Summary
Lung ultrasound could facilitate the triage of patients with suspected COVID‐19 infection admitted to the emergency room. We developed a predictive model for COVID‐19 diagnosis based on lung ultrasound and clinical features. We used ultrasound to image the lung bilaterally at two anterior sites, one and two hands below each clavicle, and a posterolateral site that was the posterior transverse continuation from the lower anterior site. We studied 100 patients, 31 of whom had a COVID‐19 positive reverse transcriptase polymerase chain reaction. A positive test was independently associated with: quick sequential organ failure assessment score ≥1; ≥3 B‐lines at the upper site; consolidation and thickened pleura at the lower site; and thickened pleura line at the posterolateral site. The model discrimination was an area (95%CI) under the receiver operating characteristic curve of 0.82 (0.75–0.90). The characteristics (95%CI) of the model’s diagnostic threshold, applied to the population from which it was derived, were: sensitivity, 97% (83–100%); specificity, 62% (50–74%); positive predictive value, 54% (41–98%); and negative predictive value, 98% (88–99%). This model may facilitate triage of patients with suspected COVID‐19 infection admitted to the emergency room.
Keywords: COVID‐19, lung ultrasound, triage
Introduction
The current coronavirus disease 2019 (COVID‐19) pandemic is caused by the severe acute respiratory syndrome coronavirus 2 strain (SARS‐CoV‐2), with many patients admitted to the emergency room with suspected COVID‐19 infection [1]. The results of RNA reverse transcriptase polymerase chain reaction (RT‐CR) diagnostic oropharyngeal swabs may be unavailable for 48 h after collection [2]. This delay can lead to unnecessary isolation of many patients that may exceed a hospital’s resources.
Lung ultrasound can contribute to the diagnosis of acute respiratory failure in the emergency room, for instance the ‘bed‐side lung ultrasound in emergency’ (BLUE) protocol [3]. Lung ultrasonographic characteristics of COVID‐19 disease have been recently described [4]. Lung ultrasound could facilitate rapid, simple and reliable triage of patients with suspected COVID‐19 infection admitted to the emergency room and may inform prognosis [5].
We aimed to develop a model for COVID‐19 diagnosis in patients presenting to the emergency room with possible infection, based on the association of lung ultrasound and clinical features with positive viral swabs. Our secondary objectives were to study the associations between these and admission to the intensive care unit, respiratory complications and mortality.
Methods
The University Medical Centre review board approved this observational study, which we conducted from March to April 2020 and that we report as strengthening the reporting of observational studies in epidemiology (STROBE) guidelines [6]. Participants gave informed consent. We studied adults admitted to the emergency room whose lungs were imaged with ultrasound by the emergency physician for suspected COVID‐19 infection as part of the BLUE protocol and who had a SARS‐CoV‐2 RT‐PCR test [3].
We excluded pregnant women or patients unable to give informed consent or patients with suspected or proven chronic interstitial lung disease. We did not analyse patients whose ultrasound scans were poor due to an acoustic barrier, for instance pneumothorax or subcutaneous emphysema.
We used a convex array transducer and ultrasound system (C5‐2s™ and TE7, Mindray™; Shenzhen, China) to identify abnormalities consistent with possible COVID‐19 disease: thickening of the pleural line with irregularity; B‐lines in a variety of patterns, including focal, multifocal and confluent; and consolidation in a variety of patterns (Fig. 1a) [4, 7, 8]. The ‘bed‐side lung ultrasound in emergency’ (BLUE) protocol interrogates the lung bilaterally at upper and lower anterior sites and at a posterolateral site (Fig. 1b) [9]. We only counted the number of B‐lines at the upper and lower sites as they are present in around 25% of healthy subjects elsewhere [8, 10]. The number of B‐lines was counted in a short‐axis scan between two ribs. Two experts who had performed at least 50 lung ultrasound scans interpreted stored images, unaware of patients’ SARS‐CoV‐2 RT‐PCR status (SB, PG) [11].
Figure 1.
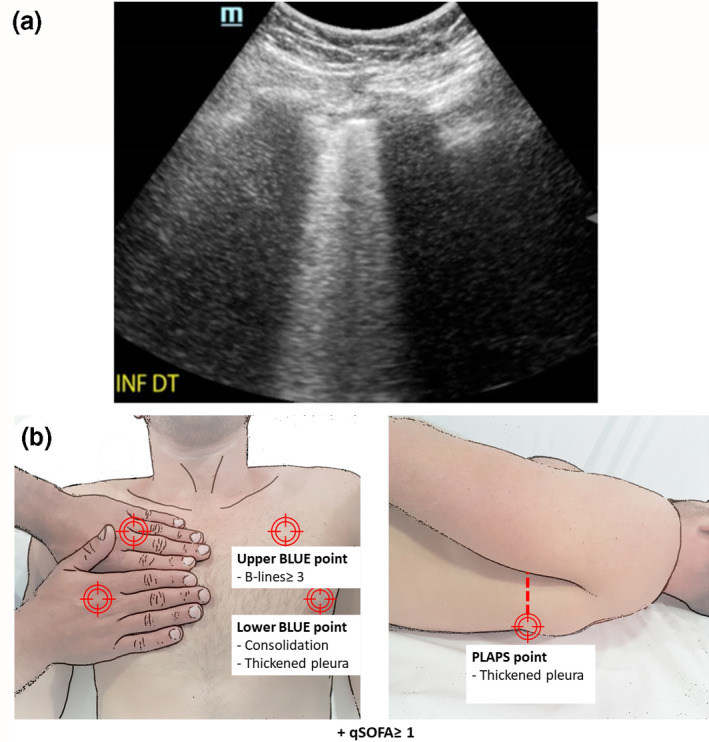
(a) Lower anterior chest subpleural consolidation associated with thickened pleura. (b) The ‘bed‐side lung ultrasound in emergency’ (BLUE) protocol interrogates three points in each hemithorax. The two anterior sites are under one (upper) and two (lower) hands placed below each clavicle. The posterolateral site is the posterior transverse continuation from the lower anterior site, interrogated as posterior as possible in the supine patient
We recorded baseline characteristics, including age, sex, BMI, medical history and medications. We also recorded heart rate, mean arterial pressure and pulsed oxygen saturation. We calculated the quick sequential organ failure assessment (qSOFA) score and the Glasgow coma scale, respiratory rate and systolic arterial pressure [12]. We measured lymphocyte count, C‐reactive protein and the ratio of arterial oxygen partial pressure to inspired fraction oxygen (PaO2/FIO2). The primary outcome was the SARS‐CoV‐2 RT‐PCR result, which we defined as negative if COVID‐19 was not detected by two RT‐PCRs [13]. The secondary outcomes were admission to intensive care; respiratory complications (acute respiratory distress syndrome (ARDS), pulmonary embolism and secondary bacterial infection); and mortality 14 days after inclusion, recorded by one investigator (AL) who was not informed of lung ultrasound results or COVID‐19 status.
We calculated that we would need 100 patients to have a 80% power to demonstrate an ultrasound diagnostic accuracy of 18% and 12% for sensitivity and specificity, assuming their true values to be 50%, at an alpha threshold of 5%, if 30% of patients presenting to the emergency room with suspected COVID‐19 had SARS‐CoV‐2 detected by up to two RT‐PCR. We used the Agostino‐Pearson test for normality of data distribution. We used Student's t‐test, Mann–Whitney test, Chi‐square test or Fisher test, as appropriate. We used logistic regression to model the associations of lung ultrasound and clinical features with admission to intensive care, respiratory complications and mortality. We constructed a multivariate logistic model with ultrasound variables that associated with outcome (p < 0.05), constrained by elastic net penalisation, with the L2 ridge parameter α set to 0.9 and the optimal L1 Lasso parameter λ determined by 10‐fold cross‐validation [14, 15]. In this multivariate logistic model, we categorised the number of B‐lines ≥ 3 and < 3 in accordance with the international definition of interstitial syndrome [7]. We used the area under the receiver operating characteristic curve and the highest Youden index to define model discrimination and diagnostic threshold, respectively. Inter‐observer agreement between the two experts concerning qualitative ultrasound signs (signs present or absent) was evaluated using a Kappa concordance coefficient and agreement on quantitative evaluations (number of B‐lines detected at the upper and lower sites) using an intraclass correlation coefficient. We used R for analyses (Core Team®, 2017, Vienna, Austria).
Results
We included 100 adults of whom 31 had a positive SARS‐CoV‐2 RT‐PCR (Table 1 and Fig. 2). The qSOFA score (≥ 1) and four ultrasound signs were independently associated with a positive test (Table 2). The area (95%CI) under the receiver operating characteristic curve for the multivariate equation was 0.82 (0.75–0.90). The optimal model value for diagnosis was −1.35, recursively characterised (95%CI) in the derivation population by: sensitivity, 97% (83–100%); specificity, 62% (50–74%); positive predictive value, 54% (41–98%); and negative predictive value, 98% (88–99%).
Table 1.
Characteristics of 100 patients presenting to the emergency room with possible COVID‐19 infection. Values are mean (SD), number (proportion) or median (IQR [range])
| SARS‐CoV‐2 RT‐PCR | p value | ||
|---|---|---|---|
| Positive (n = 31) | Negative (n = 69) | ||
| Age; years | 66.8 (16.3) | 68.7 (16.4) | 0.98 |
| Females | 20 (65%) | 39 (56%) | 0.60 |
| BMI; kg.m−2 | 30.0 (3.19) | 26.4 (3.98) | 0.06 |
| Medical history | |||
| High blood pressure | 21 (68%) | 36 (52%) | 0.22 |
| Coronary heart disease | 2 (6%) | 13 (19%) | 0.19 |
| Smoking | 2 (6%) | 21 (30%) | 0.01 |
| Peripheral arterial disease | 2 (6%) | 4 (6%) | 0.74 |
| Stroke | 7 (23%) | 9 (13%) | 0.36 |
| Diabetes | 3 (10%) | 7 (10%) | 0.77 |
| Dyslipidaemia | 10 (32%) | 21 (30%) | 0.96 |
| Medication | |||
| ACE inhibitor | 5 (16%) | 11 (16%) | 0.79 |
| Angiotensin receptor blocker | 8 (26%) | 10 (15%) | 0.28 |
| NSAID | 0 | 1 (1%) | 0.68 |
| qSOFA score | 1 (0‐1 [0‐2]) | 0 (0‐1 [0‐1]) | 0.003 |
| Heart rate; min−1 | 97 (80‐115 [70‐127]) | 88 (80‐105 [67‐134]) | 0.22 |
| Mean arterial pressure; mmHg | 96.0 (12.9) | 97.5 (17.4) | 0.68 |
| Oxygen saturation; % | 95 (93‐98 [85‐100]) | 97 (93‐99 [82‐100]) | 0.22 |
| Lymphocyte count; 109.l−1 | 1.5 (1.0‐2.1 [0.6‐3.6]) | 2.0 (1.8‐2.2 [0.6‐3.9]) | 0.01 |
| C‐reactive protein; mg.l−1 | 118 (71‐151 [14‐327]) | 42 (12‐125 [0‐29]) | 0.005 |
| PaO2/FIO2 | 298 (119) | 338 (105) | 0.12 |
| Chest ultrasound sites | |||
| Upper and lower anterior | |||
| B lines | 6 (2‐10 [0‐30]) | 3 (1‐7 [0‐16]) | 0.04 |
| Confluent B‐lines | 3 (10%) | 0 | 0.04 |
| Thickened pleural line | 24 (77%) | 26 (38%) | < 0.001 |
| Consolidation | 17 (54%) | 11 (16%) | < 0.001 |
| Posterolateral | |||
| Confluent B‐lines | 10 (32%) | 8 (12%) | 0.03 |
| Thickened pleural line | 24 (77%) | 26 (38%) | < 0.001 |
| Consolidation | 18 (58%) | 23 (33%) | 0.04 |
ACE, angiotensin‐converting‐enzyme; BLUE, bed‐side lung ultrasound in emergency; NSAID, nonsteroidal anti‐inflammatory drug; PaO2/FiO2, arterial oxygen partial pressure to fractional inspired oxygen; qSOFA, quick sequential organ failure assessment; SARS‐CoV‐2 RT‐PCR, severe acute respiratory syndrome coronavirus 2 reverse transcription polymerase chain reaction
Figure 2.
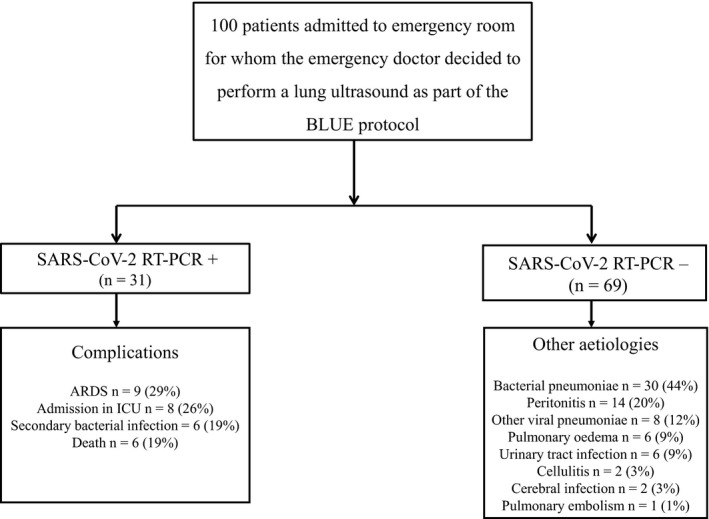
Study flow chart detailing complications in case of positive RT‐PCR and other aetiologies in case of negative RT‐PCR. ARDS, acute respiratory distress syndrome; ICU, intensive care unit; RT‐PCR, reverse transcription polymerase chain reaction
Table 2.
Lung ultrasound characteristics and qSOFA score independently associated with COVID‐19
| Coefficients | Odds ratio (95%CI) | |
|---|---|---|
| Intercept | −1.95 | |
| qSOFA score ≥ 1 | 0.05 | 1.05 (1.01‐1.10) |
| Chest ultrasound site findings | ||
| Upper sites B lines ≥ 3 | 0.42 | 1.52 (1.31‐1.79) |
| Lower sites thickened pleura | 0.55 | 1.73 (1.49‐1.98) |
| Lower sites consolidation | 0.87 | 2.39 (2.07‐2.69) |
| Posterolateral sites thickened pleura | 0.68 | 1.97 (1.72‐2.22) |
qSOFA, quick sequential organ failure assessment
Nine patients (29%) with a positive SARS‐CoV‐2 RT‐PCR developed ARDS, six (19%) developed a secondary bacterial infection and none developed a pulmonary embolism. The odds ratio (95%CI) for subsequent ARDS in patients with COVID‐19 was independently associated with three variables: ≥ 3 upper site B‐lines, 1.7 (1.3–2.3), p = 0.001; ≥ 3 lower site B‐lines, 1.0 (1.0–1.1), p = 0.03; and PaO2/FIO2 ratio, 1.00 (1.00–1.01), p = 0.006. The same variables were associated with admission to the ICU, OR (95%CI): 1.6 (1.2–2.1), p = 0.003; 1.0 (1.0–1.1), p = 0.016; and 1.00 (1.00–1.01], p = 0.02, respectively. No associations were found between other respiratory complications and lung ultrasound variables.
Six patients (19 %) with a positive SARS‐CoV‐2 RT‐PCR died during the study period. Mortality was not associated with lung ultrasound variables. The inter‐observer agreement was good, with a Kappa concordance coefficient of 0.89 (95%CI [0.67–1.00]). The intraclass correlation coefficient for the agreement on quantitative evaluations was 0.92 (95%CI [0.81–0.97]).
Discussion
We found that a combination of clinical features and lung ultrasound signs were independently associated with positive SARS‐CoV‐2 RT‐PCR. Subsequent development of adult respiratory distress syndrome and ICU admission were also associated with lung ultrasound signs.
Chest computed tomography (CT) imaging has been strongly recommended because it is very sensitive for detecting early disease [16]. The early stages of COVID‐19 infection are characterised by bilateral ground glass opacification, accompanied by interlobular thickening and consolidation, predominantly in the peripheral and subpleural middle and lower lobes [17, 18]. However, the transportation of potentially infectious and unstable patients for CT limits its use [4].
Lung ultrasound has several advantages compared with CT. It is non‐irradiating and non‐invasive. It can be learned quickly and its use in the emergency room has generated great interest [7, 19, 20, 21, 22, 23]. Decontamination of the equipment is straightforward [24]. The abnormalities observed on CT are accompanied by ultrasound signs, which include B‐lines that become more extensive with disease progression, accompanied by pleural thickening and subpleural consolidation [25, 26].
The signs associated with COVID‐19 diagnosis in our model are consistent with other studies [4, 25]. The most common sign was thickening of the pleural line in the inferior and posterolateral sites, which is indicative of pneumonia or ARDS [7]. Consolidation is common to bacterial pneumonitis and did not independently associate with COVID‐19 diagnosis [7]. Occasional B‐lines may indicate chronic changes and are common to a number of diseases, but at least three lines indicate interstitial syndrome and greater numbers are associated with disease severity and higher mortality [4, 5, 7, 8, 10, 26, 27, 28]. The qSOFA score is a simplified version of the SOFA score that aims to identify patients more likely to suffer serious outcomes after infection [12]. Few patients with COVID‐19 are haemodynamically compromised, which may limit its utility [29, 30].
Our study had several limitations. The first was the interpretation of B‐lines in the upper chest, as a diffuse and heterogeneous distribution of B‐lines with thickening of the pleura also occurs with chronic interstitial lung disease, whom we excluded from the study [31]. Lung ultrasound can distinguish between cardiogenic and non‐cardiogenic pulmonary oedema, particularly through careful examination of the pleura [32, 33]. Lung ultrasound signs are not particularly specific for infections, although the bilateral distribution of changes in COVID‐19 can help differentiate it from influenza and bacterial pneumonias [7, 34, 35]. The performance of our model will be limited in part by the sensitivity of the SARS‐CoV‐2 RT‐PCR test, which misdiagnoses one quarter of COVID‐19 patients as negative, a rate that we tried to reduce by performing two tests on each patient. It is possible that the performance of a model might be improved by imaging with ultrasound more lung areas, but any improvement might not justify the additional time [3, 5, 31, 36].
In conclusion, the association of BLUE protocol lung ultrasound signs and qSOFA with COVID‐19 diagnosis could facilitate more effective triage of patients presenting to emergency departments with suspected COVID‐19 infection. This model should be tested on an independent cohort.
Acknowledgements
We retrospectively registered this study, 29 April 2020 (Clinicaltrials.gov, NCT04368338). No external funding or competing interests declared.
Contributor Information
S. Bar, Email: stephane.bar.sb@gmail.com.
E. Arnaud, @CreatixEA.
P. Gosset, @gosset_pierre.
References
- 1. Park M, Cook AR, Lim JT, Sun Y, Dickens BL. A systematic review of COVID‐19 epidemiology based on current evidence. Journal of Clinical Medicine 2020; 9: 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Loeffelholz MJ, Tang Y‐W. Laboratory diagnosis of emerging human coronavirus infections – the state of the art. Emerging Microbes and Infections 2020; 9: 747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008; 134: 117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chinese Critical Care Ultrasound Study Group (CCUSG) , Peng Q‐Y, Wang X‐T, Zhang L‐N. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Medicine 2020; 46: 849–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith MJ, Hayward SA, Innes SM, Miller A. Point‐of‐care lung ultrasound in patients with COVID‐19 ‐ a narrative review. Anaesthesia 2020; 75: 1096–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. International Journal of Surgery 2014; 12: 1495–9.25046131 [Google Scholar]
- 7. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Medicine 2012; 38: 577–91. [DOI] [PubMed] [Google Scholar]
- 8. Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet‐tail artifact: an ultrasound sign of alveolar‐interstitial syndrome. American Journal of Respiratory and Critical Care Medicine 1997; 156: 1640–6. [DOI] [PubMed] [Google Scholar]
- 9. Lichtenstein D, Mezière G. The BLUE‐points: three standardized points used in the BLUE‐protocol for ultrasound assessment of the lung in acute respiratory failure. Critical Ultrasound Journal 2011; 3: 109–10. [Google Scholar]
- 10. Frasure SE, Matilsky DK, Siadecki SD, Platz E, Saul T, Lewiss RE. Impact of patient positioning on lung ultrasound findings in acute heart failure. European Heart Journal: Acute Cardiovascular Care 2014; 4: 326–32. [DOI] [PubMed] [Google Scholar]
- 11. Arbelot C, Dexheimer Neto FL, Gao Y, et al. Lung ultrasound in emergency and critically Ill patients: number of supervised exams to reach basic competence. Anesthesiology 2020; 132: 899–907. [DOI] [PubMed] [Google Scholar]
- 12. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis‐3). Journal of the American Medical Association 2016; 315: 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases. Radiology 2020; 296: e32–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pavlou M, Ambler G, Seaman SR, et al. How to develop a more accurate risk prediction model when there are few events. British Medical Journal 2015; 351: h3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kessler RC, Hwang I, Hoffmire CA, et al. Developing a practical suicide risk prediction model for targeting high‐risk patients in the Veterans health Administration. International Journal of Methods in Psychiatric Research 2017; 26: e1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Revel M‐P, Prosch H, Silva M, Sverzellati N, Gleeson F, Brady A. COVID‐19 patients and the radiology department – advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). European Radiology 2020; 30: 4903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jin Y‐H, Cheng Z‐S, Cheng H, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019‐nCoV) infected pneumonia (standard version). Military Medical Research 2020; 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang W, Sirajuddin A, Zhang X, et al. The role of imaging in 2019 novel coronavirus pneumonia (COVID‐19). European Radiology 2020. Epub 15 April. 10.1007/s00330-020-06827-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noble VE, Lamhaut L, Capp R, et al. Evaluation of a thoracic ultrasound training module for the detection of pneumothorax and pulmonary edema by prehospital physician care providers. BMC Medical Education 2009; 9: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inchingolo R, Smargiassi A, Moro F, et al. The diagnosis of pneumonia in a pregnant woman with COVID‐19 using maternal lung ultrasound. American Journal of Obstetrics and Gynecology 2020; 223: 9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jambrik Z, Monti S, Coppola V, et al. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. American Journal of Cardiology 2004; 93: 1265–70. [DOI] [PubMed] [Google Scholar]
- 22. Platz E, Pivetta E, Merz AA, Peck J, Rivero J, Cheng S. Impact of device selection and clip duration on lung ultrasound assessment in patients with heart failure. American Journal of Emergency Medicine 2015; 33: 1552–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wee LE, Fua T‐P, Chua YY, et al. Containing COVID‐19 in the emergency department: the role of improved case detection and segregation of suspect cases. Academic Emergency Medicine 2020; 27: 379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abramowicz JS, Basseal JM. World federation for ultrasound in medicine and biology position statement: how to perform a safe ultrasound examination and clean equipment in the context of COVID‐19. Ultrasound in Medicine and Biology 2020; 46: 1821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xing C, Li Q, Du H, Kang W, Lian J, Yuan L. Lung ultrasound findings in patients with COVID‐19 pneumonia. Critical Care 2020; 24: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fiala MJ. Ultrasound in COVID‐19: a timeline of ultrasound findings in relation to CT. Clinical Radiology 2020; 75: 553–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lomoro P, Verde F, Zerboni F, et al. COVID‐19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single‐center study and comprehensive radiologic literature review. European Journal of Radiology Open 2020; 7: 100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sakka SG, Klein M, Reinhart K, Meier‐Hellmann A. Prognostic value of extravascular lung water in critically ill patients. Chest 2002; 122: 2080–6. [DOI] [PubMed] [Google Scholar]
- 29. Su Y, Tu G, Ju M, et al. Comparison of CRB‐65 and quick sepsis‐related organ failure assessment for predicting the need for intensive respiratory or vasopressor support in patients with COVID‐19. Journal of Infection 2020; 81: 647–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ihle‐Hansen H, Berge T, Tveita A, et al. COVID‐19: symptoms, course of illness and use of clinical scoring systems for the first 42 patients admitted to a Norwegian local hospital. Tidsskrift for Den norske legeforening 2020; 140. [DOI] [PubMed] [Google Scholar]
- 31. Volpicelli G, Gargani L. Sonographic signs and patterns of COVID‐19 pneumonia. Ultrasound Journal 2020; 12: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovascular Ultrasound 2008; 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferré A, Guillot M, Lichtenstein D, et al. Lung ultrasound allows the diagnosis of weaning‐induced pulmonary oedema. Intensive Care Medicine 2019; 45: 601–8. [DOI] [PubMed] [Google Scholar]
- 34. Wang H, Wei R, Rao G, Zhu J, Song B. Characteristic CT findings distinguishing 2019 novel coronavirus disease (COVID‐19) from influenza pneumonia. European Radiology 2020; 30: 4910–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsung JW, Kessler DO, Shah VP. Prospective application of clinician‐performed lung ultrasonography during the 2009 H1N1 influenza A pandemic: distinguishing viral from bacterial pneumonia. Critical Ultrasound Journal 2012; 4: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soldati G, Smargiassi A, Inchingolo R, et al. Proposal for international standardization of the use of lung ultrasound for COVID‐19 patients; a simple, quantitative, reproducible method. Journal of Ultrasound in Medicine 2020; 39: 1413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


