Abstract
As the coronavirus disease 2019 (COVID-19) pandemic spread across the globe, transplant programs suffered a setback. We report the first experience of COVID-19 infection within 1 month of living donor kidney transplant (LDKT). We describe 2 LDKT recipients who were detected positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at day 19 and day 7 posttransplant. They had minimal symptoms at diagnosis and did not develop any respiratory complications or allograft dysfunction. Immunosuppression was de-escalated; however, nasopharyngeal swab real-time reverse transcription polymerase chain reaction (rRT-PCR) remained positive for SARS-CoV-2 for a prolonged time. Younger age, absence of other comorbidities, and lower dose of anti-thymocyte globulin (ATG) used as induction possibly contributed to good outcome in our recent LDKT recipients compared with earlier published cases of recent deceased donor kidney transplant recipients with COVID-19.
KEYWORDS: clinical research/practice, complication: infectious, immunosuppressive regimens, infection and infectious agents – viral, infectious disease, kidney transplantation/nephrology
Abbreviations: COVID-19, coronavirus disease 2019; DDKT, deceased donor kidney transplant; ICU, intensive care unit; LDKT, living donor kidney transplant; rRT-PCR, real-time reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
Worldwide, transplant programs suffered a setback in the coronavirus disease 2019 (COVID-19) pandemic and most have temporarily suspended transplant activity voluntarily or under government advisory and regulations.1 , 2 This pertains specifically to living donor kidney transplant (LDKT), where a person can continue on dialysis while awaiting a more conducive time for a transplant. However, with repeated exposure in a center-based hemodialysis program, COVID-19 infection is likely in these immunosuppressed individuals, and the accompanying problem of finding a COVID-19-positive hemodialysis unit can be challenging and life threatening.
Sooner or later we will need to restart transplant programs, both LDKT and deceased donor kidney transplant (DDKT), as the acute era transitions to a post-COVID-19 new normal, where severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection will be a possibility. Nationwide lockdown was announced in India on March 24, 2020. As of May 23, 2020, there have been 124 794 cases and 3726 deaths in India due to COVID-19, with 28 634 cases and 949 deaths in the city of Mumbai.3 , 4 In India, 2.8 million tests have been performed for the diagnosis of SARS-CoV-2 infection until May 23, 2020.5 We present our single-center Indian experience and look at published data of recent kidney transplant recipients (<3 months posttransplant) with COVID-19.
2. CASE REPORT
At Jaslok Hospital and Research Centre, Mumbai, India, 7 kidney transplants were performed from March 1 to March 25, 2020. Of these, 5 were LDKT and 2 were DDKT. The last transplant was performed on March 25, 2020, almost 2 weeks after the first reported case of COVID-19 in the state. Following this, transplant activity was suspended and patients already on dialysis were continued on the same modality, whereas patients awaiting preemptive LDKT were managed conservatively. During the further follow-up over the next 2 months, 2 of these 7 patients tested positive for SARS-CoV-2 by nasopharyngeal swab real-time reverse transcription polymerase chain reaction (rRT-PCR), 3 tested negative, and 2 were not tested because they were asymptomatic.
2.1. Case 1
The first patient was a 35-year-old man who underwent LDKT on March 20, 2020 with an ABO-compatible spousal donor. His previous medical history included chronic kidney disease of unknown cause and hypertension for the past 2 years. He had been on hemodialysis for 12 months. Triple immunosuppression consisting of prednisolone, tacrolimus, and mycophenolate mofetil was started 2 days prior to the transplant and 1 mg/kg of induction agent anti-thymocyte globulin (ATG) was given on the day of surgery. His serum creatinine reached a nadir of 1 mg/dL by day 3 and he was discharged on day 10 posttransplant.
He developed dry cough on day 19 posttransplant; however, he was afebrile with no shortness of breath. On evaluation, oxygen saturation was 99% in ambient air, blood pressure 134/80 mm Hg, and respiratory rate 20 breaths per minute. Nasopharyngeal swab rRT-PCR was ordered in view of his symptoms and it was positive for SARS-CoV-2. He was readmitted to the hospital as prevalent local government protocol required hospital-based isolation of COVID-19-positive cases. There were no abnormalities on chest radiograph. Laboratory evaluation revealed a total leukocyte count of 5.1 × 109/L, absolute lymphocyte count of 0.4 × 109/L, C-reactive protein 10.8 mg/L, procalcitonin <0.05 ng/mL, and no growth on blood culture.
He was started on hydroxychloroquine and azithromycin as per prevalent institutional protocol. Immunosuppression dose reduction included rapid tapering of prednisolone to 15 mg/d and tacrolimus dose adjustment to maintain a trough level of 4-6 ng/mL. Mycophenolate mofetil was stopped in view of lymphopenia. Prophylactic valganciclovir 450 mg/d was continued but cotrimoxazole 80/400 mg was reduced to alternate days. He remained afebrile throughout the course. After change in local government protocol allowing for home isolation of COVID-19-positive cases, he was discharged after 28 days from admission. His serum creatinine at discharge was 1.05 mg/dL and nasopharyngeal swab rRT-PCR was still positive for SARS-CoV-2.
2.2. Case 2
The second patient was a 45-year-old man with hepatitis B and chronic kidney disease due to membranoproliferative glomerulonephritis. He had been on hemodialysis for the past 3 years. He underwent LDKT on March 23, 2020 with an ABO-compatible spousal donor and received triple immunosuppression with ATG 1 mg/kg as induction. His serum creatinine reached 1 mg/dL by day 3 posttransplant. He was detected positive for SARS-CoV-2 on day 7 posttransplant during his indoor stay. He only had mild throat irritation at the time of diagnosis and was tested as part of contact tracing. On evaluation, oxygen saturation was 98% in ambient air, blood pressure was 126/70 mm Hg, and respiratory rate was 22 breaths per minute.
At diagnosis, a chest radiograph revealed no abnormalities and total leukocyte count was 10.9 × 109/L with absolute lymphocyte count 0.9 × 109/L. Serum procalcitonin was 0.06 ng/mL and blood culture was negative. He was shifted to the COVID-19 ward and was started on hydroxychloroquine and azithromycin. Mycophenolate mofetil was reduced to 250 mg/d; prophylactic valganciclovir 450 mg/d was continued and cotrimoxazole 80/400 mg was reduced to alternate days. He too underwent rapid tapering of prednisolone dose to 15 mg/d and tacrolimus dose adjustment to maintain a trough level of 4-6 ng/mL. His nasopharyngeal swab rRT-PCR turned negative after 48 days from exposure and subsequently he was discharged after 52 days from admission. His serum creatinine at discharge was 0.9 mg/dL.
Intensive care unit (ICU) monitoring and supplemental oxygen were not required, and kidney allograft function remained stable throughout the course of COVID-19 in both recipients. They had lymphopenia, which could, however, be attributed to recent use of ATG. No anticoagulation was given to either patient and there were no episodes of thrombosis.
At the time of these transplants, epidemiological screening and clinical screening was done for all the donors and recipients. However, prevalent local government protocol only allowed laboratory testing of symptomatic individuals, close contacts of laboratory-confirmed positive cases of COVID-19, and those with a history of international travel. Hence, protocol-based pretransplant laboratory testing for SARS-CoV-2 was not done. All patients were educated about hand hygiene and coughing etiquette, and were provided with a face mask. Pretransplant hemodialysis was done in the dialysis unit with contact and droplet precautions, environmental disinfection, spatial separation of beds, no attendant policy, and informed consent. Posttransplant, these patients were isolated in the kidney transplant unit, which is separate from the ICU.
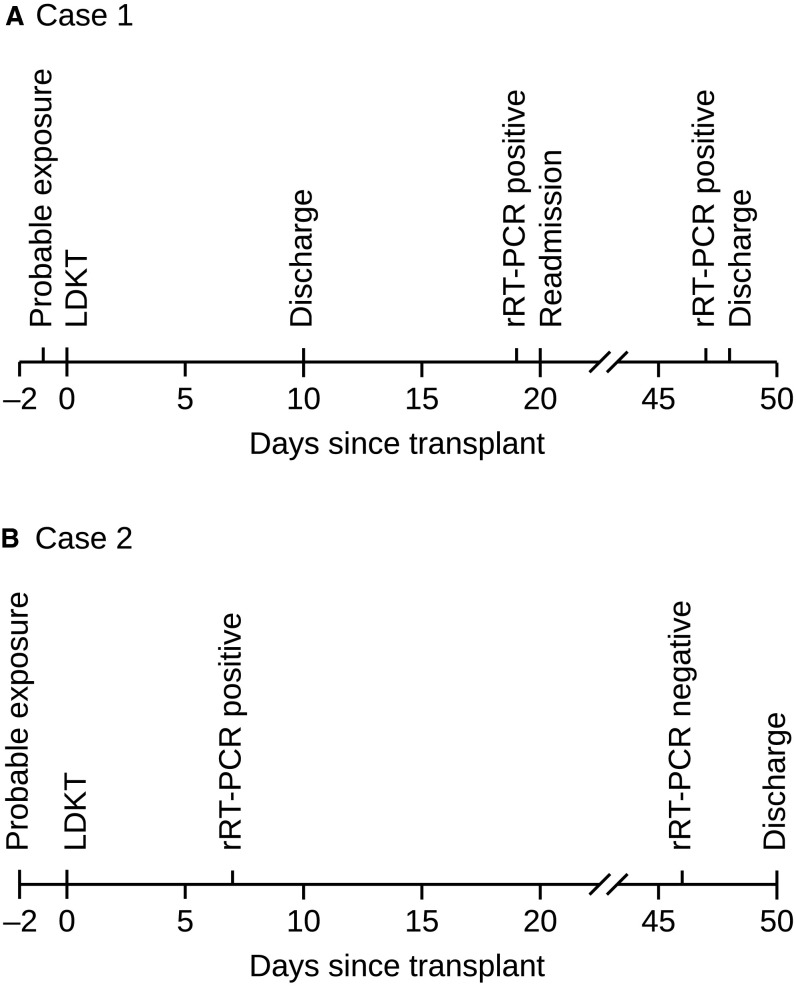
Both patients on contact tracing could have their illness attributed to pretransplant close contact with a hemodialysis patient in the nonisolation ward, who later tested positive for SARS-CoV-2. Figure 1 illustrates probable moments of exposure and diagnosis of SARS-CoV-2 with nasopharyngeal swab rRT-PCR in these patients. The first patient was asymptomatic during his posttransplant stay in the kidney transplant unit and was not tested prior to discharge. When he developed minimal symptoms after discharge, he was tested because the number of testing facilities had increased by then. It is possible that he had presumed exposure during his pretransplant inpatient stay similar to the second patient, but remained asymptomatic and hence was not diagnosed earlier. Considering the 2-14-day incubation period of COVID-19,6 exposure after discharge also remains an alternative possibility.
FIGURE 1.
Timeline of probable exposure and diagnosis of SARS-CoV-2 infection with nasopharyngeal swab rRT-PCR in case 1 (A) and case 2 (B). Both recipients were admitted 2 days prior to transplant. LDKT, living donor kidney transplant; rRT-PCR, real-time reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
3. DISCUSSION
Due to intensive immunosuppression, recent transplant recipients (<3 months posttransplant) are at increased risk of developing severe disease due to COVID-19. They are also likely to have superadded or co-infections due to other pathogens during this early posttransplant phase. In our observation, viral rRT-PCR positivity for SARS-CoV-2 can persist for a long duration in transplant recipients, which may impact their duration of stay indoors or in isolation centers. In an earlier report in a nontransplant setting, the longest viral shedding was observed for 37 days.7 In our second patient, nasopharyngeal swab rRT-PCR turned negative after 48 days from exposure.
We describe the clinical course of first reported COVID-19 cases in recent LDKT. Published studies have reported 5 cases of COVID-19 in recent DDKT. A report from New York describes poor outcome in 2 recent DDKT recipients (2/2 deaths).8 In another report from London, 1 recent DDKT recipient required ventilator support and continuous renal replacement therapy, whereas others required brief ICU stay.9 One recent DDKT recipient in a report from China was discharged at day 31 and did not require an ICU stay.10
Differences in COVID-19 mortality and complications between our experience with LDKT and the above reports could possibly be due to a higher dose of ATG used during the immediate posttransplant period in DDKT where there are greater probabilities of delayed graft function. Severe lymphopenia has been described in nonsurvivors of COVID-19.7 , 11 Preexisting lymphocyte depletion due to recent use of ATG may possibly contribute to severity of illness in transplant recipients who develop SARS-CoV-2 infection. Use of the nonlymphocyte-depleting agent basiliximab, or in the case of living-related donor transplant with low immunological risk, no induction could be alternative strategies. Favorable outcome in our patients could also be attributed to younger age and absence of other comorbidities.
Worldwide mortality due to COVID-19 is variable. As of May 23, 2020, COVID-19 mortality per 100 000 population has been lower in China (0.33) and India (0.28) than in European countries (Spain 61.27, Italy 53.97, and United Kingdom 54.86) and United States (29.34).3 Mortality differences could be attributed to genetic variation and hence differential immune response in these populations. These differences could also be due to different strains of SARS-CoV-2 prevailing in these geographical locations.12 Viral strains isolated from Indian patients have shown 99.97% homology to the original strains isolated from Wuhan, China.13 Other plausible explanations for these mortality differences include age distribution of the population, prevalence of comorbidities, access to testing, overloading of the healthcare system, and lockdown strategies.
Our recent LDKT recipients had minimal symptoms and no allograft dysfunction after developing COVID-19. Mortality and complications in recent transplant recipients with COVID-19 may be low in Asian populations, but more data are required to confirm this. In the new normal post-COVID-19 era, as transplant centers look forward to restarting their programs, our experience may be helpful. Transplant programs will require screening and laboratory testing of prospective donors and recipients to rule out COVID-19 and a pretransplant in-center or in-hospital isolation. We may start with younger recipients and use basiliximab or lower dose of ATG as induction agent. In recipients with early posttransplant SARS-CoV-2 infection, reducing or stopping mycophenolate mofetil may be a useful approach.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author and with permission from Jaslok Hospital and Research Centre, Mumbai, India. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Boyarsky BJ, Po-Yu Chiang T, Werbel WA, et al. Early impact of COVID-19 on transplant center practices and policies in the United States [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15915. [DOI] [PMC free article] [PubMed]
- 2.Bellini MI, Tortorici F, Capogni M. Kidney transplantation and the lock-down effect [published online ahead of print 2020]. Transpl Int. 10.1111/tri.13639 [DOI] [PMC free article] [PubMed]
- 3.Johns Hopkins University of Medicine. Mortality Analyses – Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/data/mortality. Published 2020. Accessed May 23, 2020
- 4.Municipal Corporation of Greater Mumbai. COVID-19 daily update. Greater Mumbai area. https://twitter.com/mybmc/status/1264261662657228800. Published May 23, 2020. Accessed May 23, 2020
- 5.Indian Council of Medical Research. Indian Council of Medical Research, New Delhi. https://www.icmr.gov.in. Published 2020. Accessed May 23, 2020
- 6.Centers for Disease Control and Prevention. Symptoms of Coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Published 2020. Accessed May 27, 2020
- 7.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee D, Popoola J, Shah S, et al. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97(6):1076–1082. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Chen Y, Yuan Q, et al. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. 2020;77(6):742–747. doi: 10.1016/j.eururo.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Hu BO, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pachetti M, Marini B, Benedetti F, et al. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J Transl Med. 2020;18(1):179. doi: 10.1186/s12967-020-02344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potdar V, Cherian SS, Deshpande GR, et al. Genomic analysis of SARS-CoV-2 strains among Indians returning from Italy, Iran & China, & Italian tourists in India. Indian J Med Res. 2020;151:255–260. doi: 10.4103/ijmr.IJMR_1058_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author and with permission from Jaslok Hospital and Research Centre, Mumbai, India. The data are not publicly available due to privacy or ethical restrictions.