To the Editor,
A novel coronavirus disease (COVID‐19), caused by infection with SARS‐CoV‐2, has swept across 31 provinces in China and beyond. Up to this day (03 June 2020), the coronavirus COVID‐19 has affected 209 countries, with more than 6 million infected patients and more than 383 000 deaths reported. 1
The most common symptoms were fever and cough, and gastrointestinal symptoms were uncommon. 2 However, severe cases of patients with COVID‐19 present clinically with respiratory distress, and most patients die of refractory hypoxemia. The mechanisms of respiratory failure are the infection of alveolar units by the COVID‐19 virus which have a tendency to be peripheral and subpleural, leading to diffuse alveolar damage with an important inflammatory response and a secondary cytokine storm, which in turn leads to pulmonary capillary leak syndrome, with a normal heart function. 2 However, and in our best knowledge, pulmonary capillary leak syndrome was not previously reported following COVID‐19 virus infection. We report two cases of acute respiratory failure due to pulmonary capillary leak syndrome following COVID‐19 virus infection.
In our department, the diagnosis of COVID‐19 infection was confirmed by reverse transcription polymerase chain reaction (RT‐PCR) test. However, in this specific pandemic situation, and according to the British Society of Thoracic Imaging expert reference group update, we took account of chest CT‐scan results in the early detection and confirmation of COVID‐19 pneumonia. 3 The first case is a 57‐year‐old woman presented with a 6‐day history of cough, fever, headache, and rhinorrhea without vomiting or diarrhea was admitted to ICU for acute respiratory failure following a COVID‐19 infection. Clinical exam on intensive care unit (ICU) admission showed that the patient febrile (39°C) with a respiratory rate was at 30 breaths per minute. Moreover, she had severe hypoxemia with anoxygen saturation measured by pulse oximetry [Spo2] at 90% under 14 L/minute oxygen via face mask. Pulmonary auscultation showed bilateral lung crackles. The blood pressure was 140/90, and the heart rate was 130 per minute. The electrocardiogram (ECG) performed on admission, showed sinus tachycardia at 125 per minute. Her admission chest x‐ray showed diffuse bilateral infiltrates without cardiomegaly. The chest computed tomographic (CT) scan obtained on ICU admission showed the presence of peripheral ground‐glass opacities associated with multilobe and posterior involvement, bilateral distribution, and right pleural effusion (Figure 1A). She had a white cell count (WCC) of 6 × 109 per L with a lymphopenia at 780 per mm3, and an elevated serum C‐reactive protein (CRP) level at 327 mg/L. The hemoglobin level and renal function were normal, and the troponin level was less than 0.01 μg/L. Protide in the pulmonary secretion was not measured. Transthoracic echocardiography showed a normal systolic function with left ventricular ejection fraction at 60% and normal diastolic function. There was no sign of left ventricular asynergy or volume overload. Due to the epidemic situation, the diagnosis of respiratory distress secondary to COVID‐19 pneumonia was obtained. Moreover, the patient was diagnosed as having pulmonary capillary leak syndrome. Due to the aggravation of respiratory distress, she was treated with invasive mechanical ventilation with a PAO2/FiO2 ratio at 140. Under treatment the patient was clearly improved, she was extubated 6 days after ICU admission. A chest computed tomographic (CT) scan performed 8 days later showed regression of diffuse bilateral infiltrates (Figure 1B). She was discharged from ICU within 14 days after admission.
Figure 1.
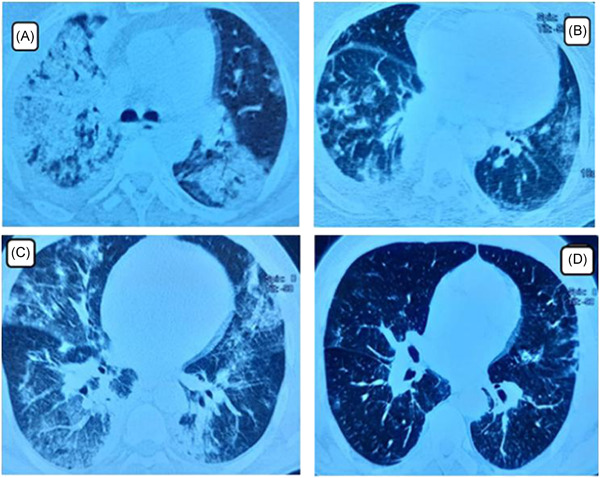
A, Computed tomographic scan of the chest of patient 1 obtained on admission to the ICU shows alveolar pulmonary edema with the presence of peripheral ground glass opacities associated with right pleural effusion. B, Computed tomographic scan for patient 1 performed 8 days later showed regression of diffuse bilateral infiltrates with great improvement. C, Computed tomographic scan of the chest of patient 2 obtained on admission to the ICU shows alveolar pulmonary edema with the presence of peripheral ground glass opacities. D, Computed tomographic scan for patient 2 performed 7 days later. It showed regression of diffuse bilateral infiltrates with great improvement. ICU, intensive care unit
A second case is a 58‐year‐old man, without pathological antecedent, presented to the COVID‐19 department with a history of 7 days of fever and anosmia. Six days later, evolution was marked by the development of dyspnea leading to acute respiratory distress and productive cough. He was admitted to ICU for respiratory distress. Examination on ICU admission showed that the temperature was at 38°C and that he had tachypnea at 32 breaths per minute and hypoxemia (Spo2 at 82% under the air room, and 94% on 8 L/min oxygen via face mask). The blood pressure was at 120/70 mm Hg, and the heart rate was 96/min. The ECG performed on admission, showed a sinus tachycardia at 92 per minute. The chest CT scan obtained on ICU admission clearly showed signs of alveolar pulmonary edema with the presence of peripheral ground glass opacities associated with multilobe and posterior involvement (Figure 1C). The WCC on ICU admission was at 16.2 × 109 per L with a moderate lymphopenia at 1320 cells/mm3and a high serum CRP level at 457 mg/L. The hemoglobin level and renal function were normal. The troponin level was less than 0.01 μg/L. Protide in the pulmonary secretion was not measured. Transthoracic echocardiography showed a normal systolic function with left ventricular ejection fraction at 65% and normal diastolic function. Therefore, the patient was diagnosed as having pulmonary capillary leak syndrome. Under treatment the patient's health was visibly better. A chest computed tomographic (CT) scan performed 7 days later showed a dispiriting of diffuse bilateral infiltrates (Figure 1D). He was discharged from ICU within 8 days after admission.
In our two patients, the mode of contamination was not found, but due to the pandemic situation, the COVID‐19 virus is probably primarily transmitted between people through respiratory droplets and contact routes. Moreover, no other signs of capillary leak syndrome (edema over the leg, neither ascites) were observed in our two cases.
Acute respiratory failure due to infection with SARS‐CoV‐2 is due fundamentally to direct pulmonary injury (due to the virus infection) followed by an important inflammatory response and a secondary cytokine storm leading to diffuse alveolar damage and acute respiratory distress. 4 The important inflammatory response in the lung leads to endothelial damage and a generalized increase in capillary permeability leading to pulmonary capillary leak syndrome 4 , 5 leading to the accumulation of protein‐rich pulmonary edema, even in the presence of normal lung vascular pressure. 5 The endothelial cells appear swollen. This hypothesis is confirmed by the histological examination of the lungs of in patients with SARS‐CoV‐2. 4 Indeed, it showed histological signs like‐minded with ARDS, 4 including bilateral acute changes with diffuse alveolar damages, vascular congestion, and extensive microvascular damages. 4 Our findings support this hypothesis. In fact, lung damages observed on chest CT‐scan, exist despite a normal cardiac function in two patients.
As there is no diagnostic test available, the positive diagnosis of pulmonary capillary leak syndrome is an exercise of exclusion. In our COVID‐19 department, the diagnosis of pulmonary capillary leak syndrome following COVID‐19 virus infection was established by a medical committee of six physicians of ours. The medical committee took into consideration the presence of clinical and radiologic features of noncardiogenic pulmonary edema and of the presence of arterial hypoxemia. Diagnosis relies upon clinical manifestations, typical radiograph findings, and the documented exclusion of heart dysfunction. In our cases, the positive diagnosis of pneumonia due to COVID‐19 virus infection was obtained on chest CT showing the presence of peripheral ground glass opacities associated with peripheral lung opacities in our two cases obtained on chest CT‐Scan results. 3
In our practice, respiratory distress due to pulmonary capillary leak syndrome following COVID‐19 infection is treated by conventional oxygen therapy. However, noninvasive and/or invasive mechanical ventilation as supportive treatment is reserved for serious cases with severe hypoxemia. Steroids (dexamethasone 20 mg/day), vitamin C (3 g/day), hydroxychloroquine (600 mg/day), azithromycine, zinc, diuretics (in the absence of shock and/or hypovolemia), and enoxaparine were equally used for all patients in the absence of contraindications.
We used corticosteroids during the SARS‐CoV outbreak due to their known capability to adjust a variety of involved cytokines (including IL‐1, IL‐6, and TNF‐α) to treat pulmonary capillary leak syndrome. 6 In fact, steroid prescription helps attenuate the severe progression of COVID‐19 with inhibition of cytokine storm (TNF α and IL6) by suppressing T cell activation. Moreover, by reducing the expression of cytokines, and other inflammatory proteins, glucocorticoids avoid the recruitment of inflammatory cells to sites of inflammation, reducing immunopathological damage. 7 As a consequence, we think that prescribing corticosteroids in patients suffering from pulmonary capillary leak syndrome following a COVID‐19 Virus Infection leads to a great improvement.
In summary, acute respiratory failure is a rare but major complication of COVID‐19 virus infection. Although we report only two cases with limited information, we think that this complication is due in part to pulmonary capillary leak syndrome. Treatment is based on good oxygenation, intravenous corticosteroids, and other symptomatic treatments. Steroids were used to treat pulmonary capillary leak syndrome.
REFERENCES
- 1. Coronavirus update. https://www.worldometers.info/coronavirus/. Accessed June 16, 2020.
- 2. etter A, Lan VuD, G L'Huillier A, Schibler M, Kaiser L, Jacquerioz F. Clinical features of covid‐19. BMJ. 2020;369:1470. 10.1136/bmj.m1470 [DOI] [PubMed] [Google Scholar]
- 3. Hare S, Rodrigues JCL, Nair A, et al. The continuing evolution of COVID‐19 imaging pathways in the UK: a British Society of Thoracic Imaging expert reference group update. Clin Radiol. 2020;20:79. 10.1016/j.crad.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. von der Thüsen Jan, van der Eerden Menno. Histopathology and genetic susceptibility in COVID‐19 pneumonia. Eur J Clin Invest. 2020. 10.1111/eci.13259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bahloul M, Dammak H, Chaari A, et al. Pulmonary capillary leak syndrome after influenza A (H1N1) virus infection. Am J Emerg Med. 2010;28(9):1063.e1‐1066.e1. [DOI] [PubMed] [Google Scholar]
- 6. Chihrin S, Loutfy MR. Overview of antiviral and anti‐inflammatory treatment for severe acute respiratory syndrome. Expert Rev Anti Infect Ther. 2005;3(2):251‐262. 10.1586/14787210.3.2.251 [DOI] [PubMed] [Google Scholar]
- 7. Russell B, Moss C, George G, et al. Associations between immune‐suppressive and stimulating drugs and novel COVID‐19—a systematic review of current evidence. Ecancermed Sci. 2020;14:1022. 10.3332/ecancer.2020.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]


