Abstract
Aims
COVID‐19 is especially severe for elderly subjects with cardiometabolic and respiratory comorbidities. Neck circumference (NC) has been shown to be strongly related to cardiometabolic and respiratory illnesses even after adjustment for body mass index (BMI). We performed a prospective study to investigate the potential of NC to predict the need for invasive mechanical ventilation (IMV) in adult COVID‐19 inpatients.
Materials and Methods
We prospectively and consecutively enrolled COVID‐19 adult patients admitted to dedicated medical wards of two Italian hospitals from 25 March to 7 April 2020. On admission, clinical, biochemical and anthropometric data, including BMI and NC were collected. As primary outcome measure, the maximum respiratory support received was evaluated. Follow‐up time was 30 days from hospital admission.
Results
We enrolled 132 subjects (55.0‐75.8 years, 32% female). During the study period, 26 (19.7%) patients underwent IMV. In multivariable logistic regression analyses, after adjusting for age, sex, diabetes, hypertension and COPD, NC resulted independently and significantly associated with IMV risk (adjusted OR 1.260—per 1 cm increase 95% CI:1.120‐1.417; P < .001), with a stronger association in the subgroup with BMI ≤30 Kg/m2 (adjusted OR 1.526; 95% CI:1.243‐1.874; P < .001). NC showed a good discrimination power in predicting patients requiring IMV (AUC 0.783; 95% CI:0.684‐0.882; P < .001). In particular, NC > 40.5 cm (>37.5 for females and >42.5 for males) showed a higher and earlier IMV risk compared to subjects with lower NC (Log‐rank test: P < .001).
Conclusions
NC is an easy to measure parameter able to predict the need for IMV in adult COVID‐19 inpatients.
Keywords: BMI, COVID‐19, invasive mechanical ventilation, neck circumference, obesity
1. INTRODUCTION
The SARS‐CoV‐2 pandemic has affected more than 2 million people with a progressively increasing global trend. 1 This new coronavirus is highly contagious and severe forms are more prevalent among elderly individuals with cardiometabolic and respiratory comorbidities such as hypertension, chronic lung disease and diabetes. 1 , 2 These types of patients have a higher risk to develop acute respiratory syndrome and/or cardiac distress requiring invasive mechanical ventilation (IMV) and prolonged hospital stays. 3
IMV is accepted as a standard of care to treat patients with severe acute respiratory failure (ARF), but this procedure is frequently associated with a high incidence of complications, greater resource consumption and needs intensive care unit (ICU) admission. 4 , 5 Use of non‐invasive mechanical ventilation (NIMV) has greatly increased in the last decades as a possible and effective alternative to IMV because of its relatively simpler management and the possibility to be applied outside the ICU. 6 Although in ARF NIMV was proven to be associated to both a lower short‐term mortality risk compared with standard oxygen therapy or IMV, 7 this procedure might be ineffective for some patients, leading to the need for a delayed invasive respiratory support. The availability of robust criteria to predict the risk of intubation and IMV since hospital admission is therefore pivotal.
The identification of predictive factors for severe course of the novel coronavirus disease (COVID‐19) mainly comes from Chinese retrospective cohorts. 8 Recently, Simonnet et al retrospectively showed a high prevalence of obesity among patients admitted in French ICUs and reported IMV need for COVID‐19 to be significantly associated with body mass index (BMI > 35 kg/m2) independent of age, diabetes and hypertension. 9 However, these findings are in contrast with the ‘obesity paradox’ for patients with Adult Respiratory Distress Syndrome (ARDS). 10 In fact, a meta‐analysis including 6268 ARDS patients documented that BMI was not associated with a higher mortality rate. 11
Several anthropometric indexes, such as waist‐to‐hip ratio, BMI, waist circumference and neck circumference (NC) are used to evaluate and estimate the ‘adiposity’. 12 Many studies have shown that upper‐body obesity has a stronger association with cardiometabolic conditions compared to lower‐body obesity. 13 , 14 NC, as an index for upper‐body subcutaneous adipose tissue distribution, has been shown to be strongly related to insulin resistance, 15 early stage atherosclerosis, 16 diabetes, 17 coronary heart diseases 18 and cardiometabolic syndrome even after adjustment for visceral adipose tissue and BMI. 19 Furthermore, NC has been demonstrated to be more strongly correlated with respiratory functions in children 20 and obstructive sleep apnea in adults when compared to other clinical parameters, such as snoring, sex, age and BMI. 21 As NC is associated with both cardiometabolic and respiratory profile, it might represent an accurate and easy to measure parameter to predict the outcome of COVID‐19 patients.
As a prompt recognition of predictive factors for respiratory outcomes is important to target high‐risk populations for adequate surveillance and management, we conducted a prospective study to investigate the potential of NC as a predictor of clinical outcomes for adult inpatients with COVID‐19.
2. MATERIALS AND METHODS
2.1. Study design and population
This multicentric study had an observational prospective design. From 25 March to 7 April 2020, we consecutively enrolled COVID‐19 patients admitted to dedicated medical wards of the following centres: Trieste (Azienda Sanitaria Universitaria Giuliano Isontina) and Latina (Santa Maria Goretti Hospital). Nasopharyngeal swab samples were obtained from all patients and tested using real‐time reverse transcriptase‐polymerase chain reaction assays. On hospital admission, COVID‐19 pneumonia was diagnosed in the whole study population according to World Health Organization guidance. 22 No patient was receiving pre‐diagnosis potential treatment (namely antiviral therapies or corticosteroids) to face COVID‐19. Following hospital admission, all patients required oxygen therapy. Patients were followed until the hospital discharge, death or at least for 30 days after the COVID‐19 diagnosis. We excluded subjects with advanced cancer and end stage renal or liver diseases. The study was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization Principles of Good Clinical Practice. The research protocol was approved by the Ethic Committee. All participants gave informed consent allowing their anonymized information to be used for a data analysis.
2.2. Study outcomes
The primary outcome was the maximum respiratory support received categorized in: (a) Venturi mask/high‐flow nasal cannula (VM/HFNC) group; (b) continuous positive airway pressure (CPAP)/non‐invasive mechanical ventilation (NIMV) group and (c) IMV group. Patients underwent IMV when clinical (fatigue or use of accessory muscles at rest), chest radiographic/CT scan and oxygenation criteria (PaO2/FiO2 ≤ 150 mmHg and or SpO2 ≤ 92% with RR > 25 per minute) have led to acute signs of respiratory distress, 23 , 24 both on hospital admission or after a trial with VM/HFNC or NIMV. The secondary outcome was the time‐interval between hospital admission and mechanical ventilation onset.
2.3. Data collection
On ward admission, trained physicians collected data on past medical history, chronic medications and clinical history. The Charlson Index was used as a comorbidity score. The following treatments administered during the hospital stay were recorded: use of angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, hydroxychloroquine, azithromycin, lopinavir/ritonavir, heparin, steroids, amiodarone, tocilizumab and remdesivir. Anthropometric measurements were taken in duplicate according to the NHANES III procedures. 25 In cases where the first two measures differed by 0.5 cm, a third measure was recorded, and the average of all measures recorded was computed. NC was measured immediately below the laryngeal prominence and perpendicular to the long axis of the neck. 25 Body weight and height were revealed to calculate the BMI. Fasting blood samples were obtained in the morning (from 8:00 to 8:30 am) to evaluate complete white blood cell count, platelet count, C‐reactive protein (CRP), albumin and D‐dimer levels.
2.4. Sample size calculation and data analysis
A minimum required sample size of 129 patients was calculated a priori for a multiple regression model including up to seven predictors to detect an anticipated effect size (f 2 ) of 0.15 (corresponding to a ‘medium’ effect size, chosen for convenience to obtain a sufficiently meaningful and realistic effect size without excessively expanding the sample size) with a probability of a type I error of 0.05 and a desired statistical power level of 0.9.
The continuous variables were displayed as medians and interquartile ranges (IQRs) and the nominal variables as numbers and percentages. Unadjusted comparisons between the groups were analysed via an χ test, nonparametric Mann‐Whitney's U test for independent samples, or Kruskal‐Wallis test adjusted by the Bonferroni correction for multiple pairwise comparisons, as appropriate. The independent association between NC and the need for IMV was tested through two multiple forward stepwise logistic regression models. The first model (#1) was adjusted for patients' age and sex, and for diabetes, hypertension, or chronic obstructive pulmonary disease (COPD) in medical history. A second model (#2) was adjusted for variables showing a statistically significant association with IMV in bivariate analyses (ie, monocytes, eosinophils, albumin, CRP, hydroxychloroquine and steroids). Since monocytes, eosinophils, serum albumin and CRP had a skewed distribution, square‐root transformations were performed to obtain more approximately normal variables.
The performance of the NC in discriminating between patients who underwent or not the IMV was tested by calculating the area under the receiver operating characteristics curve (AUC) according to the following criteria: 0.50‐0.59 = poor; 0.60‐0.69 = moderate; 0.70‐0.79 = good; 0.80‐0.89 = very good; and ≥0.90 = excellent discrimination. 26
The maximum Youden index (J) was considered as the optimal cutoff value.
‘Survival’ analysis was adopted to separate patients with longer from those with shorter intervals between hospital admission and the start of IMV. Observations were right‐censored after 30 days of observation, corresponding to the follow‐up study period. Unadjusted comparison according to NC subgroups was performed comparing crude Kaplan‐Meier curves; differences between groups was assessed with Mantel‐Cox log‐rank test.
All above analyses were repeated on subgroups of male and female patients, as well as after excluding subjects with a BMI threshold of >30 kg/m2.
Statistical analyses were performed using the software IBM SPSS Statistics, version 24.0 (Armonk, New York: IBM Corp.). For all tests, an alpha level of P < .05 was set for statistical significance.
3. RESULTS
3.1. Baseline characteristics of the study population
During the enrollment period, 162 patients were admitted to the study wards. Thirty patients were excluded according to the exclusion criteria. Finally, 132 patients constituted the study population.
The main characteristics of the enrolled patients are described in Table 1. Only 18 subjects were obese (14%). Overall, during the observational period 26 patients (19.7%) underwent IMV, while for the others the highest respiratory support was CPAP/NIMV (n = 38; 28.8%) or VM/HFNC (n = 58; 51.5%).
TABLE 1.
Baseline patients demographics, relevant comorbidities, anthropometric parameters, blood test parameters, and administered medications and comparisons according to the higher breathing support
| Variable | All patients (n = 132) | VM/HFNC (n = 68) | CPAP/NIMV (n = 38) | IMV (n = 26) | P‐value |
|---|---|---|---|---|---|
| Age (years)a | 66.0 (55.0‐75.8) | 63 (52.0‐80.0) | 70 (56.8‐75.3) | 69 (60.5‐72.0) | .931 |
| Sex (female)b | 42 (31.8%) | 25 (36.8%) | 10 (26.3%) | 7 (26.9%) | .453 |
| Active smokerb | 12 (9.1%) | 6 (8.8%) | 2 (5.3%) | 4 (16.0%) | .348 |
| COPDb | 10 (7.5%) | 4 (5.9%) | 2 (5.3%) | 4 (15.5%) | .243 |
| Diabetesb | 33 (23.3%) | 14 (20.6%) | 13 (34.2%) | 6 (23.1%) | .290 |
| Hypertensionb | 55 (41.7%) | 23 (33.8%) | 17 (44.7%) | 15 (57.7%) | .099 |
| Heart diseaseb | 24 (18.2%) | 15 (22.1%) | 6 (15.8%) | 3 (11.5%) | .448 |
| Charlson indexa , c | 3.0 (1.0‐5.0) | 2.0 (1.0‐5.0) | 3.0 (2.0‐5.0) | 3.0 (2.0‐5.0) | .298 |
| Body mass indexa | 25.7 (23.6‐27.8) | 25.3 (23.5‐27.6) | 25.7 (22.8‐28.5) | 26.3 (24.6‐27.7) | .434 |
| Neck circumferencea (cm) | 40.0 (37.0‐42.8) | 39.0 (36.3‐41.0) | 40.0 (37.0‐42.3) | 43.5 (41.0‐46.0) | <.001 |
| Albumin (g/dL)a , c | 3.5 (3.1‐3.8) | 3.7 (3.3‐4.0) | 3.4 (3.1‐3.8) | 3.2 (2.6‐3.7) | .005 |
| C‐reactive protein (mg/L)a | 17.0 (4.3‐91.8) | 6.3 (1.7‐35.0) | 29.0 (10.3‐107.3) | 59.0 (13.0‐165.0) | <.001 |
| D‐dimer (μg/mL)a , c | 0.8 (0.5‐1.6) | 0.8 (0.4‐1.3) | 0.8 (0.5‐1.8) | 1.0 (0.7‐1.9) | .135 |
| White BC (103/uL)a | 5.66 (4.29‐8.12) | 6.47 (4.32‐8.12) | 5.28 (3.89‐7.66) | 5.54 (4.33‐8.72) | .470 |
| Neutrophils (103/uL)a | 3.94 (2.76‐5.75) | 3.92 (2.78‐5.89) | 3.86 (2.29‐5.60) | 3.94 (3.06‐7.18) | .598 |
| Monocytes (103/uL)a | 0.42 (0.25‐0.56) | 0.47 (0.35‐0.63) | 0.41 (0.24‐0.53) | 0.29 (0.23‐0.41) | .002 |
| Lymphocytes (103/uL)a | 0.89 (0.59‐1.22) | 0.91 (0.67‐1.34) | 0.97 (0.53‐1.23) | 0.85 (0.55‐1.14) | .576 |
| Eosinophils (103/uL)a | 0.00 (0.00‐0.02) | 0.00 (0.00‐0.03) | 0.00 (0.00‐0.01) | 0.00 (0.00‐0.01) | .025 |
| Thrombocytes (103/uL)a | 180.5 (148.3‐258.0) | 180.0 (148.0‐261.0) | 182.5 (157.8‐260.0) | 184.0 (135.0‐247.8) | .793 |
| ACE inhibitorsb | 13 (9.8%) | 8 (11.8%) | 4 (10.5%) | 1 (3.8%) | .508 |
| Amiodaroneb | 5 (3.8%) | 2 (2.9%) | 2 (5.3%) | 1 (3.8%) | .835 |
| Azithromycinb | 58 (43.9%) | 29 (42.6%) | 20 (52.6%) | 9 (34.6%) | .345 |
| Hydroxychloroquineb | 120 (90.9%) | 63 (92.6%) | 31 (81.6%) | 26 (100%) | .033 |
| Lopinavir/ritonavirb | 58 (43.9%) | 32 (47.1%) | 13 (34.2%) | 13 (50.0%) | .347 |
| Sartanb | 12 (9.1%) | 3 (4.4%) | 4 (10.5%) | 5 (19.2%) | .077 |
| Steroidsb | 90 (68.2%) | 34 (50.0%) | 37 (97.4%) | 19 (73.1%) | 0.001 |
| Tocilizumabb | 22 (16.7%) | 8 (11.8%) | 6 (15.8%) | 8 (30.8%) | .085 |
Note: Significance of bold values are P < 0.05.
Abbreviations: ACE, angiotensin converting enzyme; BC, blood cells; COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; IMV, invasive mechanical ventilation; NIMV, non‐invasive mechanical ventilation; VM/HFNC, Venturi mask/high‐flow nasal cannula.
Median (interquartile range).
Number (percentage).
n = 130.
3.2. NC and maximum respiratory support received
In the bivariate analysis, the NC differed significantly (P < .001) according to the highest level of breathing support needed by the patient. Moreover, the NC differed significantly (P < .001) for patients undergoing IMV or VM/HFNC in all sex and BMI threshold subgroup comparisons, while NC differences between the other respiratory support modalities gave uneven results (Figure 1).
FIGURE 1.
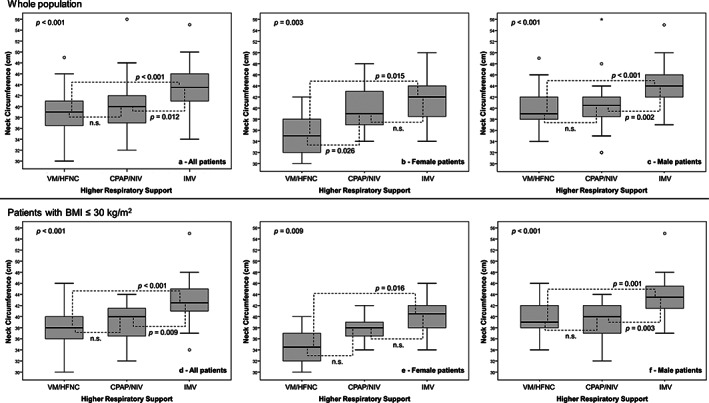
Differences in neck circumference according to the highest level of respiratory support provided during the observation time for general population and for male or female patients, both in the whole study population and after excluding patients with a BMI of >30 kg/m2
In multivariable logistic regression analyses, after adjusting the model (#1) for age, sex, presence of diabetes, hypertension and COPD, NC showed to be independently and significantly associated to IMV risk (adjusted OR 1.260; CI:1.120‐1.417, P < .001). We confirmed an independent and significant association with IMV risk even in the subgroup of patients with a BMI of ≤30 (adjusted OR 1.526; CI:1.243‐1.874, P < .001). NC showed a similar independent association with IMV risk when the regression models (#2) were adjusted for variables (ie, monocytes, eosinophils, albumin, CRP, hydroxychloroquine and steroids) (Table 1) that showed a statistically significant association with this outcome in bivariate analyses (all patients: adjusted OR 1.328; CI: 1.126‐1.565, P = .001; patients with BMI ≤30: adjusted OR 1.371; CI:1.133‐1.658, P = .001). Results of the multivariable analyses are reported in Table 2.
TABLE 2.
Adjusted multiple forward stepwise logistic regression models for the association between neck circumference and invasive mechanical ventilation in 132 patients with COVID‐19 infection
| Model | Determination coefficient | Predictors a | Adjusted OR (95% CI) | P‐value |
|---|---|---|---|---|
| #1 | R 2 0.222; P < .001 | Neck circumference | 1.260 (1.120–1.417) | <.001 |
| #1a | R 2 0.337; P < .001 |
Neck circumference Female sex |
1.526 (1.243‐1.874) 3.857 (0.971‐15.316) |
<.001 .055 |
| #2 | R 2 0.540; P < .001 |
Neck circumference Albumin Monocytes |
1.328 (1.126‐1.565) 0.122 (0.030‐0.490) 0.896 (0.807‐0.994) |
.001 .003 .039 |
| #2a | R 2 0.490; P < .001 |
Neck circumference Albumin |
1.371 (1.133‐1.658) 0.127 (0.030‐0.530) |
.001 .005 |
Note: Stepwise multiple logistic regression of invasive mechanical ventilation on neck circumference, adjusted for age, sex, diabetes, hypertension, and chronic obstructive pulmonary disease (Model #1) and for monocytes, eosinophils, albumin, C‐reactive protein, hydroxychloroquine, and steroids (Model #2). Results of the same models applied on the population of patients with body mass index of ≤30 kg/m2 are reported as Models #1a and #2a, respectively.
Variables included in the final models (P < .05).
The NC showed a good discrimination power (AUC 0.783; 95% CI: 0.684‐0.882; P < .001) at separating patients who needed IMV from those who did not. Compared to the whole population, the discrimination power was accurate for both male (AUC 0.801; 95% CI: 0.693‐0.909; P < .001) and female patients (AUC 0.767; 95% CI: 0.581‐0.953; P = .027). The optimal cutoff value of NC to predict the need for IMV in the whole study population was 40.5 cm (J: 0.456), while different thresholds were found according to patient's sex (male: 42.5 cm; female 37.5 cm). After ruling out patients with BMI > 30 kg/m2, the NC threshold did not change. When performing ROC analysis in patients with a BMI of ≤30 kg/m2, a further improvement in the discrimination power of NC with respect to IMV occurrence was found, particularly in the female population (all pts: AUC 0.799, 95% CI: 0.691‐0.910, P < .001; male: AUC 0.816, 95% CI: 0.698‐0.934, P < .001; female: AUC 0.816, 95% CI: 0.624‐1.000, P = .016). Figure 2 shows the ROC curves for all tested subgroups.
FIGURE 2.
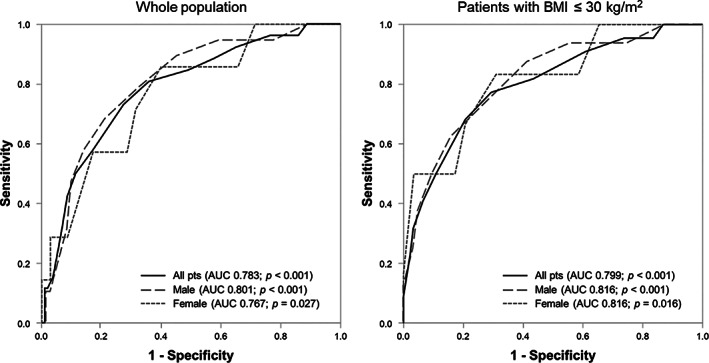
ROC curves for all tested subgroups
Figure 3 shows the Kaplan‐Meier curves for the risk of IMV in patients belonging to the NC groups according to the identified risk threshold and to patient's sex. In the whole study population, patients with a NC > 40.5 cm showed a higher and earlier risk for IMV (Log‐rank test: P < .001) compared to subjects in the lower circumference group (Figure 3A). Similar results were found after separately analysing the female and male populations (Log‐rank test: P = .028 and P < .001, respectively) according to the respective circumference thresholds (Figure 3B,C), and even better results were confirmed (Log‐rank test: P < .001, P = .017 and P < .001, respectively for all, female and male patients) after repeating the same analyses in the subgroup of patients with a BMI of ≤30 kg/m2 (Figure 3D‐F).
FIGURE 3.
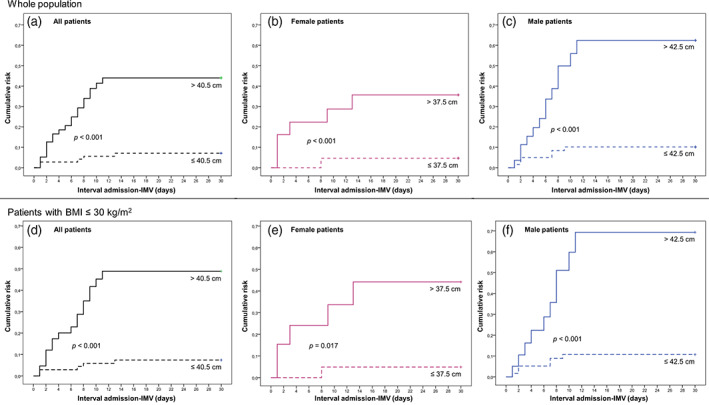
Kaplan‐Meier curves for the risk of IMV in patients belonging to the NC groups according to the identified risk threshold and to patient's sex. IMV, invasive mechanical ventilation; NC, neck circumference
4. DISCUSSION
Our findings have demonstrated that NC is an independent predictor for IMV in adult COVID‐19 inpatients. In particular, in our study population, the risk of being subjected to IMV increases by 26% for each centimetre increase in NC, increasing up to 53% in patients with a BMI of ≤30 kg/m2.
The COVID‐19 outbreak has pushed worldwide scientific efforts to identify patients at higher risk of developing critical illness requiring advanced supportive care.
The first reports and retrospective evaluations indicated elderly patients with underlying cardiometabolic (diabetes, hypertension) and respiratory diseases to have a greater risk to experience severe COVID‐19. 1 Later on, it was shown that obesity can negatively affect the progression of COVID‐19. 9 , 27 , 28 It has worldwide accepted the use of BMI to define the grade of obesity, however BMI, as well as the Waist Circumference (WC), is a valid surrogate measure especially for abdominal adiposity. 14 Many studies demonstrated that specific patterns of ‘excess fat distribution’ conferred different metabolic risk, pointing out the pro‐inflammatory role of visceral adipose tissue (VAT) and upper‐body adiposity. 29 It has been shown that NC, as a proxy of upper‐body subcutaneous fat, is indicative of pathogenic fat depot independent to but, at the same time, synergistic with VAT. 19 Therefore, NC may represent an easy, valid measure of adiposity and it might be even a better marker of metabolic risk than standard parameters such as WC and BMI. 19 , 30
In our study, unlike BMI, NC significantly differed according to the highest level of respiratory support required and it showed a good discrimination power at separating patients who needed IMV, even after adjustment for age, sex, COPD, hypertension and diabetes. Several reasons might explain the detrimental effect of this ‘excess ectopic fat’ on subjects with COVID‐19: firstly, it can enhance the prothrombotic state (including disseminated intravascular coagulation and venous thromboembolism), 31 which is a common feature of COVID‐19; secondly, it can worsen the lung function reducing low forced expiratory volume and forced vital capacity 32 ; lastly, this fat excess can impair the immune response through a chronic basal inflammatory status. 33
According to our results, NC confirms its ability to predict COVID‐19 clinical outcomes in both female and male populations using different thresholds; most importantly, although there is a relationship between BMI and NC, the measurement of upper‐body subcutaneous fat performs even better in the subgroup with BMI < 30 mg/k2. While BMI appears to be a good predictor of clinical outcomes for severe obesity, 9 this finding would make NC a useful tool for both overweight and normal weight subjects. Indeed, it has been shown that NC represents a more precise VAT estimate 34 , 35 and epicardial fat thickness, even in non‐obese subjects, 36 and this kind of adipose tissue might be strictly related to the COVID‐19 related cytokine storm phenomenon. 37
Furthermore, NC is a practical and easy to measure anthropometric parameter, especially useful for bedridden patients where traditional measurements may be challenging or not meaningful.
The main strengths of our study are the prospective and multicentric design and the novelty of using a simple, easy to measure and costless anthropometric parameter to predict clinical outcome and therefore potential health care needs for COVID‐19 patients. Instead, the main limitation is the relatively small patient sample size.
In conclusion, NC appears to be an easy and valuable tool able to stratify the COVID‐19 patients' risk to develop respiratory worsening requiring IMV. The ‘triagist use’ of NC measurement on admission would allow the allocation of patients to wards with adequate intensity of care ideally prior to the development of COVID‐19 complications. Moreover, our findings indirectly support the fundamental role of metabolic impairment in contributing to the pathogenic process of COVID‐19. Further and larger studies are needed to confirm our results and to test the accuracy of this measurement to predict mortality.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Stefano Di Bella, Roberto Cesareo, Paolo De Cristofaro, Andrea Palermo and Gianfranco Sanson researched and analysed the data and wrote and edited the manuscript. Erik Roman‐Pognuz, Verena Zerbato, Silvia Manfrini, Donatella Giacomazzi, Elisabetta Macchini, Francesco Quintavalle, Giuseppe Campagna, Renato Masala, Luigi Ottaviani, Cosmo Del Borgo, Lorenzo Ridola and Frida Leonetti researched data and reviewed the manuscript. Eugenia Dal Bo, Giorgio Berlot, Roberto Luzzati and Gianfranco Sanson contributed to the discussion and reviewed the manuscript. Stefano Di Bella, Roberto Cesareo, Paolo De Cristofaro, Andrea Palermo and Gianfranco Sanson contributed to write the manuscript and conceived the study. Stefano Di Bella is the guarantor of the study, conceived the study and takes full responsibility for the work. All authors have read and approved the final manuscript.
ACKNOWLEDGEMENTS
We thank Dr Andrea De Cristofaro and the Morphogram Pro web app (app.morphogram.com) for his kind help in database management and anthropometric data collection. We are indebted with Dr Maria Crapulli of Campus Bio‐Medico University of Rome for her patience and her kind bibliographic support through the years. This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
Di Bella S, Cesareo R, De Cristofaro P, et al. Neck circumference as reliable predictor of mechanical ventilation support in adult inpatients with COVID‐19: A multicentric prospective evaluation. Diabetes Metab Res Rev. 2021;37:e3354. 10.1002/dmrr.3354
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request from the authors.
REFERENCES
- 1. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory‐confirmed coronavirus disease 2019 – COVID‐NET, 14 states, March 1‐30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458‐464. 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab Res Rev. 2020;e3319. 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carter SJ, Baranauskas MN, Fly AD. Considerations for obesity, vitamin D, and physical activity amidst the COVID‐19 pandemic. Obesity. 2020;oby.22838. 10.1002/oby.22838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corl KA, Dado C, Agarwal A, et al. A modified Montpellier protocol for intubating intensive care unit patients is associated with an increase in first‐pass intubation success and fewer complications. J Crit Care. 2018;44:191‐195. 10.1016/j.jcrc.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilcox ME, Vaughan K, Chong CAKY, Neumann PJ, Bell CM. Cost‐effectiveness studies in the ICU: a systematic review. Crit Care Med. 2019;47(8):1011‐1017. 10.1097/CCM.0000000000003768. [DOI] [PubMed] [Google Scholar]
- 6. Cabrini L, Esquinas A, Pasin L, et al. An international survey on noninvasive ventilation use for acute respiratory failure in general non‐monitored wards. Respir Care. 2015;60(4):586‐592. 10.4187/respcare.03593. [DOI] [PubMed] [Google Scholar]
- 7. Liu Q, Gao Y, Chen R, Cheng Z. Noninvasive ventilation with helmet vs control strategy in patients with acute respiratory failure: a systematic review and meta‐analysis of controlled studies. Crit Care. 2016;20(1):265. 10.1186/s13054-016-1449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020. 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020. 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jose RJ, Manuel A. Does COVID‐19 disprove the obesity paradox in ARDS? Obesity. 2020;28(6):1007. 10.1002/oby.22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ni Y‐N, Luo J, Yu H, et al. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta‐analysis. Crit Care. 2017;21(1):36. 10.1186/s13054-017-1615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Capizzi M, Leto G, Petrone A, et al. Wrist circumference is a clinical marker of insulin resistance in overweight and obese children and adolescents. Circulation. 2011;123(16):1757‐1762. 10.1161/CIRCULATIONAHA.110.012898. [DOI] [PubMed] [Google Scholar]
- 13. Saneei P, Shahdadian F, Moradi S, Ghavami A, Mohammadi H, Rouhani MH. Neck circumference in relation to glycemic parameters: a systematic review and meta‐analysis of observational studies. Diabetol Metab Syndr. 2019;11(1):50. 10.1186/s13098-019-0445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joshipura K, Muñoz‐Torres F, Vergara J, Palacios C, Pérez CM. Neck circumference may be a better alternative to standard anthropometric measures. J Diabetes Res. 2016;2016:6058916. 10.1155/2016/6058916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liang J, Teng F, Li Y, et al. Neck circumference and insulin resistance in Chinese adults: the cardiometabolic risk in Chinese (CRC) study. Diabetes Care. 2013;36(9):e145‐e146. 10.2337/dc13-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liang J, Wang Y, Li H, Liu X, Qiu Q, Qi L. Neck circumference and early stage atherosclerosis: the cardiometabolic risk in Chinese (CRC) study. Cardiovasc Diabetol. 2014;13(1):107. 10.1186/s12933-014-0107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cho NH, Oh TJ, Kim KM, et al. Neck circumference and incidence of diabetes mellitus over 10years in the Korean genome and epidemiology study (KoGES). Sci Rep. 2015;5:18565. 10.1038/srep18565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang G‐R, Dye TD, Zand MS, Fogg TT, Yuan S‐Y, Li D. Association between neck circumference and coronary heart disease: a meta‐analysis. Asian/Pacific Isl Nurs J. 2019;4(1):34‐46. 10.31372/20190401.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Preis SR, Massaro JM, Hoffmann U, et al. Neck circumference as a novel measure of cardiometabolic risk: the framingham heart study. J Clin Endocrinol Metab. 2010;95(8):3701‐3710. 10.1210/jc.2009-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akın O, Arslan M, Haymana C, Karabulut E, Hacihamdioglu B, Yavuz ST. Association of neck circumference and pulmonary function in children. Ann Allergy, Asthma Immunol. 2017;119(1):27‐30. 10.1016/j.anai.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 21. Ahbab S, Ataoǧlu HE, Tuna M, et al. Neck circumference, metabolic syndrome and obstructive sleep apnea syndrome; Evaluation of possible linkage. Med Sci Monit. 2013;19(1):111‐117. 10.12659/MSM.883776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anon. No Title . Clinical Management of Severe Acute Respiratory Infection When COVID‐19 Is Suspected. 2020.
- 23. Asai T. Airway management inside and outside operating rooms—circumstances are quite different. Br J Anaesth. 2018;120(2):207‐209. 10.1016/j.bja.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 24. Kangelaris KN, Ware LB, Wang CY, et al. Timing of intubation and clinical outcomes in adults with acute respiratory distress syndrome. Crit Care Med. 2016;44(1):120‐129. 10.1097/CCM.0000000000001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manual CDC. Anthropometry procedures. National Health and Nutrition Examination Survey. Centre for Disease Control and Prevention. National Health and Nutrition Examination Survey; 2007. https://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/anthro.pdf. Accessed October 12, 2016. [Google Scholar]
- 26. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29‐36. 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 27. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60years is a risk factor for Covid‐19 hospital admission. Clin Infect Dis. 2020;ciaa415. 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Watanabe M, Risi R, Tuccinardi D, Baquero CJ, Manfrini S, Gnessi L. Obesity and SARS‐CoV‐2: a population to safeguard. Diabetes Metab Res Rev. 2020;e3325. 10.1002/dmrr.3325. [DOI] [PubMed] [Google Scholar]
- 29. Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the framingham heart study. Circulation. 2007;116(1):39‐48. 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 30. Namazi N, Larijani B, Surkan PJ, Azadbakht L. The association of neck circumference with risk of metabolic syndrome and its components in adults: a systematic review and meta‐analysis. Nutr Metab Cardiovasc Dis. 2018;28(7):657‐674. 10.1016/j.numecd.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 31. Yang G, de Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2(4):499‐509. 10.4236/ojpm.2012.24069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12(9):755‐767. 10.1080/17476348.2018.1506331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Green WD, Beck MA. Obesity impairs the adaptive immune response to influenza virus. Ann Am Thorac Soc. 2017;14:S406‐S409. 10.1513/AnnalsATS.201706-447AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pinho CPS, Diniz A da S, De Arruda IKG, Leite APDL, Petribú M de MV, Rodrigues IG. Predictive models for estimating visceral fat: The contribution from anthropometric parameters. PLoS One. 2017;12(7):e0178958. 10.1371/journal.pone.0178958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borel A‐L, Coumes S, Reche F, et al. Waist, neck circumferences, waist‐to‐hip ratio: Which is the best cardiometabolic risk marker in women with severe obesity? The SOON cohort. PLoS One. 2018;13(11):e0206617. 10.1371/journal.pone.0206617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Küçük U, Küçük HO, Cüce F, Balta S. Relationship between neck circumference and epicardial fat thickness in a healthy male population. Arq Bras Cardiol. 2016;107(3):266‐270. 10.5935/abc.20160112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request from the authors.


