Abstract
Background
Given the growing importance of patient‐reported outcomes (PROs) as part of “big data” in improving patient care, there is a need to provide a state‐of‐the‐art picture of the added value of using PROs in prostate cancer (PCa) randomized controlled trials (RCTs). We aimed to synthetize the most recent high‐quality PRO evidence‐based knowledge from PCa RCTs and to examine whether quality of PRO reporting in PCa research improved over time.
Methods
We conducted a systematic literature search using PubMed, from April 2012 until February 2019. For benchmarking purposes, we also included RCTs identified in our previously published review of RCTs (2004‐2012). Methodology for study identification and evaluation followed standardized criteria and a predefined data extraction form was used to abstract information. PRO quality of the studies was evaluated using the International Society of Quality of Life Research (ISOQOL) recommended criteria.
Results
A total of 55 new RCTs were published between April 2012 and February 2019. About 24 (43.6%) RCTs were found to be of high‐quality regarding PRO assessments. Of these, 13 (54.2%) have been reported in the most recent European Association of Urology (EAU) PCa Guidelines. Overall QoL and sexual, urinary, and bowel function were the most commonly reported PROs. FACT‐P, EPIC‐26, and EORTC QLQ‐C30 and/or its module PR25 were most frequently used as measurement tools. An overall improvement in the completeness of PRO reporting was noted over time.
Conclusion
Many PRO trials are currently not included in the EAU guidelines. Our findings suggest that there has to be a better consensus on the use of PRO data for PCa patients, which will then be reflected in the PCa Guidelines and future data collection. Homogeneity in PROs collection and measurement tools will in turn enable “big data” Consortia to increase the patients’ voice in clinical research.
Keywords: outcome measurement, PROs, prostate cancer, quality of life, RCT
Patient reported outcomes need to become the standard in prostate cancer RCTs. There is need for consensus on the use of PRO data, which will reflect their use in the guidelines and future data collection.
1. INTRODUCTION
Receiving a diagnosis of cancer and undergoing its subsequent treatments does not only have a detrimental impact on physical function, but may also affect psychological and social well‐being of the individual. Patient‐reported outcomes (PROs) addressing these domains of well‐being (including both physical and psychosocial components) are increasingly being incorporated in randomized controlled trials (RCTs) to assess the effectiveness of cancer treatments. A recent Cochrane systematic review on psychosocial well‐being and care needs of people with cancer 1 concluded that there is a need not only for more uniformity in outcomes and reporting, but importantly also for combining PROs with objective clinical outcomes. These conclusions are also in line with a recent report of the US Food and Drug Administration and the Critical Path Institute—as they are “committed to collaborate with international drug development stakeholders to identify rigorous methods to incorporate the patient perspective into the development of cancer therapeutics.” 2
In the context of prostate cancer (PCa), we have previously evaluated the completeness of PRO reporting of 65 RCTs published between January 2004 and March 2012, which reported on PROs. 3 Significant improvements in PRO quality reporting over time were observed and it was estimated that about 20% of PRO RCTs had provided solid PRO data to allow health policy makers and clinicians to make a critical appraisal. Since 2012, over 800 new studies have been published on PCa and PROs, according to PubMed. Since then, PIONEER, an IMI2 funded pan‐European public private partnership led by the European Association of Urology (EAU), is in the process of defining core outcome sets for PCa in the context of the patient's treatment pathway—whereby PROs from RCTs as well as real world evidence will be included. Core outcome sets for PCa will provide homogeneity in clinician reported outcomes and patient‐reported outcomes measures (PROMs) (in terms of outcomes and their definitions) to help Guidelines Offices with summarizing study findings and generate evidence‐based recommendations for the PCa treatment pathway. PIONEER’s goal is to ensure the optimal care for all European men diagnosed with PCa by unlocking the potential of “big data” and “big data analytics.” 4 , 5
Given the increasing importance of the use of PROs as part of “big data” in improving patient care, 6 there is a need to provide evidence‐based PRO information that may be used to facilitate clinical decision‐making. The main objective of this study was to synthetize most recent high‐quality PRO data from PCa RCTs, that is, those studies most likely to robustly inform patient care through for example inclusion in the EAU PCa guidelines. The latter is a useful indication of quality of clinical studies with an impact on the PCa patient experience as these urological clinical guidelines are used worldwide to disseminate recommended PCa treatment pathways. The EAU PCa guidelines are updated on an annual basis using a broad and comprehensive literature search and are based on modified version of the Oxford Center for Evidence‐based Medicine Levels of Evidence. Any flaws in the evidence used to support any given recommendation are taken into account and hence reflect on the quality of PRO reported information. Details of the search methodology can be found here. 7 A secondary objective of the current study was to assess whether completeness of PRO reporting in PCa research improved over time.
2. MATERIALS AND METHODS
2.1. Search strategy and identification of studies
We conducted a systematic literature search using PubMed, from April 2012 until February 2019. Methodology for study identification and evaluation followed standardized criteria used in the Patient‐Reported Outcomes Measurements Over Time In Oncology (PROMOTION) Registry (http://promotion.gimema.it). Study abstracts and additional records identified through hand searching of the literature were screened by two independent reviewers, following study selection criteria (see further for additional details). Then, full‐text articles selected were assessed for eligibility. This was previously described in the systematic review on PROs in PCa RCTs covering the years 2004‐April, 2012. 3 For the purpose of the current review, we used the same search terms as in our previous work 3 : ("quality of life" OR “health related quality of life” OR "health status" OR “health outcomes” OR “patient outcomes” OR “depression” OR “anxiety” OR “emotional” OR “social” OR “psychosocial” OR “psychological” OR “distress” OR “social functioning” OR “social wellbeing” OR “emotional” OR “patient reported symptom” OR "patient reported outcomes" OR pain OR fatigue OR “patient reported outcome” OR "PRO" OR "PROs" OR "HRQL" OR "QOL" OR "HRQOL" OR “symptom distress” OR “symptom burden” OR “symptom assessment” OR “functional status” OR sexual OR functioning) AND prostate. The search strategy was restricted to RCTs. In case of multiple publications from the same RCT, all relevant data possibly published in secondary articles were combined.
2.2. Selection criteria
Only English‐language reports of RCTs comparing conventional treatments and involving adult men with PCa were included—irrespective of disease stage. The minimum, overall sample size (combined treatment arms) was set at 50 patients. Screening studies or those involving patients with benign disease were excluded. We did not consider conference abstracts as these typically report insufficient information on PRO methodology and outcomes. RCTs of interventions that were psychological, behavioral, complementary, or alternative were also excluded.
We included all studies evaluating a PRO either as a primary or secondary endpoint—either as a multidimensional QoL outcome or a single dimensional outcome, such as symptoms. Those studies evaluating only treatment adherence or satisfaction were excluded. Details on search strategy and selection process were documented according to the PRISMA guidelines. 8
2.3. Methods of evaluation of studies
Three reviewers (FS, LM, and KB) extracted information from the identified studies. Each study was evaluated independently by two of these reviewers. All data were entered by the reviewers into a password protected online database (REDCap) 9 by completing a predefined electronic‐data extraction form (eDEF). Full details on information contained in the PROMOTION eDEF are reported in the appendix. A double‐blind data entry procedure was performed as each reviewer completed the eDEF independently. Discrepancies in evaluations were electronically recorded and when disagreements occurred in the evaluation of any item included in the eDEF, the reviewers revisited the paper to reconcile any differences. If no consensus was achieved, a fourth reviewer (FE) was consulted. For every included RCTs, their inclusion in the EAU PCa guidelines was checked by manual searches of the references and corresponding sections.
2.4. Type of data extraction and data analysis
For the purpose of this review, the following types of information were considered: (a) basic trial characteristics, (b) clinical and PRO characteristics, and (c) elements of PRO reporting based on recommendations from the International Society of Quality of Life Research (ISOQOL) 10 and the CONSORT‐PRO extension. 11 Quality of PRO reporting was evaluated with the ISOQOL checklist, which comprises a common set of 17 key issues regardless of PRO being a primary or secondary endpoint. Eleven additional issues were considered when a PRO was a primary endpoint of the study. Each item of the ISOQOL checklist was rated as “yes” if documented in the publication (scored as 1) or “no” if not documented (scored 0). To further refine the investigation of the accuracy of reporting, we divided the ISOQOL item addressing the problem of missing data into two (ie reporting the extent of missing data and reporting statistical approaches for dealing with missing data). We thus rated each RCT with a score ranging from 0 to a maximum of 18 (RCT with PRO as a secondary endpoint) or 29 (PRO as primary endpoint); in both cases, the higher the score the better the quality of the PRO reporting. Identification of high‐quality PRO studies was based on previously defined criteria. 3 Specifically, we defined as high‐quality PRO studies those which, at the same time, satisfied at least two‐thirds of the recommended criteria (12 for RCT with PRO as secondary endpoint and 20 for PRO as primary endpoint) and addressed three mandatory issues: study patients characteristics and baseline PRO scores described, documentation of PRO instrument validity, and missing data reported. In addition, we checked whether those studies considered as high‐quality RCTs were included in the most recent EAU PCa Guidelines. 12 , 13
Main characteristics of eligible studies (eg disease stage, type of PRO endpoint) were reported by proportions and means, according to the type of variable. Differences between studies were assessed using the chi‐square test. Based on the ISOQOL checklist score, comparisons between RCTs selected for this review and those included in a previous study 3 were performed to examine whether the completeness of PRO reporting in PCa trials has improved over time. We also compared the overall level of PRO reporting according to the CONSORT‐PRO extension, between the RCTs published before and after the publication of this guideline. All tests were two‐sided and statistical significance was set at α = 0.05. Analyses were performed by SAS software v. 9.4 (SAS Institute Inc).
3. RESULTS
A total of 55 new RCTs were published between April 2012 and February 2019 (Figure 1), of which the majority were international trials (ie more than one country) (60%). An overview of trial characteristics, as compared to the data from January 2004 to March 2012 is shown in Table 1. The duration of PRO assessment has increased, with 63.6% of trials reporting a period of more than 1 year as compared to 43.1% of trials previously (P < .006). Most RCTs reported on PROs as a secondary endpoint (70.9% vs 60% previously; P = .212). However, the prevalence of RCTs with a sample size ≥200 has increased over time, with 69.1% of RCTs including more than 200 men with PCa, as compared to 52.3% previously (P = .062).
FIGURE 1.
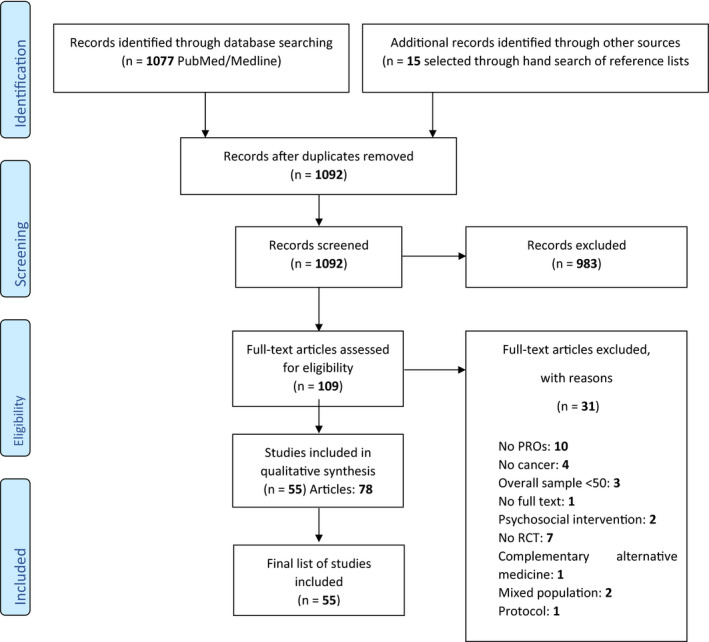
PRISMA flow chart of literature search results of Prostate Cancer Randomised Controlled Trials. PRO, patient‐reported outcomes
TABLE 1.
Overview of RCT characteristics as compared to our previous systematic review a
| Variable |
RCTs January 2004‐March 2012 (n=65) |
RCTs April 2012‐February 2019 (n=55) |
Total n (%) |
P value (two sided) |
|---|---|---|---|---|
| International (if more than one country) | ||||
| No | 46 (70.8) | 22 (40) | 68 (56.7) | <.001 |
| Yes | 19 (29.2) | 33 (60) | 52 (43.3) | |
| Industry supported (fully or in part) | ||||
| No | 31 (47.7) | 16 (29.1) | 47 (39.2) | .038 |
| Yes | 34 (52.3) | 39 (70.9) | 73 (60.8) | |
| Disease stage | ||||
| Only metastatic/advanced | 21 (32.3) | 27 (49.1) | 48 (40) | .160 |
| Only nonmetastatic/local | 31 (47.7) | 21 (38.2) | 52 (43.3) | |
| Both | 13 (20) | 7 (12.7) | 20 (16.7) | |
| Secondary paper on PRO | ||||
| No | 47 (72.3) | 37 (67.3) | 84 (70) | .549 |
| Yes | 18 (27.7) | 18 (32.7) | 36 (30) | |
| Length of PRO assessment during RCT | ||||
| Up to 6 mo | 22 (33.8) | 10 (18.2) | 32 (26.7) | .006 |
| Up to 1 y | 15 (23.1) | 6 (10.9) | 21 (17.5) | |
| More than 1 y | 28 (43.1) | 35 (63.6) | 63 (52.5) | |
| Unknown | 0 (0) | 4 (7.3) | 4 (3.3) | |
| Overall study sample size | ||||
| ≤200 | 31 (47.7) | 17 (30.9) | 48 (40) | .062 |
| >200 | 34 (52.3) | 38 (69.1) | 72 (60) | |
| Type of treatment used b | ||||
| Radiotherapy | 21 (32.3) | 17 (30.9) | 38 (31.7) | |
| Surgery | 10 (15.4) | 4 (7.3) | 14 (11.7) | |
| Chemotherapy | 13 (20) | 10 (18.2) | 23 (19.2) | |
| Targeted therapy | 0 (0) | 12 (21.8) | 12 (10) | |
| Hormonal therapy | 25 (38.5) | 24 (43.6) | 49 (40.8) | |
| Other | 22 (33.9) | 4 (7.3) | 26 (21.7) | |
| PRO endpoint | ||||
| Primary | 26 (40) | 16 (29.1) | 42 (35) | .212 |
| Secondary | 39 (60) | 39 (70.9) | 78 (65) | |
| Most frequently used PRO instruments b | ||||
| EORTC Questionnaires | 18 (27.7) | 16 (29.1) | 34 (28.3) | |
| FACT Questionnaires | 13 (20) | 21 (38.2) | 34 (28.3) | |
| BPI | 5 (7.7) | 13 (23.6) | 18 (15) | |
| VAS or LASA Questionnaires | 8 (12.3) | 3 (5.5) | 11 (9.2) | |
| IPSS | 2 (3.1) | 7 (12.7) | 9 (7.5) | |
| SF‐36 Questionnaires | 2 (3.1) | 6 (10.9) | 8 (6.7) | |
| EPIC | 0 (0) | 7 (12.7) | 7 (5.8) | |
| EQ‐5D | 1 (1.5) | 5 (9.1) | 6 (5) | |
Abbreviations: BPI, Brief Pain Inventory; EORTC, European Organization for Research and Treatment of cancer; EPIC, Expanded Prostate Cancer Index Composite; EQ‐5D, EuroQol‐5 Dimensions; FACT, Functional Assessment of Cancer Therapy; LASA, Linear Analog Scale Assessment; PRO, Patient‐Reported Outcomes; RCT, Randomized Controlled Trial; VAS, Visual Analog Scale.
Efficace et al Eur Urol. 2014;66(3):416‐427.
More than one category can be chosen.
To assess the clinical impact of the RCTs, we first identified the proportion of high‐quality RCTs with respect to PRO reporting, which was 43.6%, an increase since 2012 when it was 20% (P < .01). Table 2 provides more information about these high‐quality RCTs (n = 47 reports—describing study results of 24 different RCTs). The majority of trials (15/24) were conducted in the advanced PCa setting (ie locally advanced PCa, metastatic PCa, metastatic castration‐resistant and nonmetastatic castration‐resistant PCa). Some RCTs included patients with different PCa stages, so that it was not possible to categorize these RCTs according to localized, locally advanced, or metastatic PCa. Six studies focussed exclusively on men with localized PCa. In these high‐quality RCTs, PROs encompassed Health‐Related Quality of Life (HRQoL) or symptoms such as erectile, bladder, or bowel function. The most frequently used measures were Functional Assessment of Cancer Therapy‐Prostate (FACT‐P) (9/24), EORTC QLQ‐C30 and/or its module QLQ‐PR25 (5/24), and Expanded Prostate Cancer Index Composite (EPIC) (5/24).
TABLE 2.
Patient‐Reported Outcomes (PROs) and PRO measurements (PROMs) in the high‐quality PRO RCTs for prostate cancer
| Name of study | First author | PCa risk category | PRO | PROM |
|---|---|---|---|---|
| PREVAIL, 2015 | Loriot 27 | mCRPC |
HRQoL Pain |
FACT‐P EQ‐5D Brief Pain Inventory Short |
| PREVAIL, 2014 a | Beer 28 | mCRPC | QoL | FACT‐P |
| PREVAIL, 2017 | Devlin 29 | mCRPC | HRQoL | EQ‐5D |
| REACTT, 2015 | Patel 30 | Nonmetastatic PCa (GS ≤ 7, PSA < 10) |
Erectile function Patients’ treatment satisfaction Self‐esteem (baseline) Prostate‐specific QoL |
IIEF‐EF EDITS questionnaire SEAR questionnaire EPIC‐26 |
| REACTT, 2013 | Montorsi 31 | Nonmetastatic PCa (GS ≤ 7, PSA < 10) |
Erectile Function Sexual encounter |
IIEF‐EF score SEP‐1/ SEP‐2 questions |
| RTOG‐0126, 2015 | Bruner 32 | cT1b‐2b, (+GS2‐6, PSA10‐20 OR GS7, PSA < 15) |
Bladder function Bowel function Erectile function |
FACE questionnaire FACE questionnaire IIEF questionnaire |
| RTOG‐0126, 2013 | Michalski 33 | cT1b‐2b, (+GS2‐6, PSA10‐20 OR GS7, PSA < 15) |
Acute GI/GU toxicity Late GI/GU toxicity |
RTOG/EORTC late morbidity scoring system |
| RTOG‐0126, 2018 a | Michalski 34 | cT1b‐2b, (+GS2‐6, PSA10‐20 OR GS7, PSA < 15) | / | / |
| AFFIRM, 2014 | Fizazi 35 | mCRCP |
Pain HRQoL improvement HRQoL deterioration |
BPI‐SF FACT‐P FACT‐P |
| AFFIRM, 2015 | Cella 36 | mCRPC | HRQoL | FACT‐P |
| AFFIRM, 2012 a | Scher 37 | mCRPC | QoL | FACT‐P |
| AFFIRM, 2017 | Armstrong 38 | mCRPC | / | / |
| COU‐AA‐302 phase 3, 2013 | Basch 39 | mCRPC |
Pain HRQoL |
BPI‐SF questionnaire FACT‐P questionnaire |
| COU‐AA‐302 phase 3, 2012 a | Ryan 40 | mCRPC |
HRQoL Pain |
FACT‐P BPI‐SF |
| PROTECT, 2013 | Beer 41 | Nonmetastatic androgen dependent PCa with PSA relapse and GS ≥ 7 after RP | QoL |
BFI score LASA score, GRoC scale, symptoms checklist |
| PROTECT, 2011 | Beer 42 | Nonmetastatic androgen dependent PCa with PSA relapse and GS ≥ 7 after RP | / | / |
| SWOG‐9346, 2013 a | Hussain 43 | mCRPC | HRQoL | QoL questionnaire (SWOG) |
| NR | Mason 44 | T2b‐T4N0M0, GS ≥ 7, or PSA ≥ 10 |
LUTS relief QoL (urinary symptoms) |
IPSS |
| TROG 03.04 RADAR, 2012 | Denham 18 | cT2b–4N0M0 or T2a + GS≥7 + PSA≥10 | QoL | EORTC QLQ‐C30 + EORTC QLQ‐PR‐25 questionnaires |
| TROG 03.04 RADAR, 2014 | Denham 45 | cT2b–4N0M0 or T2a + GS≥7 + PSA≥10 | / | / |
| TROG 03.04 RADAR, 2012 | Denham 46 | cT2b–4N0M0 or T2a + GS≥7 + PSA≥10 | Dysfunctional rectal symptoms | EORTC QLQ‐PR25 questionnaire |
| NCT00884273, 2012 | Axcrona 47 | PCa all stages |
LUTS relief QoL improvement |
IPSS |
| CHHiP, 2015 a | Wilkins 17 | pT1b–T3aN0M0 |
Overall bowel bother Overall urinary bother Overall sexual bother General HRQoL |
UCLA‐PCI, EPIC instrument FACT‐P, SF‐36, SF‐12 |
| CHHiP, 2016 a | Dearnaley 48 | pT1b–T3aN0M0 | Patient reported outcome | UCLA‐PCI, EPIC instrument |
| ACTRN12611000661976 a | Yaxley 15 | ≤T2cN0M0 |
Urinary function Sexual function Pain Physical and mental functioning Fatigue Bowel function Cancer‐specific distress Psychological distress Time to return to work |
EPIC score EPIC/ IIEF score Surgical pain scale SF‐36 Vitality domain SF‐36 EPIC score RIES scale HADS score / |
| NCT00866554 | Gaudet 49 | T1c‐T2b, GS: 6 or 7(3 + 4), PSA ≤ 15 |
Acute and late effects on sexual function Urinary toxicity |
IPSS + EPIC score IPSS + EPIC score |
| TROG 03.06 and VCOG PR 01‐03, 2017 | Duchesne 50 | PSA relapse after treatment or group two of asymptomatic men unsuitable for curative treatment because of age, comorbidity, or locally advanced disease | Global HRQoL | EORTC QLQ‐C30, EORTC QLQ‐PR25 |
| TROG 03.06 and VCOG PR 01‐03, 2016 a | Dushesne 51 | PSA relapse after treatment or group two of asymptomatic men unsuitable for curative treatment because of age, comorbidity, or locally advanced disease | Global HRQoL | EORTC QLQ‐C30, EORTC QLQ‐PR25 |
| MRC PATCH trial (PR09), 2016 | Gilbert 52 | cT3‐4cN + M0 or TxNxM1 |
Global health status/QoL Urinary, bowel and sexual symptoms and function and hormone‐related symptoms |
EORTC QLQ‐C30, QLQ‐PR25 |
| MRC PATCH trial (PR09), 2013 | Langley 53 | cT3‐4cN + M0 or TxNxM1 | Adverse events (not patient reported) | Not applicable |
| MRC PATCH trial (PR09), 2016 | Langley 54 | cT3‐4cN + M0 or TxNxM1 | / | / |
| ASCENDE‐RT trial, 2016 | Rodda 55 | High‐risk PCa: T3aN0M0, GS 8‐9, PSA > 20 | HRQoL | SF36v2 |
| ASCENDE‐RT trial, 2013 a | Morris 56 | High‐risk PCa: T3aN0M0, GS 8‐9, PSA > 20 | / | / |
| PROSPER, 2018 a | Hussain 57 | nmCRPC | QoL assessments | / |
| PROSPER, 2019 | Tombal 58 | nmCRPC |
Pain progression HRQoL |
BPI‐SF questionnaire EORTC QLQ‐PR25 |
| RTOG 0938, 2018 | Lukka 59 | Low risk PCa (cT1‐2aN0M0, PSA < 10, GS 2‐6) |
Bowel and urinary PROs % of patients with >5 point reduction in the EPIC bowel domain at 1 y % of patients with >2 point reduction in EPIC urinary domain at 1 y Sexual and hormonal toxicity Acute/Late GI/GU toxicity |
EPIC‐50 EPIC 50 EPIC 50 EPIC 50 EPIC 50 |
| COMET‐2, 2018 | Basch 60 | mCRPC | Rate of pain response | BPI reports |
| SPARTAN, 2018 | Saad 16 | nmCRPC |
HRQoL: PCa symptoms, pain‐related symptoms, and overall QoL HRQoL: mobility, self‐care, usual activities, pain, discomfort, and anxiety or depression |
EQ‐5D‐3L FACT‐P |
| SPARTAN, 2018 a | Smith 61 | nmCRPC | / | / |
| NCT 02135357, 2018 | Khalaf 62 | mCRPC |
Patient reported HRQoL Depression symptoms Cognitive function |
FACT‐P PHQ‐9 MoCA |
| NCT 02135357, 2018 | Annala 63 | mCRPC | / | / |
| SWOG S0421, 2018 | Unger 64 | mCRPC |
Palliation of worst pain Improvement of functional status Vitality General QoL Analgesic use |
BPI inventory FACT‐P TOI SF‐36 Energy/vitality scale EORTC QLQ‐C30 (Pain medication logs) |
| SWOG S0421, 2013 | Quinn 65 | mCRPC | / | / |
| CHAARTED, 2015 a | Sweeney 66 | Metastatic hormone‐sensitive PCa | / | / |
| CHAARTED, 2018 | Morgans 67 | Metastatic hormone‐sensitive PCa |
Overall QoL Treatment and disease‐related QoL Adverse effect of taxanes treatment Fatigue Pain |
FACT‐P FACT‐Taxane FACIT‐Fatigue BPI |
| LATITUDE, 2017 a | Fizazi 68 | Metastatic hormone‐sensitive PCa | Time to pain progression | BPI‐SF |
| LATITUDE, 2018 | Chi 69 | Metastatic hormone‐sensitive PCa |
Pain Fatigue Disease‐related QoL HRQoL |
BPI‐SF BFI FACT‐P EQ‐5D‐5L |
Abbreviations: BPI, Brief Pain Inventory; EDITS, Erectile Dysfunction Inventory of Treatment Satisfaction; EORTC QLQ‐C30, European Organization for Research and Treatment of Cancer Quality of life questionnaire‐Core 30; EORTC QLQ‐PR25, European Organization for Research and Treatment of Cancer Quality of life questionnaire‐Prostate 25; EPIC, Expanded Prostate Index Composite questionnaire; EQ‐5D, EuroQol 5‐dimension; EQ‐5D‐3L, EuroQol 5‐dimension, 3‐level questionnaire; FACE, Functional Alterations due to Changes in Elimination; FACT‐P, Functional assessment of Cancer Therapy‐Prostate Cancer; HADS, Hospital Anxiety and Depression Scale; IIEF‐EF, International Index of Erectile Function‐Erectile Function; IPSS, International Prostate Symptom Score; MoCA, Montreal Cognitive Assessment;PHQ‐9, Patient Health Questionnaire‐9; RIES, Revised Impact of Events; SEAR, Self‐Esteem and Relationship questionnaire; SF‐36, Short‐Form 36; UCLA‐PCI, UCLA Prostate Cancer Index.
Included in the EAU prostate cancer guidelines.
To further assess the clinical impact of these RCTs, Table 3 reports on the influence of the reported HRQoL on the final treatment recommendation (as defined by the authors of each published RCT). As most RCTs reported clinical and HRQoL specific outcomes separately, Table 3 aims to combine the results as one final recommendation for each RCT. In the metastatic PCa group, new treatment options (ie second‐generation antiandrogens like enzalutamide and abiraterone) and the use of docetaxel resulted in significant improvements in both clinical (overall, progression‐free, and metastatic‐free survival) and HRQoL outcomes.
TABLE 3.
Evaluation of HRQoL outcomes/recommendations for included high‐quality PROs
| Name of trial | Treatment | HRQoL outcome | Clinical outcome | Treatment recommendations |
|---|---|---|---|---|
|
PREVAIL* Loriot 27 Beer 28 Devlin 29 |
Enzalutamide (160 mg/d) vs placebo | Enza: reduced risk of and delayed time to HRQoL deterioration, pain progression, and occurrence of SREs. | Enza is recommended in asymptomatic and minimally symptomatic, chemo‐naïve patients with mCRPC due to its positive effects on survival and HRQoL benefits. | |
| Significant delay in radiographic disease progression or death and need for cytotoxic chemotherapy | ||||
| Significant benefits in terms of Pain/Discomfort and Anxiety/Depression (EQ‐5D) | ||||
|
REACTT Patel 30 Montorsi 31 |
9 mo tadalafil 5 mg once daily vs tadalafil 20 mg on demand vs placebo | Early chronic dosing after nsRP increases and accelerates EF recovery and improves patients’ QoL. | Tadalafil treatment may contribute to the recovery of EF after RP | |
| Improvements in IIEF‐EF and successful intercourse during 9 mo tadalafil once daily were not sustained 6w after drug cessation. | Protection from penile length loss | |||
|
RTOG‐0126* Bruner 32 Michalski 33 Michalski 34 |
3D‐CRT vs IMRT | No difference in patient‐reported bowel, bladder, or sexual functions | The decision to deliver high radiation dose must be balanced against the risk of morbidity in the individual patient. | |
| IMRT: Lower incidence of acute GI or GU toxicity and a lower cumulative incidence of late grade 2b rectal toxicity | ||||
| Increase in late grade 2 or greater GI and GU toxic effects. | Improvement in biochemical failure and distant metastases, but no improvement in OS. | |||
|
AFFIRM* Fizazi 35 Cella 36 Scher 37 Armstrong 38 |
Enzalutamide (160 mg/d) vs placebo | Reduction of the risk of SREs. Reduction of pain and increase in time to HRQoL deterioration | Enza improves both OS and well‐being and everyday functioning of patients with mCRPC (postchemo) | |
| Stabilization of patient HRQoL | ||||
| Prolonged OS | ||||
| PSA declines of any, ≥30%, and ≥50% within 90 d of Enza were strongly associated with the clinical benefit | ||||
|
COU‐AA‐302 phase 3* Basch 39 Ryan 40 |
Abiraterone acetate + prednisone vs prednisone alone | Delay in patient‐reported pain progression and HRQoL deterioration | Abi + prednisone can be recommended for patients with mCRPC (prechemo) | |
| Improvement of radiographic PFS, a trend toward improvement of OS, and significant delay in clinical decline and initiation of chemotherapy | ||||
|
PROTECT Beer 41 Beer 42 |
Sipuleucel‐T vs placebo | No clinically significant negative impact on QoL | Long‐term FU is needed to determine the effect on clinically important events | |
| No difference in biochemical failure | ||||
|
SWOG‐9346, 2013* Hussain 43 |
Intermittent vs continuous ADT | Intermittent therapy was associated with improved EF and mental health at 3 mo but not thereafter. | Too few events occurred to rule out significant inferiority of intermittent therapy | In this noninferiority trial, findings were statistically inconclusive |
|
NR Mason 44 |
Degarelix + RT vs Goserelin with bicalutamide + RT | Degarelix had more pronounced effects on LUTS in symptomatic patients | Noninferior efficacy of degarelix in terms of prostate shrinkage | Degarelix provides an alternative treatment for PCa patients who need neoadjuvant ADT before RT, especially for those having LUTS problems. |
|
TROG 03.04 RADAR Denham 18 Denham 45 Denham 46 |
STAS (6 mo) vs STAS + ZA vs ITAS (12 mo) vs ITAS + ZA | ITAS + RT causes adverse effects on some PROs but not on global QoL scores. Only hormone treatment‐related symptoms persisted at marginally higher frequencies. HDR‐BT boost adversely affected emotional function and financial problems. | Further follow‐up of the RADAR trial is needed before we can take our findings to the clinic | |
| No difference in PCa‐specific mortality between the 4 groups | ||||
| ADT, ZA and increasing EBRT dose did not increase rectal or urinary dysfunction. The use of HDR‐BT increased urinary dysfunction. | ||||
|
Axcrona 47 |
12 wk of degarelix (240/80 mg) vs goserelin (3.6 mg) + 28 d of bicalutamide. |
Degarelix showed superiority in LUTS relief in symptomatic patients | Same reduction in total prostate volume | Degarelix can be considered as a useful approach to combined GnRH agonist plus antiandrogen for PCa patients in need of short‐term neoadjuvant ADT. |
|
CHHiP* Wilkins 17 Dearnaley 48 |
Hypofractionated RT vs conventional RT | PROs were not significantly different between treatment groups for any of the endpoints | Hypofractionated RT using 60 Gy in 20 fractions is recommended as new standard of care for EBRT of localized PCa. | |
| Hypofractionated schedule is noninferior to the conventionally fractionated schedule for time to biochemical or clinical failure | ||||
|
ACTRN12611000661976* Yaxley 15 |
RARP vs RRP | No difference in domain‐specific QoL outcomes at 12 wk | No difference in pathological outcomes at 12 wk | Long‐term follow‐up is needed |
|
Gaudet 49 |
Dutasteride 0.5 mg + Bicalutamide 50 mg + Tamoxifen 10 mg daily vs LHRH agonist + Bicalutamide daily | Less sexual toxicity compared to LHRH agonists prior to BT and for the first 6 mo after BT | Noninferior efficacy to LHRH agonist based regimens for prostate volume reduction prior to BT | D + B is therefore an option to be considered for prostate volume reduction prior to BT |
|
TROG 03.06 and VCOG PR 01‐03* Duchesne 50 G Dushesne 51 |
Immediate vs delayed ADT (PSA relapse only) | Early detriments in specific hormone treatment‐related symptoms with immediate ADT, but with no other demonstrable effect on overall functioning or HRQoL | Progression is delayed, but at a small cost in global QoL. The option can be discussed with men with a PSA relapse. | |
| Immediate receipt of ADT significantly improved OS | ||||
|
MRC PATCH trial (PR09) Gilbert 52 Langley 53 RE Langley 54 |
Transdermal estradiol vs LHRH agonist | estradiol: better self‐reported QoL outcomes at 6 mo but increased gynecomastia | Provides further supporting evidence for the ongoing phase 3 trial | |
| Castrate testosterone concentrations similar to those achieved with LHRHa | ||||
| Mitigating BMD loss | Castration levels of testosterone comparable with LHRHa administration | |||
|
ASCENDE‐RT trial* Rodda 55 Morris 56 |
LDR‐BT vs DE‐EBRT | LDR‐PB boost: more moderate to severe GU toxicity, urinary incontinence, and need for catheterization and a larger mean decline in HRQoL for physical and urinary function at 6 y. | Treatment should be individualized and requires careful consideration of the potential risks and benefits. | |
| LDR‐PB patients were twice as likely to be free of BF at a median follow‐up of 6.5 y | ||||
|
PROSPER* Hussain 57 Tombal 58 |
Enzalutamide vs placebo | Significantly increased metastasis‐free survival | Enza is a treatment option that should be discussed in high‐risk, nmCRPC | |
| Benefit in delaying pain progression, symptom worsening, and decrease in functional status | ||||
|
RTOG 0938, 2018 Lukka 59 |
Two ultrahypofractionated RT schemes | Both schemes are well tolerated and bowel, urinary, and sexual PROs are comparable to those for standard RT | Longer follow‐up is required | |
|
COMET‐2, 2018 Basch 60 |
Cabozantinib vs mitoxantrone‐prednisone | Cabozantinib treatment did not improve pain palliation | Enrollment was terminated | |
|
SPARTAN* Saad 16 MR Smith 61 |
Apalutamide vs placebo | HRQoL was maintained after initiation of apalutamide treatment | Apalutamide provides clinical benefit in the treatment of men with nmCRPC | |
| Metastasis‐free survival and time to symptomatic progression were significantly longer | ||||
|
NCT 02135357 Khalaf 62 Annala 63 |
Abiraterone vs enzalutamide | PROs favored Abi with differences in FACT‐P and PHQ‐9 scores. Differences in the total FACT‐P score only in the elderly subgroup. | Abi and Enza are standard first‐line treatment options for mCRPC with similar efficacy but different side‐effect profiles. Administration should be discussed with each patient individually. | |
| Enza: superior PSA responses but no differences in progression‐free survival | ||||
|
CHAARTED* Sweeney 66 Morgans 67 |
ADT + Docetaxel vs ADT alone | Six cycles of docetaxel at the beginning of ADT resulted in significantly OS than ADT alone. | For patients with hormone‐sensitive metastatic PCa, who are fit enough ADT + docetaxel can be considered | |
| Both arms reported a similar minimally changed QoL over time, suggesting that ADT + Docetaxel is not associated with a greater long‐term negative impact on QoL | ||||
|
LATITUDE* Fizazi 68 Chi 69 |
ADT + abiraterone acetate + prednisone vs ADT alone | Addition of abiraterone acetate increased OS and radiographic progression‐free survival | Treatment with ADT plus abiraterone acetate and prednisone could be considered a new option for standard of care for patients with metastatic castration‐naïve PCa | |
| Addition of abiraterone acetate improved overall PROs by consistently showing a clinical benefit in the progression of pain, PCa symptoms, fatigue, functional decline, and overall HRQoL. | ||||
|
SWOG S0421 Unger 64 Quinn 65 |
Docetaxel + Atrasentan vs Docetaxel + placebo | No substantial treatment arm differences for pain and functional status | Docetaxel remains one of the standard options for CRPC. Endothelin inhibitors do not have an established role. | |
| Atrasentan did not improve PFS or OS |
To date, 14 of the 47 publications have been included in the EAU PCa Guidelines 12 , 13 —reflecting 13 different RCTs (Table 2).
In terms of methodology, there was still a lack of information on mode of administration of the PRO tool and methods of data collection over time (83.6% vs 76.9%; P = .360). However, there was an improvement in the reporting of the evidence for PRO instrument validity and reliability (80% vs 66.1%; P = .007). More RCTs also identified PROs in the trial protocol and post hoc analyses (67.3% vs 20%; P < .001). In general, there was an improvement in terms of reporting methods and results for PROs in PCa RCTs. A detailed overview of the methodological assessment, as compared to our previous systematic review, 3 is provided in Table 4.
TABLE 4.
Level of patient‐reported outcomes (PRO) reporting as compared to our previous systematic review 3
| Variable | Category |
RCTs January 2004‐March 2012 n = 65 (%) |
RCTs April 2012‐February 2019 n = 55 (%) |
Total n (%) |
P value (two sided) |
|---|---|---|---|---|---|
| Title and abstract | |||||
| The PRO is identified as an outcome in the abstract | No | 6 (9.2) | 4 (7.3) | 10 (8.3) | .699 |
| Yes | 59 (90.8) | 51 (92.7) | 110 (91.7) | ||
| (Additional standards only for PRO as primary outcome) The title of the paper is explicit as to the RCT including a PRO a | No | 10 (38.5) | 9 (56.3) | 19 (45.2) | .252 |
| Yes | 16 (61.5) | 7 (43.8) | 23 (54.8) | ||
| Introduction, background, and objectives | |||||
| The PRO hypothesis is stated and should specify the relevant PRO domain if applicable | No | 11 (16.9) | 19 (34.5) | 30 (25) | .082 |
| Yes | 24 (36.9) | 15 (27.3) | 39 (32.5) | ||
| N/A (if explorative) | 30 (46.2) | 21 (38.2) | 51 (42.5) | ||
| (Additional standards only for PRO as primary outcome) The introduction contains a summary of PRO research that is relevant to the RCT a | No | 3 (11.5) | 7 (43.7) | 10 (23.8) | .031 |
| Yes | 23 (88.5) | 9 (56.3) | 32 (76.2) | ||
| (Additional standards only for PRO as primary outcome) Additional details regarding the hypothesis are provided including the rationale for the selected domains, the expected directions of change, and the time points for assessment. a | No | 22 (84.6) | 12 (75) | 34 (81) | .346 |
| Yes | 4 (15.4) | 4 (25) | 8 (19) | ||
| Methods | |||||
| Outcomes | |||||
| The mode of administration of the PRO tool and the methods of collecting data are described | No | 50 (76.9) | 46 (83.6) | 96 (80) | .360 |
| Yes | 15 (23.1) | 9 (16.4) | 24 (20) | ||
| Electronic mode of PRO administration | No | 15 (23.1) | 5 (9.1) | 20 (16.7) | .044 |
| Yes | 0 (0) | 2 (3.6) | 2 (1.6) | ||
| N/A | 50 (76.9) | 48 (87.3) | 98 (81.7) | ||
| The rationale for choice of the PRO instrument used is provided | No | 24 (36.9) | 26 (47.3) | 50 (41.7) | .252 |
| Yes | 41 (63.1) | 29 (52.7) | 70 (58.3) | ||
| Evidence of PRO instrument validity and reliability is provided or cited | No | 22 (33.9) | 11 (20) | 33 (27.5) | .007 |
| Yes, for all PRO instruments | 25 (38.4) | 37 (67.3) | 62 (51.7) | ||
| Yes, only for some PRO instruments | 18 (27.7) | 7 (12.7) | 25 (20.8) | ||
| The intended PRO data collection schedule is provided | No | 6 (9.2) | 5 (9.1) | 11 (9.2) | .979 |
| Yes | 59 (90.8) | 50 (90.9) | 109 (90.8) | ||
| PROs are identified in the trial protocol; post hoc analyses are identified | No | 52 (80) | 18 (32.7) | 70 (58.3) | <.001 |
| Yes | 13 (20) | 37 (67.3) | 50 (41.7) | ||
| The status of PRO as either a primary or secondary outcome is stated | No | 9 (13.8) | 3 (5.5) | 12 (10) | .106 |
| Yes | 48 (73.9) | 49 (89) | 97 (80.8) | ||
| Unclear | 8 (12.3) | 3 (5.5) | 11 (9.2) | ||
| (Additional standards only for PRO as primary outcome) A citation for the original development of the PRO instrument is provided a | No | 11 (42.3) | 3 (18.8) | 14 (33.3) | .086 |
| Yes | 7 (26.9) | 10 (62.4) | 17 (40.5) | ||
| Yes, only for some PRO instruments | 8 (30.8) | 3 (18.8) | 11 (26.2) | ||
| (Additional standards only for PRO as primary outcome) Windows for valid PRO responses are specified and justified as being appropriate for the clinical context a | No | 7 (26.9) | 14 (87.5) | 21 (50) | <.001 |
| Yes | 19 (73.1) | 2 (12.5) | 21 (50) | ||
| Sample size | |||||
| (Additional standards only for PRO as primary outcome) There is a power sample size calculation relevant to the PRO based on a clinical rationale a | No | 10 (38.5) | 5 (31.2) | 15 (35.7) | .412 |
| Yes | 16 (61.5) | 11 (68.8) | 27 (64.3) | ||
| Statistical methods | |||||
| There is evidence of appropriate statistical analysis and tests of statistical significance for each PRO hypothesis tested | No | 2 (3.1) | 3 (5.5) | 5 (4.2) | .418 |
| Yes | 22 (33.8) | 13 (23.6) | 35 (29.2) | ||
| N/A (If PRO hypotheses were not stated) | 41 (63.1) | 39 (70.9) | 80 (66.6) | ||
| The extent of missing data is stated | No | 18 (27.7) | 17 (30.9) | 35 (29.2) | .699 |
| Yes | 47 (72.3) | 38 (69.1) | 85 (70.8) | ||
| Statistical approaches for dealing with missing data are explicitly stated | No | 53 (81.5) | 35 (63.6) | 88 (73.3) | .027 |
| Yes | 12 (18.5) | 20 (36.4) | 32 (26.7) | ||
| (Additional standards only for PRO as primary outcome) The manner in which multiple comparisons have been addressed is provided a | No | 19 (73.1) | 11 (68.8) | 30 (71.4) | .439 |
| Yes | 7 (26.9) | 5 (31.2) | 12 (28.6) | ||
| Results | |||||
| Participant flow | |||||
| A flow diagram or a description of the allocation of participants and those lost to follow‐up is provided for PROs specifically | No | 41 (63.1) | 24 (43.6) | 65 (54.2) | .033 |
| Yes | 24 (36.9) | 31 (56.4) | 55 (45.8) | ||
| The reasons for missing data are explained | No | 42 (64.6) | 37 (67.3) | 79 (65.8) | .760 |
| Yes | 23 (35.4) | 18 (32.7) | 41 (34.2) | ||
| Baseline data | |||||
| The study patients characteristics are described including baseline PRO scores | No | 23 (35.4) | 14 (25.5) | 37 (30.8) | .241 |
| Yes | 42 (64.6) | 41 (74.5) | 83 (69.2) | ||
| Outcomes and estimation | |||||
| Are PRO outcomes also reported in a graphical format? | No | 26 (40) | 15 (27.3) | 41 (34.2) | .143 |
| Yes | 39 (60) | 40 (72.7) | 79 (65.8) | ||
| (Additional standards only for PRO as primary outcome) The analysis of PRO data accounts for survival differences between treatment groups if relevant a | No | 1 (3.8) | 1 (6.2) | 2 (4.8) | .002 |
| Yes | 0 (0) | 7 (43.8) | 7 (16.6) | ||
| N/A (if not relevant) | 25 (96.2) | 8 (50) | 33 (78.6) | ||
| (Additional standards only for PRO as primary outcome) Results are reported for all PRO domains(if multidimensional)and items identified by the reference instrument a | No | 3 (11.5) | 7 (43.7) | 10 (23.8) | .031 |
| Yes | 23 (88.5) | 9 (56.3) | 32 (76.2) | ||
| (Additional standards only for PRO as primary outcome) The proportion of patients achieving predefined responder definitions is provided where relevant a | No | 1 (3.9) | 3 (18.7) | 4 (9.5) | .198 |
| Yes | 7 (26.9) | 2 (12.5) | 9 (21.4) | ||
| N/A (if not relevant) | 18 (69.2) | 11 (68.8) | 29 (69.1) | ||
| Discussion | |||||
| Limitations | |||||
| The limitations of the PRO components of the trial are explicitly discussed | No | 42 (64.6) | 33 (60) | 75 (62.5) | .603 |
| Yes | 23 (35.4) | 22 (40) | 45 (37.5) | ||
| Generalizability | |||||
| Generalizability issues uniquely related to the PRO results are discussed | No | 28 (43.1) | 26 (47.3) | 54 (45) | .645 |
| Yes | 37 (56.9) | 29 (52.7) | 66 (55) | ||
| Interpretation | |||||
| Are PRO interpreted?(Not only restated) | No | 19 (29.2) | 12 (21.8) | 31 (25.8) | .355 |
| Yes | 46 (70.8) | 43 (78.2) | 89 (74.2) | ||
| The clinical significance of the PRO findings is discussed | No | 44 (67.7) | 35 (63.6) | 79 (65.8) | .641 |
| Yes | 21 (32.3) | 20 (36.4) | 41 (34.2) | ||
| Methodology used to assess clinical significance | Anchor based (eg minimal important difference) | 15 (23.1) | 13 (23.6) | 28 (23.3) | .251 |
| Distribution based (e.g effect size) | 6 (9.2) | 3 (5.5) | 9 (7.5) | ||
| Both | 0 (0) | 3 (5.5) | 3 (2.5) | ||
| Other | 0 (0) | 1 (1.8) | 1 (0.8) | ||
| Missing | 44 (67.7) | 35 (63.6) | 79 (65.9) | ||
| The PRO results is discussed in the context of the other clinical trial outcomes | No | 4 (6.1) | 15 (27.3) | 19 (15.8) | .002 |
| Yes | 61 (93.9) | 40 (72.7) | 101 (84.2) | ||
| Other information | |||||
| Protocol | |||||
| (Additional standards only for PRO as primary outcome) A copy of the instrument is included if it has not been published previously (It could be found in the article appendix or in the online version of the paper) a | No | 13 (50) | 2 (12.5) | 15 (35.7) | <.001 |
| Yes | 13 (50) | 0 (0) | 13 (31) | ||
| N/A (if the instrument is already published or known in the literature) | 0 (0) | 14 (87.5) | 14 (33.3) | ||
Percentage for these items was calculated by considering only the RCTs with PRO as primary endpoint (n = 42), that is, 26 RCTs published between January 2004 and March 2012 and 16 RCTs published from April 2012 to February 2019.
Evaluating the level of PRO reporting according to the CONSORT‐PRO extension, we observed, for all of the items, an improvement in the studies published after the publication of the CONSORT‐PRO extension, compared with those published before. Major differences were observed in two key items: the statistical methods for dealing with missing data were reported in 36.6% of newer RCTs as compared to 21.5% of older RCTs and PRO‐specific limitations were discussed in 46.3% vs 32.9% of RCTs, respectively (Table 5).
TABLE 5.
Level of PRO reporting by year (2013) of publication of the CONSORT‐PRO extension
| CONSORT‐PRO Extension Item |
RCTs before CONSORT‐PRO publication (2004‐2013) (n = 79) No (%) |
RCTs after CONSORT‐PRO publication (2014‐2019) (n = 41) No (%) |
|---|---|---|
| P1b. The PRO should be identified in the abstract as a primary or secondary outcome. | 72 (91.1) | 38 (92.7) |
| P2b. The PRO hypothesis should be stated, and relevant domains should be identified if applicable. a | 25 (31.7) | 14 (34.2) |
| P6a. Evidence of PRO instrument validity and reliability should be provided or cited if available. b | 55 (69.6) | 32 (78.0) |
| P6aa. This is the mode of administration, including the person completing the PRO and the methods of data collection (paper, telephone, electronic, and other). b , c | 15 (19.0) | 9 (22.0) |
| P12a. Statistical approaches for dealing with missing data are explicitly stated. | 17 (21.5) | 15 (36.6) |
| P20/21. PRO‐specific limitations and implications for generalizability and clinical practice should be discussed. | 26 (32.9) | 19 (46.3) |
Abbreviations: CONSORT, Consolidated Standards of Reporting Trials; PRO, patient‐reported outcome; RCT, randomized controlled trial.
This percentage was calculated on the basis of all studies (including those explicitly reporting an exploratory evaluation, for which this item would be rated as not applicable).
Items P6a and P6aa were combined in the original CONSORT‐PRO extension; however, for the purposes of this study, to better appraise the proportion of RCTs providing evidence on the validity of the PRO instrument but not further describing how this was administered to patients, this item was split into two items.
In case of studies using multiple PRO measures, we evaluated this as “yes” if at least one measure was validated.
4. DISCUSSION
Between April 2012 and February 2019, a total of 55 PCa RCTs have been published using PRO, of which nearly half (43.6%) can be considered as high‐quality RCTs for use of PROs. Of these 24 trials, only 13 (54.2%) have been reported in the most recent EAU Guidelines for PCa. The majority of RCTs were conducted in the advanced and metastatic PCa setting. Six RCTs were specifically conducted in men with localized PCa. Overall QoL and erectile, urinary, and bowel function were the most commonly reported PRO. The FACT‐P, EPIC‐26, and EORTC QLQ‐C30 and/or QLQ‐PR25 were the most commonly used measurement tools. An overall improvement in the reporting of the evidence for PROs was noted.
As highlighted by Kluetz et al cancer clinical trials have mainly focused on overall survival and measures of tumor growth or reduction to assess the efficacy of a specific treatment. 14 However, the balance between improving disease symptoms and introducing symptomatic toxicity affects how patients function in their daily lives and hence affects their HRQoL. Kluetz and colleagues suggest to focus on three distinct measures of well‐defined concepts: symptomatic adverse events, physical function, and disease‐related symptoms. As shown in Table 2, these outcomes are measured in several of the included PCa RCTs, however, only the ACTRN12611000 15 and the SPARTAN 16 trials specifically reported on all three outcomes.
It is important to note that the majority of trials used validated tools, with FACT‐P, EPIC‐26, and EORTC QLQ‐C30 being the most commonly used. Those studies that were included in the EAU guidelines had all used at least one of these three measurement tools. However, these validated HRQoL questionnaires were less common in trials of men with localized PCa. The latter focused more on specific adverse events such as erectile or bowel function. Nevertheless, the CHHiP trial measured general HRQoL with FACT‐P 17 and the TROG 03.04 RADAR trial measured QoL with EORTC QLQ‐C30 and its PCa module. 18
There seems to be a need for PRO‐specific guidance for the different disease‐specific stages of PCa. There are emerging international standards 19 to help generate robust data with more focus on patient engagement and the EAU is already undertaking work to develop core outcome sets for PCa, including both clinician‐reported outcomes and PROs. 4 , 5 , 20 With improved methodology and practice, and increasing patient engagement, high quality and clinically meaningful generation of PRO data will become the norm for PCa clinical studies 21 and help increase their clinical impact in every disease stage. This will ensure that more high‐quality PRO studies will also be incorporated in the recommendations of guidelines offices, which also uses robust evidence assessment methods. A modified Grading of Recommendations Assessment, Development and Evaluation (GRADE) is currently used by the EAU. This approach allows for a transparent assessment of how recommendation statements have been developed, whereby overall quality of the evidence along with magnitude of effect, certainty of the results, balance between desirable and undesirable outcomes, impact of patient values, and preferences on the intervention, and certainty of those patient values and preferences result in guideline recommendations. 7 Inclusion of PRO studies into the guidelines thus provides a reflection on the quality of the evidence and the reporting of PROs.
It is important to note that a patient‐centred treatment recommendation should consider both clinical (ie survival) and HRQoL outcomes. Considering these HRQoL aspects relies on understanding patients’ values, needs, and experience of the disease and integrate them to formulate an optimal treatment strategy. Quality of life remains subjective and validated PROMs are of critical importance to guide these tailored interventions to improve patients’ well‐being. The rising importance of PROs is already captured for localized disease by a systematic review, coordinated by the EAU PCa Guidelines panel, evaluating the effect of primary treatment on HRQoL. 22 The same PROMs as reported in our systematic review were captured, emphasizing their importance and systematic use. Such evaluation and recommendations are lacking for advanced and metastatic disease, where HRQoL becomes even more important. Methodology for reporting of PRO and HRQoL for these different disease stages does not need to differ, but there is a clear need for a broader consensus on its use in all disease stages so that it can be integrated with the existing clinical outcomes.
In addition to RCT data, it is of interest to note that there is an increased interest in using real world data to support regulatory decision‐making—including the EAU guidelines. Information about how a patient feels and functions, as captured directly from patients themselves, is however also often missing in real world data (eg observational data or hospital data). For example, PROs were only collected in 14% of recent postauthorisation safety studies. 23 Harnessing the patient voice through the use PROs in “big data” indeed implies the need to align PRO measurements (PROMs) between RCTs and real world data. 24
With respect to the methodological assessment of PRO use in PCa RCTs, data from this study suggests that completeness of PRO reporting has improved over the last years, as documented by the higher proportion of high‐quality RCTs published from 2012 onward. Indeed, while only 20% of PCa RCTs published between 2004 and 2012 were considered as high quality, this percentage has more than doubled for the more recent RCTs. This may be partly explained by the publication of the ISOQOL recommended standards 10 and the subsequent CONSORT‐PRO criteria 11 in 2013, which may have guided and helped investigators to increase the completeness of PRO reporting. Indeed, journal endorsement and author use of CONSORT‐PRO extension has been demonstrated to be associated with improved PRO reporting. 25
This study has limitations. First, despite our comprehensive search strategy, it is possible that some RCTs with a PRO component might have been missed. Another limitation is the exclusion of non‐English language published papers. However, it is unlikely that such omission would have significantly altered the conclusion of this review. 26 A strength of the current review is that we used a formal, replicable approach to evaluate PRO reporting of PCa RCTs. Since all studies use different reporting criteria and methods, the information was extracted and assessed by two independent researchers. In case of inconsistencies, a third arbiter helped achieving consensus. Also, by using state of the art and well‐established international recommendations for PRO reporting, we were able to identify the proportion of studies that are most likely to robustly inform patient care.
5. CONCLUSION
We observed an important improvement in the reporting of the evidence for PROs during the last 7 years, of which only a small proportion of high‐quality PRO trials made it into the EAU PCa Guidelines. The most commonly used measurements focused on overall HRQoL and were predominantly used in RCTs of men with advanced PCa, whereas for RCTs in men with localized PCa the focus was more on adverse effects. Given the increasing recognition that the patients’ voice in clinical research needs to be heard, there is a need for better guidance as to how to include and measure PRO in “big data” and guidelines—an answer which may be delivered by the EAU‐led PIONEER Consortium.
CONFLICT OF INTEREST
None to be declared.
AUTHOR CONTRIBUTIONS
Data collection: FS, LM, KB; data analysis: Fs, FC, FE, MVH; manuscript drafting: MVH, LM, KB; final approval of manuscript: MVH, FS, LM, KB, FC, MS, FE.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Cancer Research UK for their support to the TOUR team of Dr Van Hemelrijck.
Van Hemelrijck M, Sparano F, Moris L, et al. Harnessing the patient voice in prostate cancer research: Systematic review on the use of patient‐reported outcomes in randomized controlled trials to support clinical decision‐making. Cancer Med. 2020;9:4039–4058. 10.1002/cam4.3018
Funding information
We are grateful to Cancer Research UK (C45074/A26553).
DATA AVAILABILITY STATEMENT
All data were taken from published manuscripts and can be obtained upon request by contacting f.sparano@gimema.it.
REFERENCES
- 1. Schouten B, Avau B, Bekkering GTE, et al. Systematic screening and assessment of psychosocial well‐being and care needs of people with cancer. Cochrane Database System Rev. 2019;3:CD012387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kluetz PG, Kanapuru B, Lemery S, et al. Informing the tolerability of cancer treatments using patient‐reported outcome measures: summary of an FDA and critical path institute workshop. Value Health. 2018;21(6):742‐747. [DOI] [PubMed] [Google Scholar]
- 3. Efficace F, Feuerstein M, Fayers P, et al. Patient‐reported outcomes in randomised controlled trials of prostate cancer: methodological quality and impact on clinical decision making. Eur Urol. 2014;66(3):416‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beyer K, Maclennan S, Lardas M, et al. PIONEER’s update and integration of a localised prostate cancer core outcome set for effectiveness trials and a standard set for clinical practice. Annual Meeting of the European Association of Urology; 15‐19 March; Barcelona, 2019.
- 5. Beyer K, MacLennan S, Lardas M, et al. PIONEER’s update and integration of a metastatic Prostate Cancer Core Outcome Set. ICHOM 2019; 2‐3 May; Rotterdam, 2019.
- 6. LeBlanc TW, Abernethy AP. Patient‐reported outcomes in cancer care – hearing the patient voice at greater volume. Nat Rev Clin Oncol. 2017;14(12):763‐772. [DOI] [PubMed] [Google Scholar]
- 7. Mottet N, van den Bergh R, Briers E, et al. EAU prostate cancer guidelines. 2019.
- 8. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006‐1012. [DOI] [PubMed] [Google Scholar]
- 9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brundage M, Blazeby J, Revicki D, et al. Patient‐reported outcomes in randomized clinical trials: development of ISOQOL reporting standards. Qual Life Res. 2013;22(6):1161‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Calvert M, Blazeby J, Altman DG, et al. Reporting of patient‐reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309(8):814‐822. [DOI] [PubMed] [Google Scholar]
- 12. Cornford P, Bellmunt J, Bolla M, et al. Guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration‐resistant prostate cancer. Eur Urol. 2017;71(4):630‐642. [DOI] [PubMed] [Google Scholar]
- 13. Mottet N, Bellmunt J, Bolla M, et al. Guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618‐629. [DOI] [PubMed] [Google Scholar]
- 14. Kluetz PG, Slagle A, Papadopoulos EJ, et al. Focusing on core patient‐reported outcomes in cancer clinical trials: symptomatic adverse events, physical function, and disease‐related symptoms. Clin Cancer Res. 2016;22(7):1553‐1558. [DOI] [PubMed] [Google Scholar]
- 15. Yaxley JW, Coughlin GD, Chambers SK, et al. Robot‐assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016;388(10049):1057‐1066. [DOI] [PubMed] [Google Scholar]
- 16. Saad F, Cella D, Basch E, et al. Effect of apalutamide on health‐related quality of life in patients with non‐metastatic castration‐resistant prostate cancer: an analysis of the SPARTAN randomised, placebo‐controlled, phase 3 trial. Lancet Oncol. 2018;19(10):1404‐1416. [DOI] [PubMed] [Google Scholar]
- 17. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate‐risk localised prostate cancer: 2‐year patient‐reported outcomes of the randomised, non‐inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Denham JW, Wilcox C, Joseph D, et al. Quality of life in men with locally advanced prostate cancer treated with leuprorelin and radiotherapy with or without zoledronic acid (TROG 03.04 RADAR): secondary endpoints from a randomised phase 3 factorial trial. Lancet Oncol. 2012;13(12):1260‐1270. [DOI] [PubMed] [Google Scholar]
- 19. Bottomley A, Pe M, Sloan J, et al. Moving forward toward standardizing analysis of quality of life data in randomized cancer clinical trials. Clin Trials. 2018;15(6):624‐630. [DOI] [PubMed] [Google Scholar]
- 20. MacLennan S, Bekema HJ, Williamson PR, et al. A core outcome set for localised prostate cancer effectiveness trials: protocol for a systematic review of the literature and stakeholder involvement through interviews and a Delphi survey. Trials. 2015;16:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MacLennan SJ, MacLennan SJ, Imamura M, et al. Urological cancer care pathways: development and use in the context of systematic reviews and clinical practice guidelines. World J Urol. 2011;29(3):291‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lardas M, Liew M, van den Bergh RC, et al. Quality of life outcomes after primary treatment for clinically localised prostate cancer: a systematic review. Eur Urol. 2017;72(6):869‐885. [DOI] [PubMed] [Google Scholar]
- 23. Engel P, Almas MF, De Bruin ML, Starzyk K, Blackburn S, Dreyer NA. Lessons learned on the design and the conduct of Post‐Authorization Safety Studies: review of 3 years of PRAC oversight. Br J Clin Pharmacol. 2017;83(4):884‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calvert M, O'Connor D, Basch E. Harnessing the patient voice in real‐world evidence: the essential role of patient‐reported outcomes. Nat Rev Drug Discovery. 2019;18(10):731‐732. [DOI] [PubMed] [Google Scholar]
- 25. Mercieca‐Bebber R, Rouette J, Calvert M, et al. Preliminary evidence on the uptake, use and benefits of the CONSORT‐PRO extension. Qual Life Res. 2017;26(6):1427‐1437. [DOI] [PubMed] [Google Scholar]
- 26. Morrison A, Polisena J, Husereau D, et al. The effect of English‐language restriction on systematic review‐based meta‐analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138‐144. [DOI] [PubMed] [Google Scholar]
- 27. Loriot Y, Miller K, Sternberg CN, et al. Effect of enzalutamide on health‐related quality of life, pain, and skeletal‐related events in asymptomatic and minimally symptomatic, chemotherapy‐naive patients with metastatic castration‐resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol. 2015;16(5):509‐521. [DOI] [PubMed] [Google Scholar]
- 28. Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Devlin N, Herdman M, Pavesi M, et al. Health‐related quality of life effects of enzalutamide in patients with metastatic castration‐resistant prostate cancer: an in‐depth post hoc analysis of EQ‐5D data from the PREVAIL trial. Health Qual Life Outcomes. 2017;15(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel HR, Ilo D, Shah N, et al. Effects of tadalafil treatment after bilateral nerve‐sparing radical prostatectomy: quality of life, psychosocial outcomes, and treatment satisfaction results from a randomized, placebo‐controlled phase IV study. BMC urology. 2015;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Montorsi F, Brock G, Stolzenburg J‐U, et al. Effects of tadalafil treatment on erectile function recovery following bilateral nerve‐sparing radical prostatectomy: a randomised placebo‐controlled study (REACTT). Eur Urol. 2014;65(3):587‐596. [DOI] [PubMed] [Google Scholar]
- 32. Bruner DW, Hunt D, Michalski JM, et al. Preliminary patient‐reported outcomes analysis of 3‐dimensional radiation therapy versus intensity‐modulated radiation therapy on the high‐dose arm of the Radiation Therapy Oncology Group (RTOG) 0126 prostate cancer trial. Cancer. 2015;121(14):2422‐2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Michalski JM, Yan Y, Watkins‐Bruner D, et al. Preliminary toxicity analysis of 3‐dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high‐dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys. 2013;87(5):932‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Michalski JM, Moughan J, Purdy J, et al. Effect of standard vs dose‐escalated radiation therapy for patients with intermediate‐risk prostate cancer: the NRG oncology RTOG 0126 randomized clinical trial. JAMA Oncol. 2018;4(6):e180039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fizazi K, Scher HI, Miller K, et al. Effect of enzalutamide on time to first skeletal‐related event, pain, and quality of life in men with castration‐resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15(10):1147‐1156. [DOI] [PubMed] [Google Scholar]
- 36. Cella D, Ivanescu C, Holmstrom S, Bui CN, Spalding J, Fizazi K. Impact of enzalutamide on quality of life in men with metastatic castration‐resistant prostate cancer after chemotherapy: additional analyses from the AFFIRM randomized clinical trial. Ann Oncol. 2015;26(1):179‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187‐1197. [DOI] [PubMed] [Google Scholar]
- 38. Armstrong AJ, Saad F, Phung DE, et al. Clinical outcomes and survival surrogacy studies of prostate‐specific antigen declines following enzalutamide in men with metastatic castration‐resistant prostate cancer previously treated with docetaxel. Cancer. 2017;123(12):2303‐2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Basch E, Autio K, Ryan CJ, et al. Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy‐naive men with metastatic castration‐resistant prostate cancer: patient‐reported outcome results of a randomised phase 3 trial. Lancet Oncol. 2013;14(12):1193‐1199. [DOI] [PubMed] [Google Scholar]
- 40. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy‐naive men with metastatic castration‐resistant prostate cancer (COU‐AA‐302): final overall survival analysis of a randomised, double‐blind, placebo‐controlled phase 3 study. Lancet Oncol. 2015;16(2):152‐160. [DOI] [PubMed] [Google Scholar]
- 41. Beer TM, Schellhammer PF, Corman JM, et al. Quality of life after sipuleucel‐T therapy: results from a randomized, double‐blind study in patients with androgen‐dependent prostate cancer. Urology. 2013;82(2):410‐415. [DOI] [PubMed] [Google Scholar]
- 42. Beer TM, Bernstein GT, Corman JM, et al. Randomized trial of autologous cellular immunotherapy with sipuleucel‐T in androgen‐dependent prostate cancer. Clin Cancer Res. 2011;17(13):4558‐4567. [DOI] [PubMed] [Google Scholar]
- 43. Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368(14):1314‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mason M, Maldonado Pijoan X, Steidle C, et al. Neoadjuvant androgen deprivation therapy for prostate volume reduction, lower urinary tract symptom relief and quality of life improvement in men with intermediate‐ to high‐risk prostate cancer: a randomised non‐inferiority trial of degarelix versus goserelin plus bicalutamide. Clin Oncol (R Coll Radiol). 2013;25(3):190‐196. [DOI] [PubMed] [Google Scholar]
- 45. Denham JW, Joseph D, Lamb DS, et al. Short‐term androgen suppression and radiotherapy versus intermediate‐term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): an open‐label, randomised, phase 3 factorial trial. Lancet Oncol. 2014;15(10):1076‐1089. [DOI] [PubMed] [Google Scholar]
- 46. Denham JW, Wilcox C, Lamb DS, et al. Rectal and urinary dysfunction in the TROG 03.04 RADAR trial for locally advanced prostate cancer. Radiology Oncol. 2012;105(2):184‐192. [DOI] [PubMed] [Google Scholar]
- 47. Axcrona K, Aaltomaa S, da Silva CM, et al. Androgen deprivation therapy for volume reduction, lower urinary tract symptom relief and quality of life improvement in patients with prostate cancer: degarelix vs goserelin plus bicalutamide. BJU Int. 2012;110(11):1721‐1728. [DOI] [PubMed] [Google Scholar]
- 48. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high‐dose intensity‐modulated radiotherapy for prostate cancer: 5‐year outcomes of the randomised, non‐inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gaudet M, Vigneault E, Foster W, Meyer F, Martin AG. Randomized non‐inferiority trial of Bicalutamide and Dutasteride versus LHRH agonists for prostate volume reduction prior to I‐125 permanent implant brachytherapy for prostate cancer. Radiotherapy Oncol. 2016;118(1):141‐147. [DOI] [PubMed] [Google Scholar]
- 50. Duchesne GM, Woo HH, King M, et al. Health‐related quality of life for immediate versus delayed androgen‐deprivation therapy in patients with asymptomatic, non‐curable prostate cancer (TROG 03.06 and VCOG PR 01–03 [TOAD]): a randomised, multicentre, non‐blinded, phase 3 trial. Lancet Oncol. 2017;18(9):1192‐1201. [DOI] [PubMed] [Google Scholar]
- 51. Duchesne GM, Woo HH, Bassett JK, et al. Timing of androgen‐deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01–03 [TOAD]): a randomised, multicentre, non‐blinded, phase 3 trial. Lancet Oncol. 2016;17(6):727‐737. [DOI] [PubMed] [Google Scholar]
- 52. Gilbert DC, Duong T, Kynaston HG, et al. Quality‐of‐life outcomes from the Prostate Adenocarcinoma: TransCutaneous Hormones (PATCH) trial evaluating luteinising hormone‐releasing hormone agonists versus transdermal oestradiol for androgen suppression in advanced prostate cancer. BJU Int. 2017;119(5):667‐675. [DOI] [PubMed] [Google Scholar]
- 53. Langley RE, Cafferty FH, Alhasso AA, et al. Cardiovascular outcomes in patients with locally advanced and metastatic prostate cancer treated with luteinising‐hormone‐releasing‐hormone agonists or transdermal oestrogen: the randomised, phase 2 MRC PATCH trial (PR09). Lancet Oncol. 2013;14(4):306‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Langley RE, Kynaston HG, Alhasso AA, et al. A randomised comparison evaluating changes in bone mineral density in advanced prostate cancer: luteinising hormone‐releasing hormone agonists versus transdermal oestradiol. Eur Urol. 2016;69(6):1016‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rodda S, Morris WJ, Hamm J, Duncan G. ASCENDE‐RT: an analysis of health‐related quality of life for a randomized trial comparing low‐dose‐rate brachytherapy boost with dose‐escalated external beam boost for high‐ and intermediate‐risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98(3):581‐589. [DOI] [PubMed] [Google Scholar]
- 56. Morris WJ, Tyldesley S, Rodda S, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE‐RT Trial): an analysis of survival endpoints for a randomized trial comparing a low‐dose‐rate brachytherapy boost to a dose‐escalated external beam boost for high‐ and intermediate‐risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98(2):275‐285. [DOI] [PubMed] [Google Scholar]
- 57. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration‐resistant prostate cancer. N Engl J Med. 2018;378(26):2465‐2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tombal B, Saad F, Penson D, et al. Patient‐reported outcomes following enzalutamide or placebo in men with non‐metastatic, castration‐resistant prostate cancer (PROSPER): a multicentre, randomised, double‐blind, phase 3 trial. Lancet Oncol. 2019;20(4):556‐569. [DOI] [PubMed] [Google Scholar]
- 59. Lukka HR, Pugh SL, Bruner DW, et al. Patient reported outcomes in NRG oncology RTOG 0938, evaluating two ultrahypofractionated regimens for prostate cancer. Int J Radiat Oncol Biol Phys. 2018;102(2):287‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Basch EM, Scholz M, de Bono JS, et al. Cabozantinib versus mitoxantrone‐prednisone in symptomatic metastatic castration‐resistant prostate cancer: a randomized phase 3 trial with a primary pain endpoint. Eur Urol. 2019;75(6):929‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis‐free survival in prostate cancer. N Engl J Med. 2018;378(15):1408‐1418. [DOI] [PubMed] [Google Scholar]
- 62. Khalaf DJ, Sunderland K, Eigl BJ, et al. Health‐related quality of life for abiraterone plus prednisone versus enzalutamide in patients with metastatic castration‐resistant prostate cancer: results from a phase II randomized trial. Eur Urol. 2019;75(6):940‐947. [DOI] [PubMed] [Google Scholar]
- 63. Annala M, Vandekerkhove G, Khalaf D, et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 2018;8(4):444‐457. [DOI] [PubMed] [Google Scholar]
- 64. Unger JM, Griffin K, Donaldson GW, et al. Patient‐reported outcomes for patients with metastatic castration‐resistant prostate cancer receiving docetaxel and Atrasentan versus docetaxel and placebo in a randomized phase III clinical trial (SWOG S0421). J Patient Rep Outcomes. 2017;2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Quinn DI, Tangen CM, Hussain M, et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration‐resistant prostate cancer (SWOG S0421): a randomised phase 3 trial. Lancet Oncol. 2013;14(9):893‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sweeney CJ, Chen Y‐H, Carducci M, et al. Chemohormonal therapy in metastatic hormone‐sensitive prostate cancer. N Engl J Med. 2015;373(8):737‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Morgans AK, Chen Y‐H, Sweeney CJ, et al. Quality of life during treatment with chemohormonal therapy: analysis of E3805 chemohormonal androgen ablation randomized trial in prostate cancer. J Clin Oncol. 2018;36(11):1088‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fizazi K, Tran NamPhuong, Fein L, et al. Abiraterone plus prednisone in metastatic, castration‐sensitive prostate cancer. N Engl J Med. 2017;377(4):352‐360. [DOI] [PubMed] [Google Scholar]
- 69. Chi KN, Protheroe A, Rodríguez‐Antolín A, et al. Patient‐reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration‐naive prostate cancer (LATITUDE): an international, randomised phase 3 trial. Lancet Oncol. 2018;19(2):194‐206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
All data were taken from published manuscripts and can be obtained upon request by contacting f.sparano@gimema.it.


