1. STUDY RATIONALE
Chloroquine (CQ) and hydroxychloroquine (HCQ) were both employed in the treatment of COVID‐19 in China. Based on unpublished anecdotal positive results in China, CQ is now implemented in China and the Netherlands in moderate to severe COVID‐19. 1 HCQ is an analogue of CQ with more antiviral effectivity ex vivo and a better safety and tolerability profile.
Rationale for the employment of CQ and HCQ in China comes from the fact that both reduce COVID‐19 replication ex vivo. 2 , 3 On top of this, both drugs have clear immunomodulating effects (which is used for various indications to treat rheumatologic diseases) and have shown promising results in patients with dengue and HIV. 4 , 5 , 6
The time needed to “load” the body when using CQ and thus the possible late onset of action suggests that initiation of treatment should be timely. Moreover, the hypothesized mode of action suggests that both HCQ and CQ inhibit cellular replication of COVID‐19 but do not have an intrinsic effect on the virus, thus could be best employed when the viral load is low (ie early phase of the disease) and thus safe time to improve the subsequent immune response. This underlines that both drugs should be implemented early rather than late in the course of SARS‐COV‐2 infection.
Despite the inclusion of CQ and HCQ as possible additional treatment for COVID‐19 in the Dutch treatment guideline, 1 there is no conclusion on the best treatment strategy in this population. Based on pharmacokinetic modelling, and the safety profile, HCQ seems to be more promising than CQ, but both drugs have to be studied in relation to COVID‐19. Furthermore, the availability of both drugs might differ from country to country; in the Netherlands for instance, chloroquine is widely available.
We propose a 1:1:1 cluster‐randomized controlled study evaluating the value of chloroquine and hydroxychloroquine compared to no antiviral therapy in admitted patients with moderate to severe COVID‐19.
2. POPULATION
Adult patients with confirmed COVID‐19, with moderate to severe symptoms and admitted to the hospital, will be available for the study. For definite diagnosis at least one positive SARS‐CoV‐19 PCR is required on the nasopharyngeal or pharyngeal swab. These swabs are not part of this study.
2.1. Inclusion criteria
In order to be eligible to participate in this study, a subject must meet all of the following criteria:
Patients (≥18 years of age) admitted to the hospital with confirmed COVID‐19 and not needing admission to MC or ICU
Patient has moderate to severe COVID‐19. This will be defined as patients with NEWS‐2 score ≤ 5. 7 (Table 1)
Willing and able to give written informed consent.
TABLE 1.
The NEWS‐2 scoring system
| Physiological parameter | Score | ||||||
|---|---|---|---|---|---|---|---|
| 3 | 2 | 1 | 0 | 1 | 2 | 3 | |
| Respiratory rate (per minute) | ≤8 | 9‐11 | 12‐20 | 21‐24 | ≥25 | ||
| Sp02 scale 1(%) | ≤91 | 92‐93 | 94‐95 | ≥96 | |||
| Sp02 scale 2(%) a | ≤83 | 84‐85 | 86‐87 | 88‐92 and ≥ 93 on air | 93‐94 on oxygen | 95‐96 on oxygen | ≥97 on oxygen |
| Supplemental oxygen? | Yes | No | |||||
| Systolic blood pressure (mmHg) | ≤90 | 91‐100 | 101‐110 | 111‐219 | ≥220 | ||
| Pulse (per minute) | ≤40 | 41‐50 | 51‐90 | 91‐110 | 111‐130 | ≥131 | |
| Consciousness | Alert | CVPU b | |||||
| Temperature (oC) | ≤35.0 | 35.1‐36.0 | 36.1‐ 38.0 | 38.1 ‐ 39.0 | ≥39.1 | ||
Use scale 2 when patient shows hypercapnic respiratory failure
New‐onset confusion, disorientation and/or agitation, where previously their mental state was normal
2.2. Exclusion criteria
A potential subject who meets any of the following criteria will be excluded from participation in this study:
Severe COVID‐19 defined as NEWS‐2 score > 5 or admission to the ICU needing ventilation or pressure support;
Contra‐indications for hydroxychloroquine or chloroquine;
Unable to take oral medication (chloroquine and hydroxychloroquine can be administered through tube feeding which is considered oral administration);
Pregnancy;
Identified allergies to 4‐aminoquinoline;
Severe diseases of the blood system;
6‐phosphate dehydrogenase deficiency;
History of acute myocardial infarction, unstable angina pectoris, severe arrhythmia ( ventricular tachycardia, ventricular fibrillation) in recent 6 months; New York Heart Association (NYHA) level III‐IV;
Corrected QT interval (QTc) ≥500 ms on baseline electrocardiogram;
Uncorrected severe hypokalaemia (< 2,5 mmol/L) or uncorrected severe hypomagnesemia (<0.6 mmol/L);
Pancreatitis;
Refusal to participate expressed by patient or legally authorized representative if they are present.
2.3. Informed Consent
After a patient is admitted to the hospital due to moderate to severe COVID‐19, he or she will be informed by his treating physician, or a member of the research team, about this study. If patient considers the participation, then he or she will obtain detailed information and will be offered a telephonic assessment with the investigator of this study. The existence of an impartial expert will be emphasized. If a patient agrees to participate, then informed consent will be signed by both the patient or legal representative and the treating physician or member of the research team.
As treatment of COVID‐19 must start without delay, patient has to decide in relatively short time. The decision time is set on a minimum of 1 hour and a maximum of 12 hours.
3. INTERVENTION
All three treatment arms contain standard supportive care during hospital admission within two arms an addition with either chloroquine (CQ) or hydroxychloroquine (HCQ). The optimal dosages in the different treatment arms are as follows:
Supportive care + chloroquine base arm: loading dose 600 mg, followed by 300 mg 12 hours later, followed by 300 mg bid for 4 days; total treatment duration of 5 days.
Supportive care + hydroxychloroquine arm: loading dose 400 mg bid, followed by 200mg bid for 4 days; total treatment duration of 5 days.
Supportive care only.
Dosage of chloroquine and hydroxychloroquine will be adjusted if the patients have a renal impairment with an estimated glomerular filtration rate (eGDR) <10, to 50% of the initial dosage.
Hydroxychloroquine tablets when crushed probably lose 20%‐50% biodisponibility.
The loading dose of hydroxychloroquine when administered through a nasogastric tube will thus be increased to 600 mg twice a day for 1 day in order to obtain an effective concentration in the lungs as quickly as possible. This will be followed by the same maintenance dose of 400 mg a day for 4 days.
4. OUTCOME
4.1. Main study parameter/end point
Composite end point with disease progression defined as a NEWS2score ≥ 7 or resulting in admission to Intensive/Medium Care unit or resulting in death within 14 days.
4.2. Secondary study parameters/end points
Side effects of different drugs leading to regimen change or discontinuation of the antiviral treatment.
4.3. Other study parameters
Demographic data at baseline
Symptoms at baseline and first day of symptoms
Medical history
NEWS‐2 score as measure of disease severity
Relevant laboratory results at baseline and during treatment
ECG results at baseline and during treatment
Pulmonary CT/X‐ray results at baseline
4.4. Follow‐up
The disease course of COVID‐19 shows progression after proximately 5‐7 days after onset of symptoms. Therefore, we chose a follow‐up period of 14 days to include all possible primary end points in COVID‐19 patients. 8 After 28 days, an additional adverse events assessment will take place (Figure 1).
FIGURE 1.
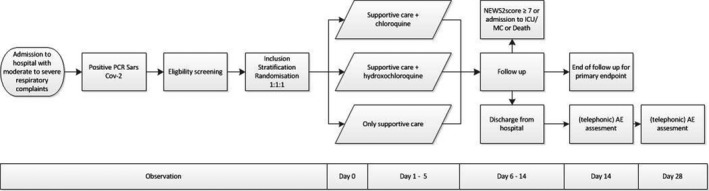
Study flow chart
If a patient uses concomitant medication that affect the drug absorption, metabolism and excretion or can induce QT interval prolongation, an electrocardiogram (ECG) is made before start and 4‐12 hours after first medication intake. Two days after start of the medication, an ECG is made in every patient who started with CQ or HCQ. If an ECG shows a prolonged corrected QT interval (>500 ms or prolongation of 25%), CQ or HCQ are discontinued and the cardiologist is consulted for advice on follow‐up. Medications which induce QT interval prolongation are as follows:
Amiodarone, disopyramide, flecainide, ibutailide, kinidine and sotalol;
Chlorpromazine, citolapram, escitalopram, haloperidol and pimozide;
Azithromycin, clarithromycin, erythromycin, moxifloxacin and trimethoprim/sulfamethoxazole;
Dimperidon and ondansetron;
Methadone;
Anagrelide, arseentrioxide, droperidol, pentamidine, sevoflurane and vandetanib.
An ECG is also made before and 4–12 hours after start of the medication if patient is known for QT interval prolongation as result of congenital long GT syndrome or other medication that induce QT interval prolongation, and if patient is known with ventricular rhythm disease, myocardial infarction or congestive heart failure.
An ECG is made in every patient treated with CQ or HCQ at day 3 of treatment (Table 2).
TABLE 2.
Schedule of assessment
| Study visits | ||||||
|---|---|---|---|---|---|---|
| Screening/D0 | D03 | D05 | Daily 2, 4 ,6‐14 | Day of discharge | D14 and D28 | |
| Contact type | Face‐to‐face | Charts | Charts | Charts | Charts | By phone or charts |
| Informed consent | X | |||||
| Eligibility | X | |||||
| BMI | X | |||||
| Medical history | X | |||||
| Randomization | X | |||||
| Laboratory safety (blood count and differential, reticulocyte, haptoglobin, creatinine, liver function, LDH, CK, CRP, Na, K. Mg, ferritin) | X | X | X | |||
| ECG (when treated with CQ or HCQ) | On indication | X | ||||
| NEWS‐2 score | X | X | X | X | X | |
| AE assessment | X | X | X | X | X | X |
5. RANDOMIZATION
Cluster randomization: in a 1:1:1 ratio of the participating hospitals, blocks are per week. Day of inclusion will define which treatment will be given to the patient.
During consecutive periods of 7 days, chloroquine, hydroxychloroquine or supportive care only is used as the preferred empirical treatment for eligible patients. With three intervention arms, 6 unique sequences are possible. Therefore, the order of interventions per hospital will be randomized in blocks of 6. Sequences are generated using computer random sequence generation. Randomization sequences will be allocated to hospitals in the order in which they start. If multiple hospitals start in the same week, the sequences will be allocated in alphabetical order of the formal hospital name. Hospitals are assigned to their sequence after approval of the study by the hospital antibiotic committee, corona crisis committee or designated authoritative committee.
6. SAMPLE SIZE CALCULATION
Based on the literature, about 25% of admitted patients develop severe disease. 9 To answer the first question whether these treatments, currently used in the practice of treating COVID‐19, are effective, we performed a sample size calculation for an RCT with 2:1 allocation, using a two‐sided alpha of 0.05, and 80% power to detect an 8% absolute risk reduction. We have not taken into account intra‐cluster correlation as these parameters are as yet unknown; we assume that the weekly alternation of intervention arms in each hospital will reduce the design effect of a putative intra‐cluster correlation to a negligible amount. The maximum total sample size to reach 80% power is 946 subjects. Hence, we aim to enrol 316 patients in each intervention arm. Since some of the assumptions are uncertain, we will perform sample size re‐estimation after enrolment of 50% of the target sample size and will perform sample size readjustment if needed and feasible to maintain adequate power.
7. STATISTICAL ANALYSIS
Data will be reported quantitatively. All analyses will be performed from the intention‐to‐treat principle. The detailed analysis will be described in a statistical analysis plan prior to the first interim analysis.
All analyses of primary and secondary end points will be adjusted for age, gender, smoking, selected comorbidities (Immunocompromised, cardiovascular disease, chronic lung disease, renal impairment and active malignancy) and baseline NEWS‐2 score. Clustering will be taken into account by including hospital as a fixed variable and study period as a random intercept. A two‐sided P‐value <= .05 will be considered statistically significant.
7.1. Primary study parameter(s)
The primary end point will be analysed as a time‐events end point using a mixed‐effects Cox regression model.
7.2. Secondary study parameter(s)
Time‐to‐event end points will be analysed in the same way as the primary end point. In case of competing events, a Fine and Gray competing events model will be used.
7.3. Interim analysis
After completing follow‐up for the primary end point in 30% of the subjects subsequently at every additional 10%, an interim analysis will be performed to analyse the primary end point and for safety. Because of the cluster‐cross‐over design, the first interim analysis will take place at the earliest after 4 weeks, to make sure all hospitals have undergone the control arm with at least one week of follow‐up in all patients. Patients with incomplete follow‐up will be administratively censored to maximize the amount of information used in each interim analysis. The trial will be stopped if superiority has been demonstrated for the primary end point based on predefined superiority bounds (Figure 2).
FIGURE 2.
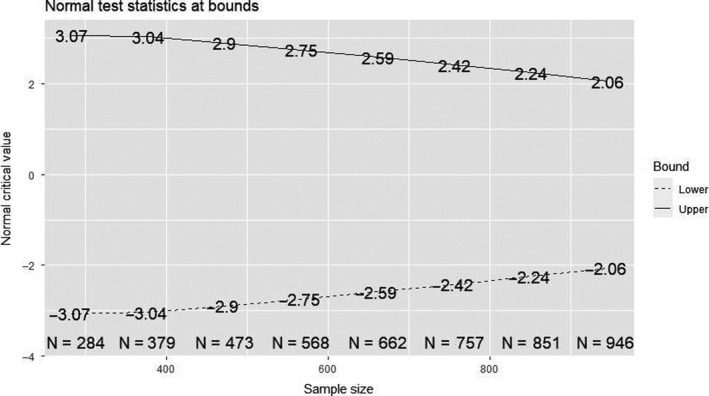
Normal test statistics at bounds
8. DISCUSSION
The study enrolled its first patient in the first week of May in het University Medical Center Utrecht. A week later the study started in two other hospitals in the Netherlands. On 14 May 2020, ten patients are enrolled in the study. We scheduled to start with the study in a total of 10 centres by the end of May. The inclusion rate in our study is uncertain due to the decline in COVID‐19 admission in the Netherlands. With the current low COVID‐19 admission rate, we estimated to enrol 950 patients in 6‐9 months.
On May 14, there are six similar clinical trials conducted or planned on clinicaltrials.gov. We already contacted different research groups in Europa, and we proposed to join our efforts for a possible meta‐interim analysis. The meeting was positive; however, more planning and agreements on this topic are needed.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interest.
FUNDING INFORMATION
The sponsor of this study is ZonMw; Netherlands organization for Health Research and Development. The study did not received any (financial) support from the pharmaceutical industry. There are no financial conflicts of interest to disclose.
REFERENCES
- 1. Vollaard A, Gieling E, van der Linden P, Sihna B, de Boer M. Medicamenteuze behandelopties bij patiënten met COVID‐19 (infecties met SARS‐CoV‐2). 2020; Available from: https://swab.nl/nl/covid‐19. Accessed April 28, 2020.
- 2. Colson P, Rolain J‐M, Lagier J‐C, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID‐19. Int J Antimicrob Agents. 2020;55(4):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30(3):269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sperber K, Louie M, Kraus T, et al. Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin Ther. 1995;17(4):622‐636. [DOI] [PubMed] [Google Scholar]
- 5. Piconi S, Parisotto S, Rizzardini G, et al. Hydroxychloroquine drastically reduces immune activation in HIV‐infected, antiretroviral therapy‐treated immunologic nonresponders. Blood. 2011;118(12):3263‐3272. [DOI] [PubMed] [Google Scholar]
- 6. Tricou V, Minh NN, Van TP, et al. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. Halstead SB, editor. PLoS Negl Trop Dis. 2010;4(8):e785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Royal College of Physicians . National Early Warning Score (NEWS) 2: Standardising the assessment of acute‐illness severity in the NHS. Updated report of a working party. Updated report of a working party. London: Royal College of Physicians; 2017. [Google Scholar]
- 8. Bouadma L, Lescure F‐X, Lucet JC, Yazdanpanah Y, Timsit J‐F. Severe SARS‐CoV‐2 infections practical consideration and management strategy for intensivists. Intensive Care Med. 2020;46:579–582. 10.1007/s00134-020-05967-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]


