ABSTRACT
The frequency of patients who switch to a second‐line therapy from a frontline second‐generation (2gen) tyrosine kinase inhibitor (TKI) such as dasatinib and nilotinib, is still substantially unknown. We retrospectively investigated a large series of chronic phase chronic myeloid leukemia (CP‐CML) patients initially treated with 2gen TKIs monitored through the Italian Medicines Agency (AIFA Agenzia Italiana del farmaco) registries. Overall, 2420 patients were analyzed over a period of 6 years. One hundred and fifty‐seven patients (16.3%) treated with dasatinib and 164 treated with nilotinib (11.3%) have switched to another drug, with an overall frequency of 13.2%. In the dasatinib cohort, 39.4% of patients changed treatment for failure and 36.3% for intolerance as compared to 45.7% and 27.4% respectively in the nilotinib cohort. Overall, the median time to switch due to resistance was 293 days, whereas it was 317 days in case of intolerance. Resistance was observed mainly in younger male patients with high‐risk features, while intolerance was not related to any baseline parameter. After resistance/intolerance to nilotinib, the majority of patients switched to dasatinib (53.8%) whereas in case of frontline dasatinib to ponatinib (43.2%). To the best of our knowledge these data provide the first report on the frequency of discontinuation of frontline 2gen TKIs and on the main causes and pattern of choice to a second‐line therapy in the real‐life setting.
Keywords: chronic myeloid leukemia, failure, intolerance, second‐generation TKIs
Overall, 2420 patients were analyzed over a period of 6 years. One hundred and fifty‐seven patients (16.3%) treated with dasatinib and 164 treated with nilotinib (11.3%) have switched to another drug, with an overall frequency of 13.2%. In the dasatinib cohort, 39.4% of patients changed treatment for failure and 36.3% for intolerance as compared to 45.7% and 27.4% respectively in the nilotinib cohort. Resistance was observed mainly in younger male patients with high‐risk features, while intolerance was not related to any baseline parameter. To the best of our knowledge these data provide the first report on the frequency of discontinuation of frontline 2gen TKIs and on the main causes and pattern of choice to a second‐line therapy in the real‐life setting.

1. INTRODUCTION
The treatment of chronic myeloid leukemia (CML) has drastically changed since the introduction of imatinib, the first‐generation tyrosine kinase inhibitor (TKI). Imatinib induced more than 85% of complete cytogenetic response (CCyR) with a major molecular response (BCR‐ABL1 ratio < 0.1% according to International Scale or 3 log‐reduction) in approximately 60% of patients. 1 , 2 However, despite these excellent results, more than 30% of treated subjects experienced treatment failure either for resistance or intolerance and had to be switched to a second‐line therapy. 3 Frontline treatment with second‐generation TKIs (2gen TKIs), namely dasatinib and nilotinib, has further improved the outcome: although no differences in terms of overall survival have been detected as compared to imatinib, both these drugs induced faster and deeper molecular responses, decreasing the number of patients experiencing a progression to advanced phases of the disease. 4 , 5 Despite the confirmed long‐term results, some patients still experienced either failure or severe intolerance to 2gen TKIs requiring therefore a subsequent therapy. According to the only published experience on the outcome of patients resistant/intolerant to a frontline 2gen TKI reported by MD Anderson Cancer Center (MDACC) out of 218 patients treated with dasatinib or nilotinib after a median follow‐up of 23 months, 40 patients (18%) discontinued therapy, 25 initially treated with nilotinib (21% of all treated with nilotinib) and 15 (15%) initially treated with dasatinib. The majority of patients switched to a subsequent line for intolerance (16 subjects) and only a minor percentage for resistance (5 patients) or for progression (4 patients). Eleven subjects received imatinib as second‐line therapy and only two patients switched to third‐generation ponatinib. 6 Considering the still persisting paucity of data on frequency and causes of discontinuation from frontline 2gen TKIs and on second‐line therapy selection, the aim of this study is to provide a real‐life picture on these crucial issues based on a large series of Italian patients from AIFA registries.
2. PATIENTS AND METHODS
For this analysis, we collected data from AIFA registries of all newly diagnosed chronic phase CML adult Italian patients treated with frontline dasatinib or nilotinib from January 2013 to December 2018. The web‐system allowed the storing and monitoring of clinical characteristics of patients eligible for treatment with 2gen TKIs according to prespecified criteria. Registered parameters for all TKIs were therapeutic drug indication, baseline characteristics (including age and Sokal score), patient outcome, treatment duration, principal reason for treatment discontinuation, occurrence of adverse reactions. According to Italian laws monitoring of these parameters does not require any informed consent or formal approval from ethical committees. Any included patient did receive, however, information about the purposes of the monitoring. Dasatinib and nilotinib eligibility form included demographic data (place of birth, age, and sex) and the Sokal risk class. 7 Prescription forms recorded the date of prescription, dose administered, and whether adverse reactions had occurred since the last prescription. Finally, the end of treatment form collected the reasons for treatment discontinuation. Time to treatment discontinuation (TTD) was defined as the time occurring between the initial prescription and the date of treatment discontinuation for any cause, including death and lost to follow‐up. A patient was defined “lost to follow‐up” in the absence of any prescription or re‐evaluation for at least 180 days after the last registry entry. Switches to a different TKI were assessed considering also bosutinib and ponatinib as destination treatment, by checking the relative AIFA monitoring registries on the basis of a patient unique identifier. Since imatinib was not actively monitored by AIFA, some uncertainties on subsequent switch to first‐generation TKI remains for the patients that have been lost to follow‐up. The time interval between the end of the first TKI treatment and the beginning of the second line was also evaluated, in order to rule out the possibility that other unmonitored therapy might have been administered between two subsequent recorded treatments.
3. RESULTS
From January 2013 to December 2018, 2.420 patients with newly diagnosed CP‐CML treated with frontline 2gen TKIs were recorded into the AIFA registries. Of them, 964 patients (39.8%) were treated with dasatinib and 1456 (60.2%) with nilotinib. A change of therapy was recorded in 321 subjects (13.2%), 157 (16.3%) of the dasatinib cohort, and 164 (11.3%) of the nilotinib cohort. The median time for switch was 354 days, with a median of 386 days for dasatinib and 323 days for nilotinib‐treated patients. Overall, 6.9% of patients switched within 365 days from start of treatment, 7.6% of the dasatinib cohort and 6.5% of the nilotinib cohort. Baseline features of CML patients treated with 2gen TKIs who change therapy are reported in Table 1. Overall, at the time of first prescription the median age was 54 years (range 21‐83) with a prevalence of male patients (59.8%). The majority of patients were aged less than 65 years (76%) with only 77 older subjects (> or equal to 65 years, 23.9%). According to Sokal risk, 103 (32%) patients were classified as low risk, 118 (36.7%) as intermediate and 100 (31.1%) as high risk (Table 1). A comparison between the dasatinib and nilotinib populations revealed a significant difference in terms of Sokal risk (P < .00001) and age at presentation, with patients treated with nilotinib being significantly younger than patients who received dasatinib (P < .00001). As far as the main reasons for discontinuation are concerned, 137 subjects (42.68%) were considered as treatment failure, 102 (31.78%) as intolerant, whereas 51 patients (15.89%) switched for nondrug‐related reasons and in 31 patients (9.6%) the cause is missing.
Table 1.
Baseline features of whole cohort
| Dasatinib | Nilotinib | Overall | |
|---|---|---|---|
| (N = 157) | (N = 164) | (N = 321) | |
| Gender—no. (%) | |||
| Female | 63 (40.13%) | 66 (40.24%) | 129 (40.19%) |
| Male | 94 (59.87%) | 98 (59.76%) | 192 (59.81%) |
| Age | |||
| Median—y | 57 | 51 | 54 |
| Range—y | 21‐82 | 21‐83 | 21‐83 |
| <65 y | 108 (68.79%) | 136 (82.93%) | 244 (76.01%) |
| ≥65 y | 49 (31.21%) | 28 (17.07%) | 77 (23.99%) |
| Sokal score | |||
| Low‐risk | 41 (26.11%) | 62 (37.80%) | 103 (32.09%) |
| Intermediate‐risk | 63 (40.13%) | 55 (33.54%) | 118 (36.76%) |
| High‐risk | 53 (33.76%) | 47 (28.66%) | 100 (31.15%) |
| Switch | 157 (16.3%) | 164 (11.3%) | 321 (13.27%) |
| Switch per 1000 y of treatment | 69.6 | 45.7 | 55.0 |
| Mean (median) days at switch | 467 (386) | 393 (323) | 430 (354) |
| Switch within 365 d from treatment start | 73 (7.6%) | 94 (6.5%) | 167 (6.9%) |
Considering the 137 patients who changed therapy for resistance/progression (Table 2), the overall median time to the switch was 293 days, 286 days for dasatinib and 293 days for nilotinib‐treated patients. Fifty‐nine percent of patients switched within 365 days from treatment start. This cohort was represented mainly by male patients (63.5%), median age 52 years, being only 25 of them (18.2%) aged ≥ 65 years. The majority of patients were classified as having at presentation a high Sokal risk (40.8%) or intermediate risk (33.5%) with only 25.5% being classified as low risk (Table 2).
Table 2.
Resistant cohort: baseline features
| Dasatinib | Nilotinib | Overall | |
|---|---|---|---|
| (N = 62) | (N = 75) | (N = 137) | |
| Gender—no. (%) | |||
| Female | 21 (33.87%) | 29 (38.67%) | 50 (36.50%) |
| Male | 41 (66.13%) | 46 (61.33%) | 87 (63.50%) |
| Age | |||
| Median—y | 57 | 51 | 52 |
| Range—y | 24‐82 | 21‐83 | 21‐83 |
| <65 y | 46 (74.19%) | 66 (88.00%) | 112 (81.75%) |
| ≥65 y | 16 (25.81%) | 9 (12.00%) | 25 (18.25%) |
| Sokal score | |||
| Low‐risk | 12 (19.35%) | 23 (30.67%) | 35 (25.55%) |
| Intermediate | 23 (37.10%) | 23 (30.67%) | 46 (33.58%) |
| High‐risk | 27 (43.55%) | 29 (38.67%) | 56 (40.88%) |
| Mean (Median) days at switch | 386 (286.5) | 335 (293) | 358 (293) |
| Switch within 365 d from treatment start | 34 (54.8%) | 47 (62.7%) | 81 (59.1%) |
One hundred and two patients switched for intolerance after a median time of 317 days, 386 for dasatinib and 189 for nilotinib‐treated subjects (Table 3). We can speculate about the short time observed in nilotinib‐treated patients implying the possible related metabolic side effects which have required discontinuation. In this cohort there was a slight difference in terms of gender with 51.9% male and 48.1% of female subjects. The median age was 57 years (range 22‐80) with 35% of patients aged ≥ 65 years; no difference among Sokal risk classification at baseline was detected, being 29.4% low risk, 45% intermediate and 25.4% as high risk (Table 3).
Table 3.
Intolerant cohort: baseline features
| Dasatinib | Nilotinib | Overall | |
|---|---|---|---|
| (N = 57) | (N = 45) | (N = 102) | |
| Gender—no. (%) | |||
| Female | 25 (43.86%) | 24 (53.33%) | 49 (48.04%) |
| Male | 32 (56.14%) | 21 (46.67%) | 53 (51.96%) |
| Age | |||
| Median—r | 60 | 51 | 57 |
| Range—y | 32‐80 | 22‐78 | 22‐80 |
| <65 y | 32 (56.14%) | 34 (75.56%) | 66 (64.71%) |
| ≥65 y | 25 (43.86%) | 11 (24.44%) | 36 (35.29%) |
| Sokal score | |||
| Low‐risk | 15 (26.32%) | 15 (33.33%) | 30 (29.41%) |
| Intermediate‐risk | 28 (49.12%) | 18 (40.00%) | 46 (45.10%) |
| High‐risk | 14 (24.56%) | 12 (26.67%) | 26 (25.49%) |
| MEAN (median) days at switch | 517 (386) | 327 (189) | 434 (317) |
| Switch within 365 d from treatment start | 26 (45.6%) | 32 (71.1%) | 58 (56.9%) |
Details on second‐line therapies after discontinuation of frontline dasatinib or nilotinib are reported in Figure 1. The majority of patients who changed from nilotinib, received as second‐line dasatinib (53.8%), ponatinib (31.8%), or in lower percentage bosutinib (14.5%) while from dasatinib the majority of subjects switched to ponatinib (43.2%), 34% to nilotinib and 22.8% to bosutinib. The choice of second line over time is represented in Figure 2.
Figure 1.
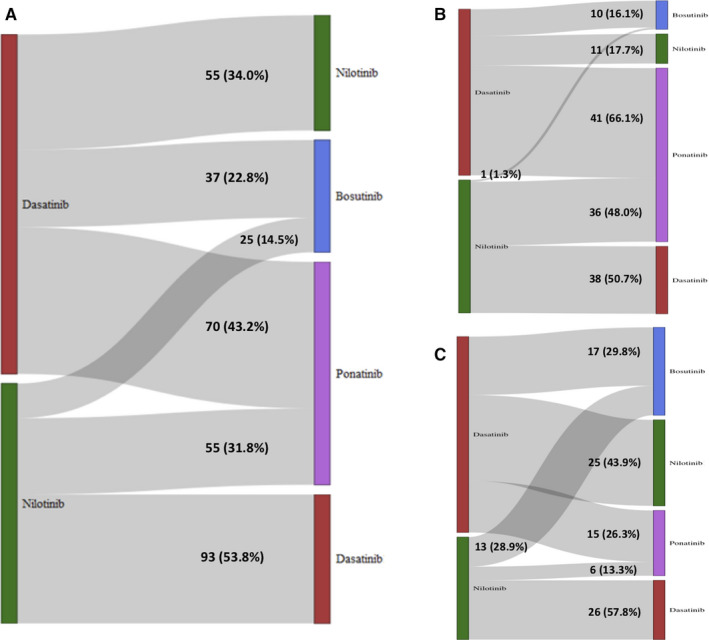
Second choice after discontinuation of first‐line dasatinib (in red) or nilotinib (in green) for any reasons (panel A), for treatment failure (panel B) or intolerance (panel C). Second TKI bosutinib and ponatinib are blue and violet colored, respectively
Figure 2.
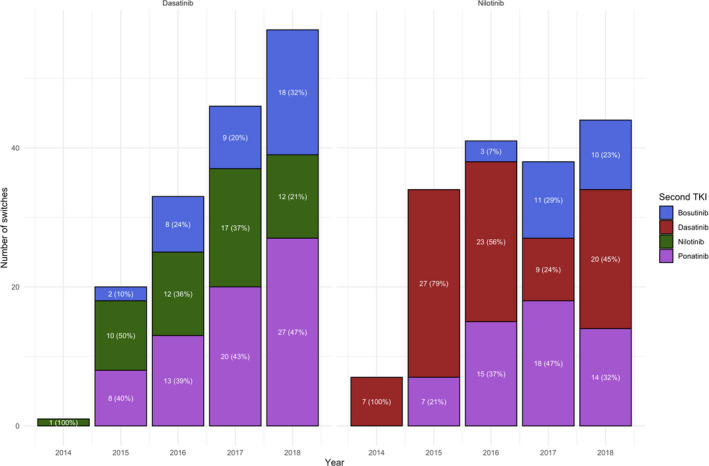
Choice of second drug reported year by year in a period of 5 y
4. DISCUSSION
Second‐generation TKIs used as frontline treatment increased the rate of faster and deeper molecular responses over time, reducing the possibility to experience a progression to advanced phase. 8 , 9 As with imatinib, some patients can experience resistance and/or intolerance to these drugs, but remains still largely unknown how many patients treated outside clinical trials discontinue the treatment permanently. Until now only a few publications reported the outcome of frontline 2gen TKI CML‐treated patients, in particular the SIMPLICITY trial 10 , 11 and the MDACC experience. 6 The SIMPLICITY trial is an observational study performed in US and six European countries with the primary aim of investigating the frontline TKI use and management patterns in routine clinical practice. Twelve‐month follow‐up data are reported from October 2010 to September 2015; out of 1242 patients enrolled, 418 received dasatinib and 408 were treated with nilotinib. 10 Treatment selection was based on the baseline comorbidity profile with a predominance for 2gen TKIs from 2014 onward. A comparison of Italian rate of discontinuation with the other European countries showed a rate of permanent discontinuation in Italy in the first 12 months of 8.9% for dasatinib cohort and 15.1% in the nilotinib‐treated cohort, whereas in the rest of European countries it was of 19.6% for dasatinib and 13.8% for nilotinib. 11
While the median time of discontinuation in Italy was 4.6 and 5.5 months for dasatinib and nilotinib, respectively, in the remaining countries it was shorter being 4.4 months for dasatinib and 1.4 months for nilotinib. Intolerance was reported as the most frequent cause of first‐line TKI discontinuation while primary resistance was the second most common one. No statistically significant predictive factors have been identified in the Italian population, whereas in the rest of European countries female and patients treated with imatinib were more likely to discontinue the treatment. While the switch to a second line occurred mostly between 3 and 9 months for dasatinib‐treated patients similarly to what occurred for nilotinib, with imatinib the majority of patients switched earlier after 3 months of treatment.
As compared to this sponsored trial not representative of the actual real‐life setting due to the criteria for selection of patients, according to our data from AIFA registries in Italy 157 patients (16.3%) treated with frontline dasatinib and 164 treated with nilotinib (11.3%) have switched to another drug, with an overall incidence of 13.2%. Differently from what usually reported in clinical trials, the main reason to discontinue a frontline 2gen TKI seems to be resistance being 39.4% in the dasatinib‐treated cohort compared to 45.7% in nilotinib‐treated patients. Interestingly, the majority of patients were aged less than 65 years (76%) with only 77 (23.9%) older subjects; it can be speculated that 2gen TKIs were used prevalently in younger patients, probably due to specific safety profile and that probably the rate of discontinuation is significantly lower, if compared to sponsored trials, due to the absence of dose modification and discontinuation forced criteria. Overall, the Sokal risk stratification showed that the majority of patients who experienced resistance and failure were classified as having at presentation a high Sokal risk (40.8%) or intermediate risk (33.5%) with only 25.5% being classified as having a low Sokal risk. This is in line with the results of sponsored clinical trials showing an increased rate of events in high‐risk patients as compared to low Sokal risk, both in terms of resistance and rate of progression; no difference was observed in Sokal risk stratification in the intolerant cohort analyzed. Median time to discontinuation was 9.5 months for dasatinib and 9.7 months for nilotinib: these data, indeed, indicate a conservative approach of physicians when starting a frontline 2gen TKIs as compared to imatinib. 12 , 13 In fact, with this latter drug a possible switch occurs more frequently after only 3 months. A real‐life experience of outcome after discontinuation of 2gen TKIs was reported by MDACC group 6 : out of 218 patients frontline treated with dasatinib and nilotinib 40 subjects (18%) discontinued the therapy, contrary to our experience, mostly for toxicity and only five patients for resistance; as in our study, the median time of discontinuation was 8 months. Overall, as per second choice of TKIs it seems that ponatinib is preferred after resistance, in particular after dasatinib failure (66% of patients switched from dasatinib to ponatinib) probably due to high potency and multitarget activity of the drug. Even after intolerance, ponatinib was chosen in 26% of patients after dasatinib and 13% after nilotinib, probably because it is possible to prescribe low dose of the drug. Since imatinib was not actively monitored by AIFA, some uncertainties on subsequent switch to first‐generation TKI remains for the patients that have been lost to follow‐up. Anyway, given the low percentage of these patients (about 4% of the overall population), the number of putative switches to imatinib might be considered to be very low.
Despite some limitations due to the retrospective nature of this investigation and the lack of some clinical data not required by the AIFA registries as for instance on the outcome of patients experiencing a switch of therapy, our study representative of the Italian real‐life setting showed that treatment discontinuation with frontline 2gen TKIs was relatively uncommon.
Further analyses are planned in order to acquire extensive data on patients treated with subsequent lines of therapy and subjects who attempt a discontinuation.
CONFLICT OF INTEREST
MB received honoraria by Novartis, Incyte, Pfizer, Celgene; all the other authors have no conflict of interest.
AUTHORS CONTRIBUTION
MB interpreted the data and wrote the manuscript. PPO, OO, PF, AC, AC, MC, LG, and VS collecting the data, finalized the statistical analysis and revised the final version. AI and FP collecting the data and revised the final version, PC and PR revised and approved the final version.
ACKNOWLEDGMENTS
The views expressed in this work are personal and may not be understood or quoted as being made on behalf of or reflecting the position of the Italian Medicines Agency or of one of their committees or working parties. Present composition of the AIFA's Monitoring Registries Group (chronologic order): Bartoccioni Giorgia, Ricagni Daniele, Di Segni Susanna, Valentini Susanna, Pugliese Sara, Angelini Valeria, D'Antrassi Paola, Celant Simone, Lido Paolo. Although the AIFA monitoring registers operate within a regulatory framework that provides for the mandatory collection of data from real clinical practice on specific treatments reimbursed by the Italian National Healthcare System (NHS), by all public health facilities, the authors are, however, grateful for the contribution of the more than 500 (534) physicians and about 700 (689) pharmacists who filled the registers of nilotinib and dasatinib. Data cannot be share because extrapolated from Italian registry of AIFA
Breccia M, Olimpieri PP, Olimpieri O, et al; on behalf of the AIFA’s Monitoring Registries Group . How many chronic myeloid leukemia patients who started a frontline second‐generation tyrosine kinase inhibitor have to switch to a second‐line treatment? A retrospective analysis from the monitoring registries of the italian medicines agency (AIFA). Cancer Med. 2020;9:4160–4165. 10.1002/cam4.3071
Pier Paolo Olimpieri and Odoardo Olimpieri contributed equally.
Members are listed in Acknowledgements section.
REFERENCES
- 1. De Lavallade H, Apperley JF, Khorashad JS, et al. Imatinb for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention‐to‐treat analysis. J Clin Oncol. 2008;26:3358‐3363. [DOI] [PubMed] [Google Scholar]
- 2. Hochhaus A, O'Brien SG, Guilhot F, et al. Six‐year follow‐up of patients receiving imatinib for the first‐line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054‐1061. [DOI] [PubMed] [Google Scholar]
- 3. Hochhaus A, Larson RA, Guilhot F, et al. Long‐term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hochhaus A, Saglio G, Hughes TP, et al. Long‐term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5‐year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5‐year study results of DASISION: the dasatinib versus imatinib study in treatment‐naïve chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34:2333‐2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eghtedar A, Kantarjian H, Jabbour E, et al. Outcome after failure of second generation tyrosine kinase inhibitors treatment as first‐line therapy for patients with chronic myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2013;13:477‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sokal JE, Cox EB, Baccarani M, et al. Prognostic discrimination in “good‐risk” chronic granulocytic leukemia. Blood. 1984;63:789‐799. [PubMed] [Google Scholar]
- 8. Jabbour E, Kantarjian H, Cortes J. Use of second‐ and third‐generation tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia: an evolving treatment paradigm. Clin Lymphoma Myeloma Leuk. 2015;15:323‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jain P, Kantarjian H, Alattar ML, et al. Long‐term molecular and cytogenetic response and survival outcomes with imatinib 400 mg, imatinib 800 mg, dasatinib, and nilotinib in patients with chronic‐phase chronic myeloid leukemia: retrospective analysis of patient data from five clinical trials. Lancet Hematol. 2015;2:11‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldberg SL, Cortes JE, Gambacorti‐Passerini C, et al. First‐line treatment selection and early monitoring patterns in chronic phase chronic myeloid leukemia in routine clinical practice: SIMPLICITY. Am J Hematol. 2017;92:1214‐1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abruzzese E, Bosi A, Breccia M, et al. Treatment patterns in patients with chronic‐phase chronic myeloid leukemia in routine clinical practice: the SIMPLICITY Italian population. Mediterr J Hematol Infect Dis. 2019;11:e2019025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Casado LF, Garcia‐Gutierrez JV, Massaguè I, et al. Switching to secon‐generation tyrosine kinase inhibitor improves the response and outcome of frontline imatinib‐treated patients with chronic myeloid leukemia with more than 10% of BCR‐ABL7ABL ratio at 3 months. Cancer Med. 2015;4:995‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonifacio M, Binotto G, Maino E, et al. Imatinib‐treated chronic myeloid leukemia patients with discordant response between cytogenetic and molecular tests at 3 and 6 month time‐points have a reduced probability of subsequent optimal response. Haematologica. 2015;100:e299‐e301. [DOI] [PMC free article] [PubMed] [Google Scholar]


