Abstract
Background
Urachal carcinoma is a rare nonurothelial malignant tumor with high rates of local recurrence and systemic metastasis. Although radical resection is widely considered the standard treatment, there is still a debate regarding the benefits of lymphadenectomy. To explore these factors, we investigated the recurrence pattern of urachal cancer and the impact of lymphadenectomy on long‐term survival.
Methods
The data of 62 patients pathologically diagnosed with urachal carcinoma at Sun Yat‐sen University Cancer Center from 2002 to 2019 were retrospectively reviewed. Lymphadenectomy was defined as lymph nodes retrieved from the obturator, internal iliac, and external iliac lymph node stations. The Kaplan‐Meier method and Cox regression model were used to identify prognostic factors. OS and DFS were the primary endpoints.
Results
Of the 47 males and 15 females included, 54 patients underwent partial cystectomy, and 27 patients underwent lymphadenectomy. The number of patients with Sheldon stage IIIA, IIIB, IIIC, IVA, and IVB were 43 (69.4%), 4 (6.5%) 3 (4.8%), 6 (9.7%), and 6 (9.7%), respectively. The median DFS was 32.7 months, and the mean OS was 114.6 months. Sheldon stage (P < .001) and tumor size (P = .001) were identified as independent prognostic factors for DFS, whereas Sheldon stage (P = .003), peritoneal metastasis (P = .006), distant metastasis (P = .024), and recurrence in pelvic lymph nodes (P = .015) were independent prognostic factors for OS.
Conclusions
Urachal carcinoma has a high recurrence rate, but only peritoneal metastasis, distant metastasis, and recurrence in pelvic lymph nodes were found to be associated with OS. Lymphadenectomy was recommended because of its role in accurately staging the disease, and further research is needed to focus on lymphadenectomy and standardized the procedure.
Keywords: lymphadenectomy, prognostic factors, recurrence pattern, Urachal carcinoma
Urachal carcinoma has a high recurrence rate, but only peritoneal metastasis, distant metastasis, and recurrence in pelvic lymph nodes were found to be associated with OS. Although lymphadenectomy was not an independent prognostic factor for DFS and OS, the data of this study showed a possibility that lymphadenectomy could improve surgical outcomes.
1. INTRODUCTION
Urachal carcinoma, an uncommon nonurothelial tumor that most often occurs at the junction of the urachal ligament and urinary bladder dome or anywhere along with the midline of the bladder, constitutes less than 1% of all urinary bladder tumors and primarily occurs in males. 1 , 2 , 3 , 4 , 5 Several published reports have shown that adenocarcinomas, including mucinous adenocarcinoma, intestinal signet‐ring cell carcinoma, and mixed signet‐ring cell carcinoma, account for nearly 90% of urachal carcinomas. However, only a few squamous cell carcinomas, transitional epithelial carcinomas, sarcomas, and undifferentiated carcinomas have been reported. 3 , 6 , 7 Due to the lack of early clinical symptoms, 9 patients with urachal carcinoma are usually diagnosed when the disease is already at an advanced stage and is often accompanied by local extension (resulting in local recurrences) and systemic metastasis (resulting in death) before any related medical intervention is even started. 8 , 9 , 10
Currently, surgery, including partial resection or radical resection, remains the mainstay therapy because it can lead to a median survival of 48 months and 5‐year survival rates of 45%‐49%. 3 , 11 , 12 Despite recent published literature reporting no significant survival differences between partial and complete resection, 1 , 11 , 13 compared to radical cystectomy, partial cystectomy has been shown to have superior oncological safety to complete resection while maintaining similar efficacy. Additionally, partial cystectomy is also accompanied by a better quality of life because of fewer postoperative complications. 13 Therefore, partial cystectomy with en bloc excision of the urachal ligament, umbilicus, and dome of the bladder is most commonly recommended in clinical practice. 2 , 3 , 10
However, whether lymphadenectomy is of any benefit to patients with urachal carcinoma is still highly debatable. 1 , 5 Some surgeons support lymphadenectomy, as it can lead to the perioperative excision of occult lymph node metastasis, which has been related to improved survival, thereby achieving an increase in the 5‐year survival rate by 25%. 11 Others insist that performing aggressive surgical resection due to the presence of enlarged lymph nodes has no significant survival benefit. 3 , 13 Given the rarity of this tumor and the absence of large‐scale studies, reaching a consensus on such topics remains challenging.
To explore this dilemma, we retrospectively reviewed all patients diagnosed and treated with urachal carcinoma at Sun Yat‐sen University Cancer Center during the last 20 years and investigated any patterns of recurrence. Moreover, we explored the impact of lymphadenectomy on the long‐term survival of patients with urachal carcinoma.
2. MATERIALS AND METHODS
2.1. Patient cohort
After obtaining approval from the ethics committee of Sun Yat‐sen University Cancer Center, we identified the records of 82 patients who presented urachal masses from 2002 to 2019. A total of 62 were pathologically diagnosed as urachal carcinoma, while of the remaining cases, nine were grossly diagnosed as inflammation, three were benign urachal lesions, six were pathologically diagnosed as bladder carcinoma, one was diagnosed as sigmoid cancer, and one was discharged early from hospital due to active pulmonary tuberculosis and his subsequent treatment data could not be retrieved.
2.2. Surgical approach
Radical or partial cystectomy with excision of the urachal ligament and umbilicus was defined as radical resection. Cytoreductive surgery and peritoneal nodule biopsy were defined as nonradical resection. Lymphadenectomy was defined as lymph nodes retrieved from the obturator, internal iliac, and external iliac lymph node stations.
2.3. Pathologic review and staging
A central pathologic assessment was carried out by at least two pathologists. Urachal carcinoma was classified as poor, moderate, or well differentiated. The primary histologic cell type, as well as any significant secondary histologic features, was also recorded. In addition, tumors at diagnosis were retrospectively staged according to the Sheldon staging system, 1 classifying the tumors as stage I, tumor confined to the urachal mucosa; stage II, tumor with invasion confined to the urachus itself; stage IIIA, tumor with local extension to the bladder; stage IIIB, tumor with local extension to the abdominal wall; stage IIIC, tumor invading the peritoneum; stage IIID, tumor invading local viscera other than the bladder; stage IVA, urachal cancer with metastasis to the lymph nodes; and stage IVB, urachal cancer with distant metastases.
2.4. Follow‐up and survival analysis
The regular follow‐up after the operation included visits to an outpatient clinic at 3‐month intervals for the first 2 years and every 6 months in subsequent years. For patients who did not visit the clinic, follow‐up information was obtained by telephone call. Patients underwent routine blood and biochemical analyses, physical examination, and abdominal and pelvic computed tomography (CT) scans. When there was any evidence of suspected recurrence, chest, abdominal, and pelvic CT scans; brain magnetic resonance imaging (MRI); bone scintigraphy; and positron emission tomography were performed. A diagnosis of recurrence was in accordance with relevant diagnostic imaging or cytological/histologic findings. The calculation of the patients’ disease‐free survival (DFS) and overall survival (OS) was carried out as our primary endpoint. DFS was defined from the time of definitive diagnosis to the day of first recurrence confirmed by radiological scan or biopsy, with detailed mention of the recurrent site. OS was calculated from the date of diagnosis to the date of death or last follow‐up visit.
2.5. Statistical analysis
Fisher's exact test was used to compare categorical data, such as lymph node dissection, pathological pelvic lymph node metastasis, postoperative pelvic lymph node stage, and postoperative pelvic lymph node recurrence. Univariate and multivariate Cox proportional hazards regression models were established to identify prognostic and independent factors. Covariates with a P < .1 in the univariate analysis were used for the multivariate analysis. DFS and OS were calculated using the Kaplan‐Meier analysis method, and comparisons of survival between different groups were assessed by the log‐rank test. Statistical analyses were performed using SPSS software, version 22 (SPSS Inc), and P < .05 was considered statistically significant.
3. RESULTS
3.1. Patient characteristics
The characteristics of the 62 enrolled patients are listed in Table 1. There were 47 males and 15 females included with a median age of 51.5 ± 13.2 (range 22 to 69) years. Forty‐five (72.6%) patients had naked‐eye hematuria, eight (12.9%) had urinary irritation, 14 (22.6%) presented with an abdominal mass, two (3.2%) had omphalorrhoea, and two (3.2%) were diagnosed upon general physical examination without any initial presenting symptoms. All patients underwent a preoperative CT, MRI, or urological ultrasound scan. The clinical median tumor diameter was 3.75 ± 2.28 cm. All of the patients underwent surgery, including 54 of 62 (87%) treated with partial resection and 43.5% (27 of 62) with lymph node dissection. The number of patients with lymph nodes dissected at stations 1, 2, 3, and 4 was 3, 8, 4, and 12, respectively. The postoperative histopathological diagnosis indicated that 95.4% (59 of 62) and 40.3% (25 of 62) of cases were well‐ or moderately differentiated carcinoma, respectively. According to the Sheldon staging system for urachal carcinoma, 1 69.4% (43 of 62) had local extension to the bladder (stage IIIA), 6.5% (4 of 62) exhibited extension to the abdominal wall (stage IIIB), 4.8% (3 of 62) invaded the peritoneum (stage IIIC), and 33.9% (21 of 62) had stage IV disease, including 6 (9.7%) with regional lymph node metastasis and 6 (9.7%) presented with distant metastases. Elevations in carcinoembryonic antigen (CEA), CA19‐9, CA125, CA724, CyFra21‐1, and neuron‐specific enolase (NSE) were observed in some patients (21%, 8.1%, 1.6%, 19.4%, 14.5%, and 4.8%, respectively).
TABLE 1.
Characteristics of 62 patients with Urachal carcinoma
| Characteristics | Median or no. of cases (%) |
|---|---|
| Gender | |
| Male | 47 (75.8) |
| Female | 15 (24.2) |
| Age (y) | 51.5 ± 13.2 |
| Tumor size (cm) | 3.75 ± 2.28 |
| Hematuria | |
| No | 17 (27.4) |
| Yes | 45 (72.6) |
| Urinary irritation | |
| No | 54 (87.1) |
| Yes | 8 (12.9) |
| Omphalorrhoea | |
| No | 60 (96.8) |
| Yes | 2 (3.2) |
| Abdominal mass | |
| No | 48 (77.4) |
| Yes | 14 (22.6) |
| Tumor calcification in imaging | |
| No | 55 (88.7) |
| Yes | 7 (11.3) |
| Preoperative metastasis | |
| No | 51 (82.3) |
| Pelvic lymph node | 4 (6.5) |
| Peritoneal metastasis | 2 (3.2) |
| Abdominal wall metastasis | 2 (3.2) |
| Lung and mediastinal lymph node metastasis | 3 (4.8) |
| Sheldon stage | |
| IIIA | 43 (69.4) |
| IIIB | 4 (6.5) |
| IIIC | 3 (4.8) |
| IVA | 6 (9.7) |
| IVB | 6 (9.7) |
| Histology | |
| Adenocarcinoma | 59 (95.4) |
| Others | 3 (4.6) |
| Differentiation degree | |
| Well or moderate | 25 (40.3) |
| Poor | 17 (27.4) |
| Not mentioned | 20 (32.3) |
| Vascular invasion | |
| Positive | 7 (11.3) |
| Negative | 22 (35.5) |
| Not mentioned | 33 (53.2) |
| Operative approach | |
| Partial resection | 54 (87.0) |
| Radical resection | 8 (13.0) |
| Lymph node dissected | |
| Yes | 27 (43.5) |
| No | 35 (56.5) |
| Postoperative chemotherapy | |
| No | 44 (71.0) |
| Yes | 18 (29.0) |
| Positive lymph node stage | |
| Yes | 6 (9.7) |
| No | 56 (90.3) |
| The second operation after recurrence | |
| No | 50 (80.6) |
| Yes | 12 (19.4) |
| CEA | |
| Abnormal | 13 (21) |
| Normal | 26 (41.9) |
| Not mentioned | 26 (37.1) |
| CA199 | |
| Abnormal | 5 (8.1) |
| Normal | 32 (51.6) |
| Not mentioned | 27 (40.3) |
| CA125 | |
| Abnormal | 1 (1.6) |
| Normal | 14 (22.) |
| Not mentioned | 46 (75.8) |
| CA724 | |
| Abnormal | 12 (19.4) |
| Normal | 20 (32.3) |
| Not mentioned | 30 (48.4) |
| Abnormal NSE | 3 (4.8) |
| Abnormal CyFra21‐1 | 9 (14.5) |
| At least 2 abnormal serum tumor markers | 14 (22.6) |
| CK20 | |
| Positive | 38 (61.3) |
| Negative | 2 (3.2) |
| Not mentioned | 22 (35.5) |
| CK7 | |
| Positive | 20 (32.3) |
| Negative | 15 (24.2) |
| Not mentioned | 27 (43.5) |
| Nuclearβ | |
| Positive | 17 (27.4) |
| Negative | 45 (72.6) |
| CDX‐2 | |
| Positive | 34 (54.8) |
| Negative | 1 (1.6) |
| Not mentioned | 27 (43.5) |
3.2. Survival and prognostic factors of survival
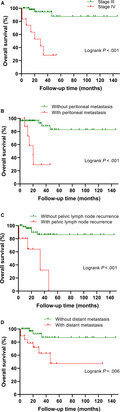
The median DFS for the entire study cohort was 32.7 months. Sheldon stage and tumor size were independent prognostic factors for DFS (P < .001, HR = 5.896, 95% CI 2.286‐15.208; P = .002, HR = 1.388, 95% CI 1.126‐1.710, respectively) (Table 2). Interestingly, although the operative approach was associated with DFS (P = .002), it was not statistically significant in the multivariate analysis. Significant survival differences in DFS and OS were found between Sheldon stages III and IV (P < .001, Figure 1; P < .001, Figure 2A).
TABLE 2.
Prognostic factors of the disease‐free survival and overall survival for patients with Urachal carcinoma
| Factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Prognostic factors of DFS | ||||
| Gender | 0.712 (0.292‐1.736) | .455 | ||
| Age (y) | 1.008 (0.980‐1.038) | .575 | ||
| Tumor size (cm) | 1.340 (1.099‐1.633) | .004 | 1.388 (1.126‐1.710) | .002 |
| Sheldon stage (III vs IV) | 3.609 (1.651‐7.889) | .001 | 5.896 (2.286‐15.208) | <.001 |
| Preoperative metastasis | 3.168 (1.430‐7.019) | .005 | ||
| Differentiation degree | ||||
| Well or moderate | Ref | |||
| Poor | 0.830 (0.359‐1.922) | .664 | ||
| Not mentioned | 0.857 (0.355‐2.068) | .732 | ||
| Vascular invasion | ||||
| No | Ref | |||
| Yes | 1.543 (0.538‐4.426) | .420 | ||
| Not mentioned | 0.381 (0.168‐0.865) | .021 | ||
| Lymph node dissected | 1.269 (0.599‐2.690) | .534 | ||
| Operative approach | 1.638 (1,202‐2.333) | .002 | ||
| Positive lymph node stage | 1.815 (0.688‐4.787) | .229 | ||
| Prognostic factors of OS | ||||
| Gender | 1.445 (0.372‐5.615) | .595 | ||
| Age (y) | 0.986 (0.939‐1.036) | .577 | ||
| Tumor size (cm) | 1.376 (1.077‐1.7559) | .011 | ||
| Sheldon stage (III vs IV) | 12.408 (3.196‐48.164) | <.001 | 12.523 (2.433‐64.460) | .002 |
| Preoperative metastasis | 9.856 (2.761‐35.188) | <.001 | ||
| Differentiation degree | ||||
| Well or moderate | Ref | |||
| Poor | 0.465 (0.094‐2.310) | .349 | ||
| Not mentioned | 0.564 (0.122‐3.145) | .564 | ||
| Vascular invasion | ||||
| No | Ref | |||
| Yes | 1.732 (0.310‐9.675) | .532 | ||
| Not mentioned | 0.446 (0.110‐1.802) | .257 | ||
| Lymph node dissected | 2.147 (0.599‐7.602) | .241 | ||
| Operative approach | 1.963 (1.281‐3.010) | .002 | ||
| Positive lymph node stage | 5.606 (1.576‐20.056) | .008 | ||
| Bladder recurrence | 2.631 (0.722‐9.444) | .138 | ||
| Abdominal wall recurrence | 3.098 (0.642‐14.947) | .159 | ||
| Postoperative peritoneal metastasis | 10.693 (2.789‐40.991) | .001 | 9.999 (1.906‐52.468) | .006 |
| Postoperative pelvic lymph node recurrence | 8.677 (2.469‐30.493) | .001 | 7.024 (1.429‐34.519) | .016 |
| Postoperative distant metastasis | 1.397 (21.022) | .015 | 8.416 (1.287‐55.036) | .026 |
| Postoperative chemotherapy | 1.407 (0.394‐5.027) | .599 | ||
| The second operation after recurrence | 0.976 (0.206‐4.620) | .976 | ||
Figure 1.
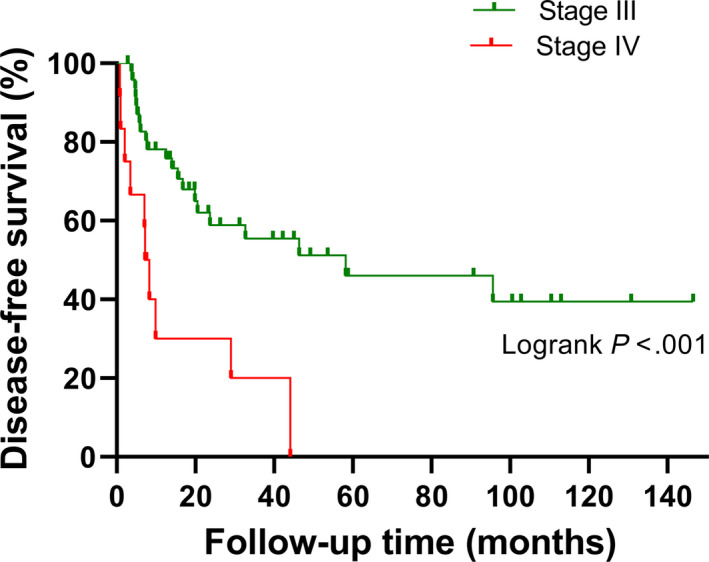
Kaplan‐Meier analysis of disease‐free survival for patients with urachal carcinoma in the Sheldon stage III and those in the Sheldon stage IV (P < .001)
Figure 2.
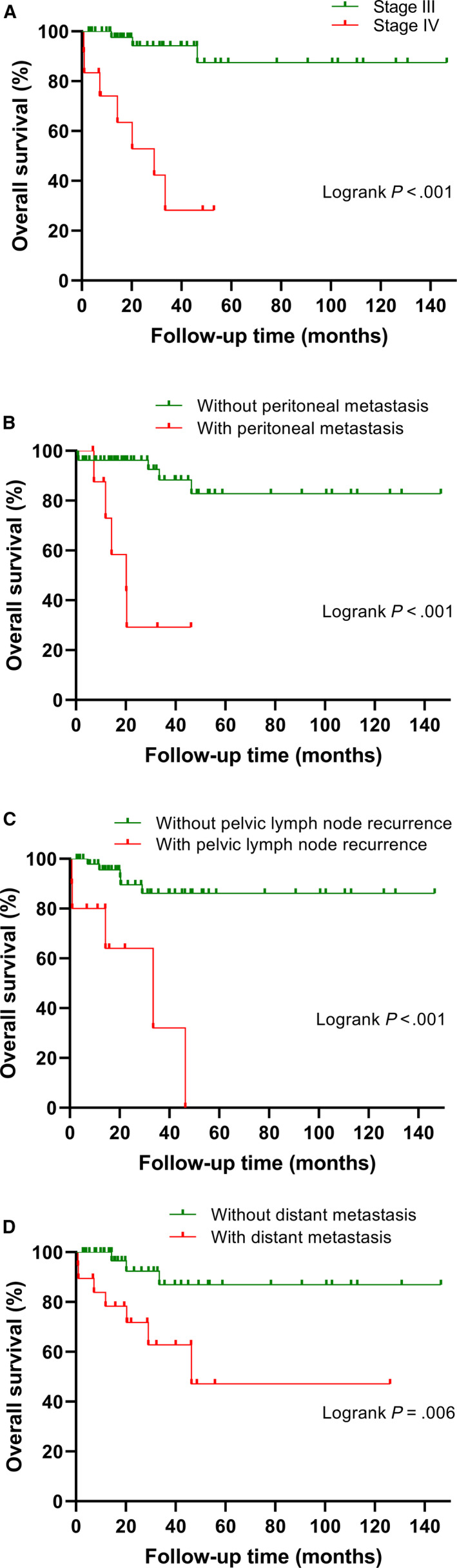
Kaplan‐Meier analysis of overall survival for patients with urachal carcinoma. A, Patients with stage IV versus stage III. B, Patients with peritoneal metastasis versus without peritoneal metastasis. C, Patients with pelvic lymph node recurrence versus without pelvic lymph node recurrence. D, Patients with distant metastasis versus without distant metastasis
Table 2 shows that Sheldon stage (P = .002, HR = 12.523, 95% CI 2.433‐64.460), postoperative peritoneal metastasis (P = .006, HR = 9.999, 95% CI 1.960‐53.539), postoperative distant metastasis (P = .028, HR = 8.416, 95% CI 1.287‐55.036), and recurrence in pelvic lymph nodes (P = .016, HR = 7.024, 95% CI 1.429‐34.519) were independent prognostic factors for impaired OS. Furthermore, we found that patients without peritoneal or distant metastasis had a significantly better OS than those with peritoneal or distant metastasis (P < .001, Figure 2B; P = .006, Figure 2D).
3.3. Relapse
Up to August 30, 2019, 31 of the initial 62 patients (50%) were alive and free of disease after surgical resection (median follow‐up 38.8 months). Conversely, 31 patients (50%) had metastases. The median time to recurrence after the primary surgery was 32.7 months. The sites of metastases are detailed in Table 3. Fifteen (24.2%) patients had metastases in their bladder, and the number of patients with metastasis in the abdominal wall, intraperitoneal cavity, and pelvic lymph nodes was 5 (8.1%), 10 (16.1%), and 10 (16.1%), respectively. Lung and mediastinal lymph nodes accounted for the majority of postoperative metastases (30.6%; 19 of 62 patients).
TABLE 3.
Sites of metastases in 31 patients after the primary resection
| The location of recurrence or metastasis | Median or case no. (%) |
|---|---|
| Bladder | 15 (24.2) |
| Abdominal wall | 5 (8.1) |
| Peritoneal metastasis | 10 (16.1) |
| Pelvic lymph node | 10 (16.1) |
| Distant metastasis | 19 (30.6) |
| Lung and mediastinal lymph nodes | 19 (30.6) |
| Bone | 1 (1.6) |
| Liver | 2 (3.2) |
| Total | 31 (50) |
The data from Table 4 indicate that 6.9% (4 of 58) of the patients had pathologically confirmed metastases in their lymph nodes, although they were diagnosed without pelvic lymph node metastasis before their surgery. Half of these patients (2 of 4 patients), who were thought to have metastasis in the pelvic lymph nodes before the surgery, were confirmed as negative for lymph node metastasis. A statistically significant difference was observed between them (Fisher's exact test P = .043). Subsequently, we found that the survival between patients with positive lymph node staging and those with negative lymph node staging was significantly different (P = .003, Figure 3). As shown in Table 5, compared with patients who underwent pelvic lymph node dissection, numerically, patients without pelvic lymph node dissection experienced a higher rate of postoperative pelvic lymph node recurrence (11.1% vs 20%), even though statistical significance was not achieved (Fisher's exact test P = .491). However, compared to patients with pelvic lymph node recurrence, patients without pelvic lymph node recurrence had significantly superior OS (P < .001, Figure 2C).
TABLE 4.
The correlation between with‐without pelvic lymph node metastasis and postoperatively pelvic lymph node stage positive or negative
| Group | Positive lymph node stage | Negative lymph node stage | Total |
|---|---|---|---|
| With pelvic lymph node metastasis | 2 | 2 | 4 |
| Without pelvic lymph node metastasis | 4 | 54 | 58 |
| Total | 6 | 56 | 62 |
Fisher's Exact Test P = .043.
Figure 3.
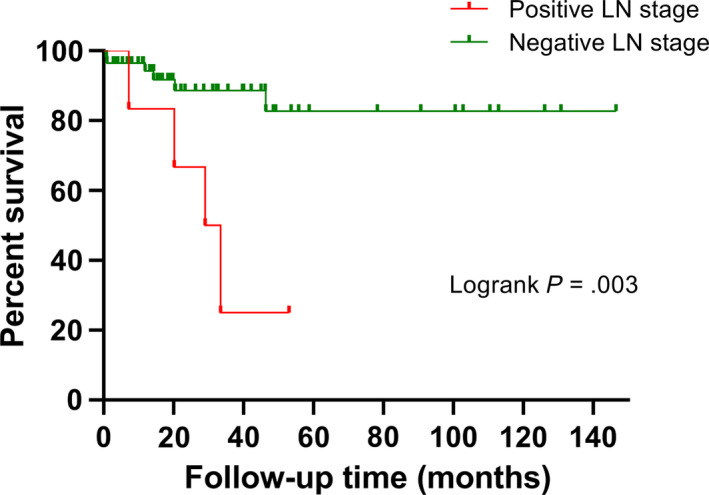
Kaplan‐Meier analysis of survival for patients with positive lymph node and patents just with negative lymph node stage (P = .003)
TABLE 5.
The correlation between with‐without pelvic lymph node dissected and with‐without postoperatively pelvic lymph node recurrence
| Group | Without pelvic lymph node recurrence | With pelvic lymph node recurrence | Total |
|---|---|---|---|
| Without pelvic lymph node dissected | 28 | 7 | 35 |
| With pelvic lymph node dissected | 24 | 3 | 27 |
| Total | 52 | 10 | 62 |
Fisher's Exact Test P = .491.
4. DISCUSSION
In this study, we retrospectively reviewed patients with confirmed urachal carcinoma who were treated at Sun Yat‐sen University Cancer Center during the last 20 years, comprehensively presented their clinical and pathological features and presented several original findings.
Elevations in some tumor markers, such as CEA, CA19‐9, and CA125, were observed in some patients in this study (12 of 32 patients, five of 37, and one of 15, respectively). Arlene et al reviewed 42 patients from the MD Anderson Cancer Center and found that 13 of 22 patients had increased CEA levels, 6 of 10 patients had increased CA19‐9 levels, and 7 of 16 patients had increased CA125 levels. 5 Although the cohort in MD Anderson Cancer Center had a higher percentage of patients with elevated tumor markers, both studies revealed the clinical similarities of urachal cancer and gastroenteric cancer. Most importantly, elevated CA724 levels were found in 37.5% (12/32) of preoperative patients, which revealed the potential value of CA724 in diagnosis. One case report about urachal carcinoma reported that increased CA724 was observed in a patient with local recurrence and ovary metastasis after resection, which showed value of detection of postoperative recurrence. 14 CA724 is a useful marker for predicting the efficacy of treatment in gastric cancer, and its prognostic value has been widely recognized. 15 , 16 Thus, given the established value of CA724 in diagnosing, staging, and prognosticating gastric cancer, we surmise the potential value of CA724 in guiding the clinical practice of urachal carcinoma, which needs further study. Additionally, similar to the immunophenotypic features in gastroenteric tumors reported by Paner et al, 17 there may be a certain similarity between urachal carcinoma and gastrointestinal neoplasms in terms of cellular structures and biological behaviors, to a certain extent. At the same time, patients with urachal carcinoma may also benefit from chemotherapy for gastrointestinal tumors. Two previous studies have revealed that chemotherapy regimens combining 5‐FU and cisplatin showed better therapeutic effects. 5 , 18 Unfortunately, the study failed to show the survival advantage of chemotherapy because of a lack of relevant data. We look forward to more advances in chemotherapy for urachal carcinoma.
Our study findings also demonstrated the preferred pattern in the site of recurrence after primary resection. In their meta‐analysis, Tibor Szarvas et al mentioned that a considerable proportion of patients develop distant metastases and lymph node metastases, with the lung, bone, and peritoneum as the most common sites of metastasis. 18 In the cohort from the MD Anderson Cancer Center, bone (13 of 26 patients), lung (12 of 26 patients), and liver (7 of 26 patients) were the three most common sites of metastasis. 5 In this study, lung and mediastinal lymph node metastasis, recurrence in the bladder, pelvic lymph node metastasis, and peritoneal metastasis were observed in 19, 15, 10, and 10 of 31 patients, respectively. These data may help oncologists monitor the progression and relapse of urachal cancer. Moreover, the multivariate analysis of OS found that some recurrence patterns had a more negative effect on survival. Peritoneal metastasis, distant metastasis, and recurrence in the pelvic lymph nodes were independent prognostic factors for OS. Compared with other patterns of recurrence, recurrence in the bladder and abdominal wall had a limited influence on OS. Although undergoing a second operation showed no statistical significance in the Cox regression model, one possible explanation is that recurrence in the bladder and abdominal wall is considered local recurrence, and patients still have a chance to completely remove these lesions during the second operation after their relapse. Distant metastasis is the most common recurrence pattern and is strongly associated with worse long‐term survival, which clearly underlines the need for effective systemic therapy.
Another key finding of this study is the role of lymphadenectomy. There is still no agreement about whether lymphadenectomy is beneficial to patients with urachal cancer. On the one hand, there is no study proving that lymphadenectomy can improve long‐term outcomes. One report reviewed 152 patients, which included 43 patients who underwent lymphadenectomy, and did not observe a positive effect of lymphadenectomy on survival. 19 Niedworok et al reviewed 26 patients and revealed that lymphadenectomy was not a prognostic factor of OS or progression‐free survival. 20 The rare positive rate of lymph node metastases and the limited number of patients in a single‐center study might explain why lymphadenectomy was not a prognostic factor. In addition, a lack of consensus on the lymphadenectomy procedure may cause discrepancies in its quality, which might have weakened its effect on OS. On the other hand, lymphadenectomy plays an important role in accurately staging urachal carcinoma. Given the general fact that preoperative imaging cannot detect all invaded lymph nodes in many kinds of cancers, 21 , 22 , 23 , 24 , 25 , 26 except for the novel findings mentioned above, we also emphasized exploring whether patients presenting obturator, internal iliac, and external iliac lymph node metastasis on imaging truly had metastasis by comparing the images to the pathology after resection. In this study, two of four patients who were clinically diagnosed with node‐positive stage disease were pathologically diagnosed with node‐negative stage disease, and four patients who were clinically diagnosed with node‐negative stage disease were pathologically diagnosed with node‐positive stage disease. Although lymphadenectomy was not an independent prognostic factor for DFS or OS, it could improve surgical outcomes. High‐quality lymphadenectomy can remove lymph nodes with micrometastases, and patients benefit from removing positive lymph nodes.
In bladder urothelial carcinoma and gastrointestinal tumors, lymph node dissection has been shown to be significant for accurately staging the disease and improving patient prognosis. 27 , 28 , 29 Given that urachal carcinoma has a similar location to bladder urothelial carcinoma and a similar histology to gastrointestinal tumors in histology, we strongly believe that pelvic lymph node dissection during the primary resection of urachal carcinoma is beneficial for patients, although we failed to find a significant predictive value of pelvic lymph node dissection in urachal carcinoma in this study.
Despite the innovation of this study as mentioned above, there are still some limitations that should be considered. The principal limitation is its small sample size and retrospective nature, and bias inevitably existed. Furthermore, loss of immunohistochemistry data in some cases limits the statistical power of this study. In addition, the lack of relevant chemotherapy data may affect the reliability of the results. However, this is a dilemma that all health‐care providers who treat rare diseases must confront. We look forward to pooling data from multiple centers into a larger common dataset in the future to carry out larger studies of urachal carcinoma.
5. CONCLUSIONS
In summary, our findings showed that the Sheldon stage, peritoneal metastasis, distant metastasis, and recurrence in pelvic lymph nodes were associated with long‐term survival. Although lymphadenectomy was not an independent prognostic factor for DFS and OS, lymphadenectomy was recommended because of its role in accurate staging. Further research is needed to focus on lymphadenectomy and make it standardized.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Fangfang Duan gathered data. Wenyu Zhai performed data analyses. Wenyu Zhai processed the figures. Fangfang Duan wrote the manuscript. Fangfang Duan, Wenyu Zhai, and Bei Zhang revised the manuscript. Shengjie Guo and Bei Zhang designed the study. All authors approved the final version of the manuscript.
Duan F, Zhai W, Zhang B, Guo S. Urachal carcinoma: Impact of recurrence pattern and lymphadenectomy on long‐term outcomes. Cancer Med. 2020;9:4166–4174. 10.1002/cam4.3059
Fangfang Duan and Wenyu Zhai contributed this work eaqually and should be regarded as co‐first authors.
Contributor Information
Bei Zhang, Email: zhangbei@sysucc.org.cn.
Shengjie Guo, Email: zhangbei@sysucc.org.cn, Email: guosjmed@163.com.
DATA AVAILABILITY STATEMENT
The key raw data have been deposited into the Research Data Deposit (http://www.researchdata.org.cn), with the Approval Number of RDDA2020001354, and the datasets used in this study are publicly available.
REFERENCES
- 1. Sheldon CA, Clayman RV, Gonzalez R, Williams RD, Fraley EE. Malignant urachal lesions. J Urol. 1984;131(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 2. Henly DR, Farrow GM, Zincke H. Urachal cancer: role of conservative surgery. Urology. 1993;42(6):635‐639. [DOI] [PubMed] [Google Scholar]
- 3. Siefker‐Radtke A. Urachal adenocarcinoma: a clinician's guide for treatment. Semin Oncol. 2012;39(5):619‐624. [DOI] [PubMed] [Google Scholar]
- 4. Jakse G, Schneider HM, Jacobi GH. Urachal signet‐ring cell carcinoma, a rare variant of vesical adenocarcinoma: incidence and pathologicalcriteria. J Urology. 1978;120(6):764‐766. [DOI] [PubMed] [Google Scholar]
- 5. Siefker‐radtke AO, Gee J, Shen YU, et al. Multimodality management of urachal carcinoma: The M. D. Anderson cancer center experience. J Urol. 2003;169(4):1295‐1298. [DOI] [PubMed] [Google Scholar]
- 6. Wright JL, Porter MP, Li CI, Lange PH, Lin DW. Differences in survival among patients with urachal and nonurachal adenocarcinomas of the bladder. Cancer. 2006;107(4):721‐728. [DOI] [PubMed] [Google Scholar]
- 7. Johnson DE, Hodge GB, Abdul‐Karim FW, Ayala AG. Urachal carcinoma. Urology. 1985;26(3):218‐221. [DOI] [PubMed] [Google Scholar]
- 8. Besarani D. Recurrent urachal adenocarcinoma. J Clin Pathol. 2003;56(11):882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. 2019;39(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Santucci RA, True LD, Lange PH. Is partial cystectomy the treatment of choice for mucinous adenocarcinoma of the urachus? Urology. 1997;49(4):536‐540. [DOI] [PubMed] [Google Scholar]
- 11. Ashley RA, Inman BA, Sebo TJ, et al. Urachal carcinoma: Clinicopathologic features and long‐term outcomes of an aggressive malignancy. Cancer. 2006;107(4):712‐720. [DOI] [PubMed] [Google Scholar]
- 12. Herr HW, Bochner BH, Sharp D, Dalbagni G, Reuter VE. Urachal carcinoma: contemporary surgical outcomes. J Urol. 2007;178(1):74‐78. [DOI] [PubMed] [Google Scholar]
- 13. Herr WH. Urachal carcinoma: the case for extended partial cystectomy. J Urol. 1994;151(2):365‐366. [DOI] [PubMed] [Google Scholar]
- 14. Zong L, Chen P. Surgical and chemotherapeutic experience regarding a urachal carcinoma with repeated relapse: case report and literature review. World J Surg Oncol. 2013;11:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nam DH, Lee YK, Park JC, et al. Prognostic value of early postoperative tumor marker response in gastric cancer. Ann Surg Oncol. 2013;20(12):3905‐3911. [DOI] [PubMed] [Google Scholar]
- 16. Zou L, Qian J. Decline of serum CA724 as a probable predictive factor for tumor response during chemotherapy of advanced gastric carcinoma. Chinese J Cancer Res. 2014;26(4):404‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paner GP, McKenney JK, Barkan GA, et al. Immunohistochemical analysis in a morphologic spectrum of urachal epithelial neoplasms: diagnostic implications and pitfalls. American J Surg Pathol. 2011;35(6):787‐798. [DOI] [PubMed] [Google Scholar]
- 18. Szarvas T, Módos O, Niedworok C, et al. Clinical, prognostic, and therapeutic aspects of urachal carcinoma – a comprehensive review with meta‐analysis of 1,010 cases. Urologic Oncol. 2016;34(9):388‐398. [DOI] [PubMed] [Google Scholar]
- 19. Bruins HM, Visser O, Ploeg M, Hulsbergen‐van de Kaa CA, Kiemeney LALM, Witjes JA. The clinical epidemiology of urachal carcinoma: results of a large, population based study. J Urol. 2012;188(4):1102‐1107. [DOI] [PubMed] [Google Scholar]
- 20. Niedworok C, Panitz M, Szarvas T, et al. Urachal carcinoma of the bladder: impact of clinical and immunohistochemical parameters on prognosis. J Urol. 2016;195(6):1690‐1696. [DOI] [PubMed] [Google Scholar]
- 21. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non‐small cell lung cancer: a review. JAMA. 2019;322(8):764‐774. [DOI] [PubMed] [Google Scholar]
- 22. Dekker E, Tanis Pieter J, Vleugels JLA, et al. Colorectal cancer. Lancet. 2019;394(10207):1467‐1480. [DOI] [PubMed] [Google Scholar]
- 23. Chen Y‐P, Chan ATC, Le Q‐T, et al. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64‐80. [DOI] [PubMed] [Google Scholar]
- 24. Koyfman SA, Ismaila N, Crook D, et al. Management of the neck in squamous cell carcinoma of the oral cavity and oropharynx: ASCO clinical practice guideline. J Clin Oncol. 2019;37(20):1753‐1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Budach V, Tinhofer I. Novel prognostic clinical factors and biomarkers for outcome prediction in head and neck cancer: a systematic review. Lancet Oncol. 2019;20:e313‐e326. [DOI] [PubMed] [Google Scholar]
- 26. Lam TBL. Optimizing the diagnosis of pelvic lymph node metastasis in bladder cancer using computed tomography and magnetic resonance imaging. Cancer Commun. 2018;38(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gschwend JE, Heck MM, Lehmann J, et al. Extended versus limited lymph node dissection in bladder cancer patients undergoing radical cystectomy: survival results from a prospective, randomized trial. European Urol. 2019;75(4):604‐611. [DOI] [PubMed] [Google Scholar]
- 28. Zhang CD, Yamashita H, Seto Y. Gastric cancer surgery: historical background and perspective in Western countries versus Japan. Annals Translational Med. 2019;7(18):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Märkl B, Olbrich G, Schenkirsch G, et al. Clinical significance of international union against cancer pN staging and lymph node ratio in node‐positive colorectal cancer after advanced lymph node dissection. Dis Colon Rectum. 2016;59(5):386‐395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The key raw data have been deposited into the Research Data Deposit (http://www.researchdata.org.cn), with the Approval Number of RDDA2020001354, and the datasets used in this study are publicly available.


