Abstract
Background
The rapid outbreak of coronavirus disease 2019 (COVID‐19) has turned into a public health emergency of international concern. Epidemiological research has shown that sex is associated with the severity of COVID‐19, but the underlying mechanism of sex predisposition remains poorly understood. We aim to study the gendered differences in inflammation reaction, and the association with severity and mortality of COVID‐19.
Methods
In this retrospective study, we enrolled 548 COVID‐19 inpatients from Tongji Hospital from 26 January to 5 February 2020, and followed up to 3 March 2020. Epidemiological, demographic and clinical features, and inflammatory indexes were collected and compared between males and females. The Cox proportional hazard regression model was applied to identify the gendered effect on mortality of COVID‐19 after adjusting for age, comorbidity, and smoking history. The multiple linear regression method was used to explore the influence of sex on inflammation reaction.
Results
Males had higher mortality than females did (22.2% vs 10.4%), with an hazard ratio of 1.923 (95% confidence interval, 1.181‐3.130); elder age and comorbidity were significantly associated with decease of COVID‐19 patients. Excess inflammation reaction was related to severity of COVID‐19. Male patients had greater inflammation reaction, with higher levels of interleukin 10, tumor necrosis factor‐α, lactose dehydrogenase, ferritin, and hyper‐sensitive C‐reactive protein, but a lower lymphocyte count than females adjusted by age and comorbidity.
Conclusions
Sex, age, and comorbidity are critical risk factors for mortality of COVID‐19. Excess innate immunity and proinflammation activity, and deficiency in adaptive immunity response promote males, especially elder males, to develop a cytokine storm, causing potential acute respiratory distressed syndrome, multiple organ failure and decease.
Keywords: age, COVID‐19, inflammation, mortality, sex
Highlights
Gender is a risk factor for mortality of COVID‐19 adjusted by age, comorbidity, and smoking history.
Excess inflammation reaction is related to severity of COVID‐19.
Male patients with COVID‐19 develop greater inflammation reaction than females do.
Gender‐specific inflammation reaction contributes to sex bias in outcome of COVID‐19.
Abbreviations
- ALI
acute lung injure
- ARDS
acute respiratory distressed syndrome
- CFR
case fatality rate
- CHD
coronary heart disease
- CKD
chronic kidney disease
- COPD
chronic obstructive pulmonary disease
- COVID‐19
coronavirus disease 2019
- CXCL10
CXCchemokine ligand 10
- HBV
hepatitis B virus
- HR
hazard ratio
- hsCRP
hyper‐sensitive C‐reactive protein
- IL
interleukin
- IMM
inflammatory monocyte macrophage
- IQR
interquartile range
- LDH
lactose dehydrogenase
- LPS
lipopolysaccharide
- MERS
Middle East respiratory syndrome
- PBMC
peripheral blood mononuclear cell
- PRR
pattern recognition receptor
- RR
relative risk
- SARS
severe acute respiratory syndrome
- SARS‐CoV‐2
coronavirus 2
- sIL‐2R
soluble interlukin‐2 receptor
- TB
tuberculosis
- TNF‐α
tumor necrosis factor α
1. INTRODUCTION
The rapid outbreak of coronavirus disease 2019 (COVID‐19) has turned into a public health emergency of international concern. COVID‐19, a newly identified infectious disease arising from coronavirus 2 (SARS‐CoV‐2), has high transmission capacity and can cause clusters of severe and even fatal pneumonia. 1 , 2 Research on the underlying mechanism of COVID‐19 has become urgent worldwide.
Previous studies showed that males were more severely affected and had a higher case fatality rate (CFR) than females in severe acute respiratory syndrome (SARS) 3 and Middle East respiratory syndrome (MERS). 4 Similarly, more male patients were observed in refractory and deceased COVID‐19 patients. 5 , 6 , 7 , 8 , 9 Meng et al 10 demonstrated that there were sex‐specific differences in the clinical characteristics and prognosis of COVID‐19 patients. Nevertheless, knowledge of the mechanism of the gendered effects on COVID‐19 is scarce.
Exaggerated activation of inflammatory cytokines (eg, tumor necrosis factor‐α [TNF‐α], interleukin‐6 [IL‐6], IL‐8, and IL‐10) and acute inflammatory proteins (eg, hyper‐sensitive C‐reactive protein [hsCRP]) were responsible for SARS‐related acute respiratory distressed syndrome (ARDS). 11 , 12 In MERS, excessive innate immune response such as a high level of proinflammatory cytokine IL‐6 was a critical factor for organ dysfunction and fatalities. 13 Besides this, in the SARS‐CoV mice model, increased accumulation of inflammatory monocyte macrophages and neutrophils in the lungs of male mice promoted CFR compared with female mice. 14 Recent research on COVID‐19 showed that severe and deceased patients had lymphopenia and proinflammatory cytokine storm (eg, high levels of serum IL‐6, hsCRP, lactose dehydrogenase [LDH]). 15 , 16
We, therefore, hypothesized that there were sex‐specific differences in inflammation reaction, which led to the sex predisposition in severity and mortality of COVID‐19. In this study, we investigated the gendered effects on inflammation reaction and the association with severity and mortality of COVID‐19.
2. METHODS
2.1. Study participants and data collection
With the approval of the Ethics Commission of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, the cohort recruited 548 inpatients with COVID‐19 admitted to the Sino‐French New City Branch of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology between 26 January 2020 and 5 February 2020, as described in our previous report. 17 All patients were diagnosed according to the World Health Organization interim guidance 1 and the diagnostic and treatment guideline for COVID‐19 issued by the Chinese National Health Committee (version 5). 18 All patients were followed up to 3 March 2020. Survival time was defined as the duration from hospital admission to decease for nonsurvivals, or the duration from hospital admission to terminate time of follow‐up (ie, 3 March 2020) for survivals. Written informed consent was waived in light of the urgent need to collect data.
The epidemiological and demographic data were obtained by face‐to‐face or telephone communications with the patients themselves or families. The laboratorial, radiological features, and outcome data from electronic medical records in the hospital were retrieved and reviewed by two trained physicians. Patients were defined to be mild or severe during hospitalization on the basis of the guidance of the American Thoracic Society and Infectious Diseases Society of America. 19 The presence of underlying comorbidities was identified based on the International Classification of Diseases and Injuries‐10 diagnostic codes. The definitions of complications were described in our previous study. 17 Serum cytokines (IL‐1β, soluble interlukin‐2 receptor [sIL‐2R], IL‐6, IL‐8, IL‐10, and TNF‐α) were measured on admission. The clinical outcomes were classified into survival and nonsurvival.
2.2. Statistical analysis
Continuous variables were expressed as medians and interquartile ranges (IQR). Categorical variables were presented as numbers and rates. Comparisons of two groups were conducted by the Mann‐Whitney U test. Pearson's χ 2 test was applied to compare categorical values of different groups. The Kruskal‐Wallis test was used for multigroup comparisons, followed by the Wilcoxon intergroup comparisons. The Kaplan‐Meier method was used to estimate survival rate grouped by sex and age, and the Log Rank test was employed for comparisons between different subgroups. The Kaplan‐Meier analysis was conducted using JMP SAS software (SAS Institute, Cary, NC). Univariable and multivariable Cox proportional hazard regression models were applied to identify the sex risk factor associated with decease, with the hazard ratio (HR) and the 95% confidence interval (CI) being reported. Age, comorbidity, and smoking history were adjusted in the univariable proportional hazard regression models because they have been previously recognized as the risk factors for severity of COVID‐19. 20 Variables with P < .10 from the results of univariable analysis were chosen for the multivariable Cox proportional hazard regression model, including sex, age, comorbidity, and smoking history. A multiple linear regression model was used to determine the association (partial regression coefficient, adjusted β) between factors and inflammation reaction, and relevant statistical significance. All statistical analyses were performed using the SPSS software version 22.0 (Chicago, IL) and P < .05 were considered statistically significant.
3. RESULTS
3.1. Subjects
In this study, 548 patients with COVID‐19 were enrolled between 26 January 2020 and 5 February 2020, all of these patients were followed up to 3 March 2020. Among all the cases, 314 (57.3%) were evaluated as severe cases and 90 died during the follow‐up period. Of the 548 patients, 279 (50.9%) patients were males and 269 (49.1%) patients were females (Table SE1).
3.2. Sex bias in CFR of COVID‐19 inpatients
As of 3 March 2020, 90 of 548 (16.4%) patients died of COVID‐19. The median follow‐up period of the cohort as a whole was 29 days (IQR, 27‐31), and it was 28 days (IQR, 27‐31) for males and 29 days (IQR, 27‐31) for females, respectively. Sex‐ and age‐specific CFR values of enrolled COVID‐19 patients are shown in Table 1. Males had a mortality of 22.2% (95% CI, 17.3%‐27.1%), which was about twice as great as females did (10.4%, 95% CI, 6.7%‐14.1%). The relative risk (RR) of mortality was 2.135 (95% CI, 1.412‐3.229, P = .0002) in males compared with females. Kaplan‐Meier survival analysis for sex‐specific patients also showed a trend towards poorer survival in male patients compared with females (χ 2 = 13.729, P = .0002; Figure 1A). Besides this, the survival rate of patients especially males dropped quickly in the first 15 days from hospitalization and then gradually turned to be stable. Compared with female patients, males had higher rates of severe patients, smokers, chronic obstructive pulmonary disease (COPD), coronary heart disease (CHD), lymphopenia, and thrombocytopenia, and greater levels of inflammation indexes, and suffered from hypoxia, worse renal and liver function, and higher frequency of complications (Table SE1).
Table 1.
Sex‐ and age‐specific case fatality rate of 548 COVID‐19 patients
| Males | Females | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Deaths | Total | Deaths | Males vs females | |||||||||
| No. | % | No. | CFR | 95% CI | No. | % | No. | CFR | 95% CI | RR | 95% CI | P | |
| All patients | |||||||||||||
| 0‐44 y | 48 | 17.2 | 1 | 2.1 | 0, 6.3 | 59 | 21.9 | 2 | 3.4 | 0.0, 8.1 | 0.615 | 0.057, 6.575 | 1.0000 |
| 45‐64 y | 118 | 42.3 | 18 | 15.3 | 8.7, 21.8 | 113 | 42.0 | 9 | 8.0 | 2.9, 13.0 | 1.915 | 0.898, 4.085 | .0847 |
| ≥65 y | 113 | 40.5 | 43 | 38.1 | 29.0, 47.1 | 97 | 36.1 | 17 | 17.5 | 9.8, 25.2 | 2.171 | 1.328, 3.550 | .0010 |
| All | 279 | 100.0 | 62 | 22.2 | 17.3, 27.1 | 269 | 100.0 | 28 | 10.4 | 6.7, 14.1 | 2.135 | 1.412, 3.229 | .0002 |
| Severe patients | |||||||||||||
| 0‐44 y | 21 | 11.8 | 1 | 4.8 | 0, 14.7 | 16 | 11.8 | 2 | 12.5 | 0.0, 30.7 | 0.381 | 0.038, 3.840 | .8054 |
| 45‐64 y | 69 | 38.8 | 18 | 26.1 | 15.5, 36.7 | 56 | 41.2 | 9 | 16.1 | 6.1, 26.0 | 1.623 | 0.791, 3.329 | .1760 |
| ≥65 y | 88 | 49.4 | 43 | 48.9 | 38.2, 59.5 | 64 | 47.0 | 17 | 26.6 | 15.4, 37.7 | 1.840 | 1.161, 2.914 | .0055 |
| All | 178 | 100.0 | 62 | 34.8 | 27.8, 41.9 | 136 | 100.0 | 28 | 20.6 | 13.7, 27.5 | 1.692 | 1.150, 2.490 | .0057 |
Abbreviations: CFR, case fatality rate; CI, confidence interval; RR, relative risk of mortality for males compared with females.
Figure 1.
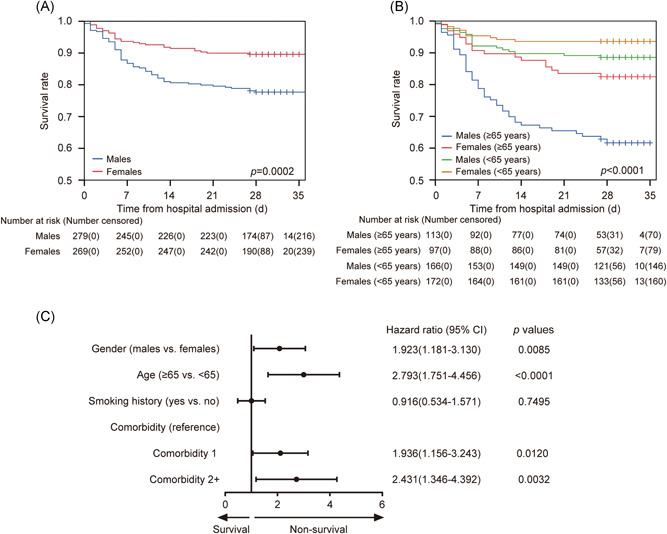
Kaplan‐Meier curves for COVID‐19 patients (A) grouped by sex (P = .0002) and (B) grouped by sex and age (P < .0001). (C) The effect of sex on mortality of COVID‐19 patients after adjusting for other potential risk factors
When dividing all patients into three subgroups based on age, that is, 0 to 44 years (young), 45 to 64 years (mid‐aged) and greater than or equal to 65 years (elder), a climbing mortality of both males and females was observed with increasing age. It was notable that no difference existed between the age distribution of males and females. The first two subgroups (young and mid‐aged) showed no difference in CFR between males and females, whereas elder male patients had greater CFR than females did (RR = 2.171, 95% CI, 1.328‐3.550, P = .0010), as their specific CFR values were 38.1% (95% CI, 29.0%‐47.1%) and 17.5% (95% CI, 9.8%‐25.2%), respectively. Considering the combined effects of sex and age, significant differences in the survival rates of four subgroups existed (χ 2 = 57.186, overall P < .0001; Figure 1B), and the survival rate curve of elder male patients remained the lowest while other curves distributed close in the plot. As male patients had a severe ratio greater than females did, the sex‐ and age‐specific CFR of 314 severe COVID‐19 patients were also analyzed (Table 1). In agreement with the results of all patients, sex, and age both had significant influences on CFR, and the elder severe male patients had the greatest CFR, that is, 48.9% (95% CI, 38.2%‐59.5%).
Recent research has demonstrated that patients with comorbidities had greater disease severity compared with those without. 19 In this study, considering sex, age, smoking history, and comorbidity, univariable and multivariable analyses to identify risk factors associated with death of all enrolled COVID‐19 patients were performed by the Cox proportional hazard regression model (Table E2). The unadjusted and adjusted results both showed that sex, age, and comorbidity were significant risk factors associated with decease of COVID‐19 patients. After adjusting for age, smoking history, and comorbidity, male patients were more likely to reach the endpoint than females did (HR, 1.923, 95% CI, 1.181‐3.130; Figure 1C). Elder patients had a higher decease risk than young and mid‐aged patients did, with a HR of 2.793 (95% CI, 1.751‐4.456). Compared with patients without comorbidity, patients with one comorbidity had an HR of 1.936 (95% CI, 1.156‐3.243), and patients with two or more comorbidities had an HR of 2.431 (95% CI, 1.346‐4.392).
3.3. Excess inflammation reaction was related to severity of COVID‐19 patients
As smoking history greatly differed between male and female COVID‐19 patients, the patients without smoking history were employed to study the combined effects of sex, age, and comorbidity on inflammation reaction. Two hundred and thirty three of 452 patients without smoking history had cytokine data available. The 233 patients were classified into three subgroups based on severity, and there were 123 mild patients, 92 severe patients, and 18 deceased patients.
Patients with excess inflammation reaction, for example, high levels of inflammatory cytokines (sIL‐2R (P = .0012), IL‐6 (P < .0001), IL‐8 (P = .0012), or IL‐10 (P < .0001)) or inflammatory proteins (LDH (P < .0001), ferritin (P = .0016), or hsCRP (P = .0008)), or low level of lymphocyte count had greater proportions of severe and deceased cases (Figure 2).
Figure 2.
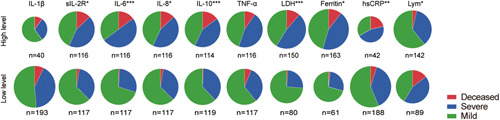
Proportions of COVID‐19 severities based on laboratory marker expressions. The cut‐off for serum cytokines were set at medians of 233 patients, that is, 5 pg/mL for IL‐1β, 737 U/mL for sIL‐2R, 16.12 pg/mL for IL‐6, 15.8 pg/mL for IL‐8, 5 pg/mL for IL‐10, and 8.5 pg/mL for TNF‐α, respectively. The cut‐off for LDH, ferritin, hsCRP, and lymphocyte count were set at 250 U/L, 400 μg/L, 100 mg/L, and 0.8 × 109/L, respectively. *P < .05; **P ≤ .001; ***P ≤ .0001. hsCRP, hyper‐sensitive C‐reactive protein; IL, interleukin; LDH, lactose dehydrogenase; Lym, lymphocyte; sIL‐2R, soluble interleukin‐2 receptor; TNF, tumor necrosis factor
3.4. Age‐related sex predisposition in inflammation reaction of COVID‐19 patients
Considering the age distribution of enrolled COVID‐19 patients, the effect of sex on inflammation reaction was studied (Figure 3). Compared with female patients, male patients had higher levels of ferritin (overall P < .0001) and hsCRP (overall P < .0001) in young, mid‐aged, and elder subgroups. Specifically, elder male patients also had higher levels of IL‐10 (P = .0235) and LDH (P = .0313) than elder females did; and similar differences in sIL‐2R, TNF‐α, and LDH were also observed in young or mid‐aged subgroups. Besides this, the lymphocyte count in peripheral blood showed a trend of lower levels in male patients. The investigated inflammatory indexes expect IL‐1β exhibited a climbing trend with aging, whereas lymphocyte count gradually decreased.
Figure 3.
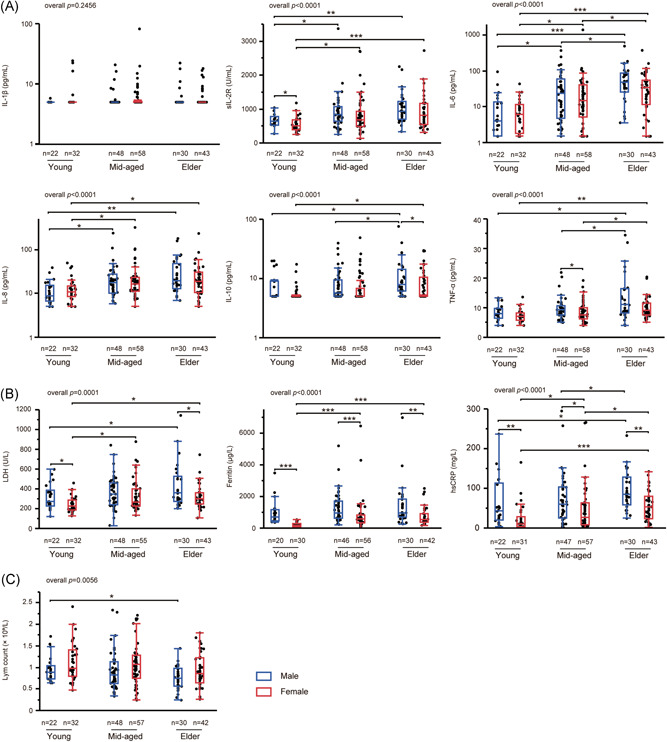
Levels of laboratory markers in patients with COVID‐19 stratified by sex and age. A, Inflammatory cytokines including IL‐1β, sIL‐2R, IL‐6, IL‐8, IL‐10, and TNF‐α. B, Inflammatory proteins including LDH, ferritin and hsCRP. C, Lymphocyte count in peripheral blood. Young, 0 to 44 years; mid‐aged, 44 to 64 years; elder, greater than or equal to 65 years. hsCRP, hyper‐sensitive C‐reactive protein; IL, interleukin; LDH, lactose dehydrogenase; Lym, lymphocyte; sIL‐2R, soluble interleukin‐2 receptor; TNF, tumor necrosis factor
3.5. Comorbidity‐related sex predisposition in inflammation reaction of COVID‐19 patients
On the basis of sex and comorbidity, the patients were divided into four subgroups: males without comorbidity, females without comorbidity, males with comorbidity, and females with comorbidity. Patients with comorbidity had greater age, severe rate and CFR, while there was no difference between comorbidity number of males and females (Table 2). There were significant differences in serum levels of sIL‐2R (overall P = .0006), IL‐6 (overall P = .0113), IL‐8 (overall P = .0063), IL‐10 (overall P = .0011), TNF‐α (overall P = .0001), and lymphocyte count (overall P = .0198), LDH (overall P = .0018), ferritin (overall P < .0001), and hsCRP (overall P < .0001) in these four subgroups. With or without comorbidity, patients in the male groups had higher levels of ferritin and hsCRP compared with female groups. For patients with comorbidity, there were significant differences in serum levels of sIL‐2R, IL‐10, TNF‐α, and lymphocyte count of males and females, which, however, were not found in patients without comorbidity. Males with comorbidity had higher serum levels of sIL‐2R, IL‐6, IL‐8, IL‐10, and TNF‐α than males without comorbidity. On the other hand, females with comorbidity were observed with increased levels of serum IL‐8, ferritin, and LDH compared with females without comorbidity.
Table 2.
Inflammatory indexes and cytokines of 233 COVID‐19 patients without smoking history
| Without comorbidity | With comorbidity | P | |||
|---|---|---|---|---|---|
| Males, n = 53 | Females, n = 82 | Males, n = 47 | Females, n = 51 | ||
| Age, median (IQR), y | 49.0 (37.5‐58.5) | 53.5 (40.8‐67.0) | 62.0 (52.0‐71.0) a | 62.0 (56.0‐69.0) b | <.0001 |
| Body mass index, median (IQR), kg/m2 | 25.5 (23.4‐26.6) | 23.9 (21.9‐25.9) | 24.2 (22.6‐27.2) | 25.6 (22.2‐28.3) | .4505 |
| Comorbidity 2+, No./total No. (%) | 0/53 (0.0) | 0/82 (0.0) | 14/47 (29.8) a | 16/51 (31.4) b | <.0001 |
| Severe patients, No./total No. (%) | 22/53 (41.5) | 32/82 (39.0) | 31/47 (66.0) | 25/51 (49.0) | .0224 |
| Death, No./total No. (%) | 2/53 (3.8) | 1/82 (1.2) | 8/47 (17.0) | 7/51 (13.7) b | .0012 |
| IL‐1β, median (IQR), pg/mL | 5.0 (5.0‐5.0) | 5.0 (5.0‐5.0) | 5.0 (5.0‐5.0) | 5.0 (5.0‐5.0) | .2347 |
| IL‐1β >5 pg/mL, No./total No. (%) | 5/53 (9.4) | 18/82 (22.0) | 7/47 (14.9) | 10/51 (19.6) | .2688 |
| IL‐2R, median (IQR), U/mL | 727.0 (554.5‐932.5) | 647.5 (470.5‐924.5) | 932.0 (694.0‐1266.0) a | 676.0 (489.0‐1107.0) c | .0006 |
| IL‐6, median (IQR), pg/mL | 12.1 (3.2‐41.7) | 11.5 (4.6‐32.3) | 35.3 (9.5‐72.0) a | 21.9 (5.9‐43.4) | .0113 |
| IL‐8, median (IQR), pg/mL | 14.2 (8.1‐20.8) | 12.6 (9.2‐20.3) | 18.5 (12.1‐30.3) a | 17.8 (11.6‐29.4) b | .0063 |
| IL‐10, median (IQR), pg/mL | 5.0 (5.0‐9.5) | 5.0 (5.0‐6.5) | 7.0 (5.1‐13.2) a | 5.0 (5.0‐8.6) c | .0011 |
| TNF‐α, median (IQR), pg/mL | 8.7 (6.6‐10.1) | 7.5 (6.2‐9.5) | 10.0 (7.9‐14.3) a | 8.2 (7.1‐10.8) c | .0001 |
| Lymphocyte count, ×109/L | 0.9 (0.7‐1.0) | 1.0 (0.7‐1.3) | 0.8 (0.6‐1.0) | 1.0 (0.6‐1.4) c | .0198 |
| Lactose dehydrogenase, median (IQR), U/L | 312.0 (250.0‐459.0) | 253.0 (208.5‐333.3) d | 363.0 (236.0‐485.0) | 303.5 (236.8‐363.8) b | .0018 |
| Ferritin, median (IQR), μg/L | 857.3 (612.9‐1414.4) | 422.8 (184.0‐563.0) d | 965.6 (664.6‐2022.0) | 569.0 (331.2‐900.2) b , c | <.0001 |
| Hyper‐sensitive C‐reactive protein, mg/L | 51.3 (26.4‐93.9) | 22.5 (8.2‐68.3) d | 78.1 (44.2‐116.0) | 40.8 (8.9‐65.5) c | <.0001 |
Note: Data are expressed as median (IQR), No., or No./total No. (%). P values comparing the four groups are from Pearson's χ 2 test, Fisher's exact test, or the Kruskal‐Wallis test followed by the Wilcoxon intergroup comparison. P values, overall P values among four groups.
Abbreviations: IL, interleukin; IQR, interquartile range; TNF, tumor necrosis factor.
Males without comorbidity vs males with comorbidity.
Females without comorbidity vs females with comorbidity.
Males with comorbidity vs females with comorbidity.
Males without comorbidity vs females without comorbidity.
Among 10 investigated comorbidities of COVID‐19 patients, that is, hypertension, diabetes, CHD, tumor, bronchiectasis, chronic kidney disease, COPD, asthma, tuberculosis (TB) and hepatitis B virus, hypertension and diabetes were prevalent comorbidities, and their numbers of cases were sufficient for further analysis (Figure 4A). In this way, sex, age, hypertension, and diabetes were chosen to perform multiple linear regression, through which the correlations between sex and inflammatory proteins and cytokines adjusted by age, hypertension, and diabetes were studied (Figure 4B). The results showed that sex (male vs female) was positively correlated with the levels of IL‐10 (P = .0463), TNF‐α (P = .0005), LDH (P = .0009), ferritin (P < .0001), and hsCRP (P < .0001), but inversely correlated with lymphocyte count (P = .0029); age had similar correlations with these cytokines except IL‐10 (ie, TNF‐α (P < .0001), LDH (P = .0009), ferritin (P = .0203), and hsCRP (P = .0006), lymphocyte count (P = .0010)), and was also positively correlated with sIL‐2R (P < .0001), IL‐6 (P = .0039), and IL‐8 (P = .0332). Besides this, there were positive correlations between hypertension and sIL‐2R (P = .0383) and TNF‐α (P = .0180), and a similar relationship existed between diabetes and IL‐10 (P = .0278).
Figure 4.
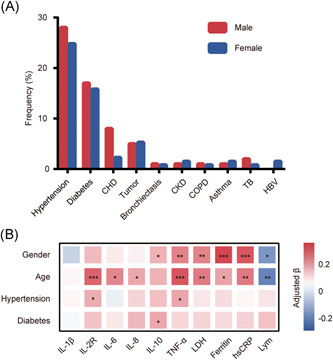
Effects of sex, age and comorbidities on laboratory markers in patients with COVID‐19. A, distribution of comorbidities in COVID‐19 patients grouped by sex. B, Correlation between the identified risk factors (sex, age, hypertension, and diabetes) and laboratory markers by multiple linear regression model; adjusted β, partial regression coefficient; *P < .05; **P ≤ .001; ***P ≤ .0001. CHD, coronary heart disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HBV, hepatitis B virus; hsCRP, hyper‐sensitive C‐reactive protein; IL, interleukin; LDH, lactose dehydrogenase; Lym, lymphocyte; sIL‐2R, soluble interleukin‐2 receptor; TB, tuberculosis; TNF, tumor necrosis factor
4. DISCUSSION
This study provided a comprehensive characterization of gendered effects on inflammation reaction and mortality of COVID‐19 patients, and the association between excess inflammation reaction and severity. In this study, the ratio of severity and death was 57.3% and 16.4%, respectively, which were higher than reported data (5.7%). 21 The possible reasons were the patients were enrolled during the highest peak of COVID‐19 outbreak; and they were from the Sino‐French New City Branch of Tongji Hospital in Wuhan, which was a designated hospital mainly for severe patients.
In this study, there were 62 of 90 (68.9%) male cases in fatal COVID‐19 patients, and males had a higher CFR than females did (22.2% vs 10.4%), with an HR of 1.923 (95% CI, 1.181‐3.130) after adjusting for age, smoking history, and comorbidity (Figure 1C and Table E2). Male and female patients both showed increasing mortality with aging, among whom elder patients were more likely to reach the endpoint, and cases with comorbidity (especially two or more comorbidities) were more susceptible to die compared to those without. Besides this, smoking history, however, had no significant influence on mortality after adjustment of gender, age, and comorbidity. As Guan et al 20 pointed out in a paradoxical result, the possible reasons could be that patients with smoking history were mainly male, but sex was not considered in their study; and the study populations were also different. Consequently, elder patients, especially males with comorbidity, were recommended to receive timely diagnosis, medical care and close monitoring.
Research on SARS patients indicated that abnormal exaggeration of inflammation reaction (eg, IL‐6, IL‐8, IL‐10, TNF‐α, and hsCRP) could lead to lung damage, ARDS, multiple organ failure, or death. 11 , 22 Severe COVID‐19 patients would likely to develop acute lung injure, ARDS, and multiple organ failure, 17 and our study indicated that the severity of COVID‐19 was associated with excess expressions of inflammatory proteins and cytokines. In viral infections, the aberrant release of proinflammatory factors could lead to lung epithelial and endothelial cell apoptosis which damaged the lung microvascular and alveolar epithelial cell barrier, causing alveolar edema, hypoxia, and even ARDS. Meanwhile, cytokine storm could also result in immunopathogenic damage to tissues and organs. 23 , 24
Gender and age were two critical factors for inflammation reaction in COVID‐19 patients. Males and females had different innate immune responses, which could be related to the innate detection of nucleic acids by pattern recognition receptors between sexes, and innate immune responses of sex hormones. 25 , 26 Previous research demonstrated that peripheral blood mononuclear cells (PBMCs) from males produced more IL‐10 than females did following virus stimulation, which was positively related to androgen concentration in males 27 ; and males had higher levels of proinflammatory cytokines (eg, TNF) and chemokines (eg, CXCchemokine ligand 10 [CXCL10]) following lipopolysaccharide stimulation. 28 , 29 , 30 In contrast, under the stimulation of PBMCs in vitro, females had higher numbers of activated CD4+ T cells and CD8+ T cells and proliferating T cells in peripheral blood compared to males. 28 , 31 Females had greater antibody responses, higher basal immunoglobulin levels and B cell numbers than males did. 31 , 32 A conclusion we could speculate on was that sex‐specific innate and adaptive immunity could result in sex differences in inflammation reaction of COVID‐19 patients. Besides this, elder patients had immune disorders including age‐related defects in T‐ and B‐cell function and excess production of inflammatory cytokines, which could lead to a deficiency in control of viral replication and more prolonged proinflammatory responses and poor outcomes. 33 Márquez et al 34 also concluded that males greater than 65 years had higher innate and proinflammatory activity and lower adaptive activity. Consequently, gender‐ and age‐driven differences in inflammation reaction could be the primary reasons of severity and mortality of COVID‐19. As have also been partially confirmed in previous studies, 35 , 36 , 37 comorbidities such as hypertension and diabetes could also affect the inflammatory cytokines including IL‐2R, IL‐10, and TNF‐α, and further contribute to mortality of COVID‐19. As innate and adaptive immunity differed from gender and age, immunotherapy considering gender differences should be developed to realize effective personal treatment of COVID‐19.
This study had some limitations. First, this retrospective study was single central, with all patients enrolled from the Tongji Hospital, and the interpretation of our findings might be limited by the sample size. Second, not all laboratory tests, especially inflammatory proteins and cytokines, were performed on all patients. Third, all the laboratory tests regarding inflammatory proteins and cytokines were conducted on admission, observation of the dynamic changes in blood cytokine levels might provide scientific insights in pathogenesis and efficacy of clinical treatment on COVID‐19. Fourth, cases with some comorbidities such as asthma and tumor were limited, there is still a lack of detailed investigation on their effects on inflammation and severity.
In summary, male patients especially elder patients with comorbidity, showed a higher risk of mortality. Males had higher innate and proinflammation reaction, and defects in adaptive immunity responses; under the combined effects of age and comorbidity, male COVID‐19 patients were prone to develop impaired immune defense and exaggerated production of inflammatory cytokines and proteins including IL‐10, TNF‐α, LDH, ferritin, and hsCRP, further leading to potential ARDS, multiple organ failure, and decease.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
MX and LQ conceived and designed the study. LQ and MX contributed to the literature search and writing of the report. XLi, JS, MY, KW, YT, and YZ contributed to data collection. MX, LQ, BW, ZY, CZ, JY, and CC contributed to data analysis and data interpretation. MX, XLiu, SX, and MZ contributed to revision of the manuscript. All authors provided a critical review of the manuscript and approved the final draft for publication.
Supporting information
Supplementary information
Qin L, Li X, Shi J, et al. Gendered effects on inflammation reaction and outcome of COVID‐19 patients in Wuhan. J Med Virol. 2020;92:2684–2692. 10.1002/jmv.26137
REFERENCES
- 1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Eng J Med. 2020;382:1199‐1207. 10.3410/f.737281536.793571806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO. Main website. https://www.who.int. Accessed May 24, 2020.
- 3. Karlberg J, Chong DSY, Lai WYY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J of Epidemiol. 2004;159:229‐231. 10.1093/aje/kwh056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alghamdi I, Hussain I, Alghamdi M, Almalki S, Alghamdi M, Elsheemy M. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int J Gen Med. 2014;7:417‐423. 10.2147/ijgm.s67061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang J, Dong X, Cao Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;1–12. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 6. Wenham C, Smith J, Morgan R. COVID‐19: the gendered impacts of the outbreak. Lancet. 2020;395:846‐848. 10.1016/s0140-6736(20)30526-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases of COVID‐19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372‐1379. 10.1164/rccm.202003-0543OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. 10.1136/bmj.m1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China. Clin Infect Dis. 2020. 10.1093/cid/ciaa270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meng Y, Wu P, Lu W, et al. Sex‐specific clinical characteristics and prognosis of coronavirus disease‐19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLOS Pathog. 2020;16:e1008520. 10.1371/journal.ppat.1008520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sheng WH, Chiang BL, Chang SC, et al. Clinical manifestations and inflammatory cytokine responses in patients with severe acute respiratory syndrome. J Formos Med Assoc. 2005;104:715‐723. [PubMed] [Google Scholar]
- 12. Wang C, Pang BS. Dynamic changes and the meanings of blood cytokines in severe acute respiratory syndrome. Chin J Tuberc Respir Dis. 2003;26:586‐589. [PubMed] [Google Scholar]
- 13. Hong KH, Choi JP, Hong SH, et al. Predictors of mortality in Middle East respiratory syndrome (MERS). Thorax. 2018;73:286‐289. 10.1136/thoraxjnl-2016-209313 [DOI] [PubMed] [Google Scholar]
- 14. Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex‐based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198:4046‐4053. 10.4049/jimmunol.1601896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS‐CoV‐2 infected patients. EBioMedicine. 2020;55:102763. 10.1016/j.ebiom.2020.102763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020. 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.New coronavirus pneumonia prevention and control program (5th ed) (in Chinese). 2020. http://117.128.6.26/cache/www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440/files/7260301a393845fc87fcf6dd52965ecb.pdf?ich_args2=464-26190622062438_8c563b9f890ddd26f265c52c792d732e_10001002_9c896c2bdfcaf8d39739518939a83798_88ccd8b555f083c7147cc824123d01d8
- 19. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community‐acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45‐e67. 10.1164/rccm.201908-1581ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guan W, Liang W, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid‐19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. 10.1183/13993003.01227-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baud D, Qi X, Nielsen‐Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID‐19 infection. Lancet Infect Dis. 2020. 10.1016/S1473-3099(20)30195-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y, Li J, Zhan Y, et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun. 2004;72:4410‐4415. 10.1128/IAI.72.8.4410-4415.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun X, Wang T, Cai D, et al. Cytokine storm intervention in the early stages of COVID‐19 pneumonia. Cytokine Growth Factor Rev. 2020;20:79. 10.1016/j.cytogfr.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID‐19 and Interleukin‐6 receptor (IL‐6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55:105954. 10.1016/j.ijantimicag.2020.105954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626‐638. 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- 26. Hannah MF, Bajic VB, Klein SL. Sex differences in the recognition of and innate antiviral responses to Seoul virus in Norway rats. Brain Behav Immun. 2008;22:503‐516. 10.1016/j.bbi.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Torcia MG, Nencioni L, Clemente AM, et al. Sex differences in the response to viral infections: TLR8 and TLR9 ligand stimulation induce higher IL10 production in males. PLOS One. 2012;7:e39853. 10.1371/journal.pone.0039853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sankaran‐Walters S, Macal M, Grishina I, et al. Sex differences matter in the gut: effect on mucosal immune activation and inflammation. Biol Sex Differ. 2013;4:10. 10.1186/2042-6410-4-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asai K, Hiki N, Mimura Y, Ogawa T, Unou K, Kaminishi M. Gender differences in cytokine secretion by human peripheral blood mononuclear cells: role of estrogen in modulating LPS‐induced cytokine secretion in an ex vivo septic model. Shock. 2001;16:340‐343. 10.1097/00024382-200116050-00003 [DOI] [PubMed] [Google Scholar]
- 30. Marriott I, Bost KL, Huet‐Hudson YM. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: a possible mechanism for gender‐based differences in endotoxic shock susceptibility. J Reprod Immunol. 2006;71:12‐27. 10.1016/j.jri.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 31. Abdullah M, Chai PS, Chong MY, et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol. 2012;272:214‐219. 10.1016/j.cellimm.2011.10.009 [DOI] [PubMed] [Google Scholar]
- 32. Furman D, Hejblum BP, Simon N, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci USA. 2014;111:869‐874. 10.1073/pnas.1321060111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Opal SM, Girard TD, Ely EW. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41:S504‐S512. 10.1086/432007 [DOI] [PubMed] [Google Scholar]
- 34. Márquez EJ, Chung CH, Marches R, et al. Sexual‐dimorphism in human immune system aging. Nat Commun. 2020;11:1‐17. 10.1038/s41467-020-14396-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang H, Luo Y, Liang Y, et al. CD4+ CD25+ T cells in primary malignant hypertension related kidney injury. Sci Rep. 2016;6:1‐9. 10.1038/srep27659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rucker AJ, Crowley SD. The role of macrophages in hypertension and its complications. Pflugers Arch. 2017;469:419‐430. 10.1007/s00424-017-1950-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Silawal S, Willauschus M, Schulze‐Tanzil G, Gögele C, Geßlein M, Schwarz S. IL‐10 could play a role in the interrelation between diabetes mellitus and osteoarthritis. Int J Mol Sci. 2019;20:768. 10.3390/ijms20030768 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


