Costanzi C., Dharia C. Developmental and tissue expression patterns of histone macroH2A1 subtypes. Journal of Cellular Biochemistry. 1997;65:107–113. doi: 10.1002/(sici)1097-4644(199704)65:1<107::aid-jcb11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 73.Sporn J.C., Jung B. Differential regulation and predictive potential of MacroH2A1 isoforms in colon cancer. Ajpa. 2012;180:2516–2526. doi: 10.1016/j.ajpath.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Timinszky G., Till S., Hassa P.O., Hothorn M., Kustatscher G., Nijmeijer B. A macrodomain-containing histone rearranges chromatin upon sensing PARP1 activation. Nature Structural & Molecular Biology. 2009;16:923–929. doi: 10.1038/nsmb.1664. [DOI] [PubMed] [Google Scholar]
- 75.Nusinow D.A., Hernández-Muñoz I., Fazzio T.G., Shah G.M., Kraus W.L., Panning B. Poly(ADP-ribose) polymerase 1 is inhibited by a histone H2A variant, MacroH2A, and contributes to silencing of the inactive X chromosome. Journal of Biological Chemistry. 2007;282:12851–12859. doi: 10.1074/jbc.M610502200. [DOI] [PubMed] [Google Scholar]
- 76.Xu C., Xu Y., Gursoy-Yuzugullu O., Price B.D. The histone variant macroH2A1.1 is recruited to DSBs through a mechanism involving PARP1. FEBS Letters. 2012;586:3920–3925. doi: 10.1016/j.febslet.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ruiz P.D., Hamilton G.A., Park J.W., Gamble M.J. MacroH2A1 regulation of Poly(ADP-ribose) synthesis and stability prevents necrosis and promotes DNA repair. Molecular and Cellular Biology. 2019;40:517. doi: 10.1128/MCB.00230-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Creppe C., Janich P., Cantarino N., Noguera M., Valero V., Musulen E. MacroH2A1 regulates the balance between self-renewal and differentiation commitment in embryonic and adult stem cells. Molecular and Cellular Biology. 2012;32:1442–1452. doi: 10.1128/MCB.06323-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wan D., Liu C., Sun Y., Wang W., Huang K., Zheng L. MacroH2A1.1 cooperates with EZH2 to promote adipogenesis by regulating Wnt signaling. Journal of Molecular Cell Biology. 2017;9:325–337. doi: 10.1093/jmcb/mjx027. [DOI] [PubMed] [Google Scholar]
- 80.Podrini C., Koffas A., Chokshi S., Vinciguerra M., Lelliott C.J., White J.K. MacroH2A1 isoforms are associated with epigenetic markers for activation of lipogenic genes in fat-induced steatosis. The FASEB Journal. 2015;29:1676–1687. doi: 10.1096/fj.14-262717. [DOI] [PubMed] [Google Scholar]
- 81.Pazienza V., Borghesan M., Mazza T., Sheedfar F., Panebianco C., Williams R. SIRT1-metabolite binding histone macroH2A1.1 protects hepatocytes against lipid accumulation. Aging. 2014;6:35–47. doi: 10.18632/aging.100632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cambronne X.A., Stewart M.L., Kim D., Jones-Brunette A.M., Morgan R.K., Farrens D.L. Biosensor reveals multiple sources for mitochondrial NAD⁺. Science. 2016;352:1474–1477. doi: 10.1126/science.aad5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hendriks I.A., Larsen S.C., Nielsen M.L. An advanced strategy for comprehensive profiling of ADP-ribosylation sites using mass spectrometry-based proteomics. Molecular & Cellular Proteomics. 2019;18:1010–1026. doi: 10.1074/mcp.TIR119.001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mehrotra P.V., Ahel D., Ryan D.P., Weston R., Wiechens N., Kraehenbuehl R. DNA repair factor APLF is a histone chaperone. Molecular Cell. 2011;41:46–55. doi: 10.1016/j.molcel.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bereshchenko O., Lo Re O., Nikulenkov F., Flamini S., Kotaskova J., Mazza T. Deficiency and haploinsufficiency of histone macroH2A1.1 in mice recapitulate hematopoietic defects of human myelodysplastic syndrome. Clinical Epigenetics. 2019:1–14. doi: 10.1186/s13148-019-0724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khurana S., Kruhlak M.J., Kim J., Tran A.D., Liu J., Nyswaner K. A macrohistone variant links dynamic chromatin compaction to BRCA1-dependent genome maintenance. Cell Reports. 2014;8:1049–1062. doi: 10.1016/j.celrep.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim J., Sturgill D., Sebastian R., Khurana S., Tran A.D., Edwards G.B. Replication stress shapes a protective chromatin environment across fragile genomic regions. Molecular Cell. 2017:1–20. doi: 10.1016/j.molcel.2017.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim J., Sun C., Tran A.D., Chin P.-J., Ruiz P.D., Wang K. The macroH2A1.2 histone variant links ATRX loss to alternative telomere lengthening. Nature Structural & Molecular Biology. 2019:1–12. doi: 10.1038/s41594-019-0192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rogakou E.P., Pilch D.R., Orr A.H., Ivanova V.S., Bonner W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. Journal of Biological Chemistry. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 90.Weyemi U., Paul B.D., Bhattacharya D., Malla A.P., Boufraqech M., Harraz M.M. Histone H2AX promotes neuronal health by controlling mitochondrial homeostasis. Proceedings of the National Academy of Sciences. 2019;116:7471–7476. doi: 10.1073/pnas.1820245116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pazienza V., Panebianco C., Rappa F., Memoli D., Borghesan M., Cannito S. Histone macroH2A1.2 promotesmetabolic health and leanness by inhibiting adipogenesis. Epigenetics & Chromatin. 2016;45:1–19. doi: 10.1186/s13072-016-0098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]