1. INTRODUCTION
Combined use of hydroxychloroquine and azithromycin was globally adopted, in part due to paucity and high cost of alternative therapies. However, the utility of these medications has been questioned; and thus safety becomes a major concern given clinical equipoise regarding efficacy. Both hydroxychloroquine and azithromycin continue to be administered in US clinical trials examining their potential role in prevention of infection, treatment of mild infection in ambulatory patients, and in combination with other medical regimens in treatment of patients with severe disease. These drugs also continue to be clinically utilized in hospitalized patients around the globe, often without continuous telemetry due to lack of resources. Concern regarding use of hydroxychloroquine without adequate rhythm monitoring in clinical trials has been recently expressed. 1 A review of clinicaltrials.gov at the time of submission of this correspondence reveals actively recruiting trials of combined hydroxychloroquine/azithromycin with or without additional COVID‐19 therapies, for both ambulatory and hospitalized patients within and outside the United States. The potential for hydroxychloroquine and azithromycin to cause QT prolongation is counterbalanced by very low risk of proarrhythmia in the general population, and emerging evidence of relatively low risk of torsades de pointes (TdP) in COVID‐19 patients. 2 , 3 , 4 , 5 Thus, delineation of the determinants of significant corrected QT interval (QTc) prolongation and proarrhythmic risk for hydroxychloroquine/azithromycin is very important, especially given the mounting evidence of inefficacy in COVID‐19 treatment.
2. METHODS
We conducted a retrospective analysis of consecutive symptomatic patients who were hospitalized for COVID‐19 infection and received treatment in combination with hydroxychloroquine/azithromycin. This study was approved by the Yale University School of Medicine Human Investigation Committee. Baseline 12‐lead ECGs were obtained before initiation of the therapy. Patients underwent continuous telemetry and QTc was measured by an electrophysiologist at baseline and then daily using Bazett's formula. Independent variables of interest were those associated with QTc prolongation or disease severity: age, gender, baseline QTc, concurrent use of other high‐risk QT‐prolonging medications, and laboratory markers of inflammation and infection (leukocytes, C‐reactive protein), disease severity (troponin T, D‐dimer), renal insufficiency (glomerular filtration rate [GFR] < 60mL/minute), and hypokalemia (serum potassium). Definition of high‐risk QTc medications was based on the classification by the Arizona Center for Education and Research on Therapeutics (AZCERT). The primary outcome was the development of significant QTc prolongation defined as an increase in baseline QTc ≥ 60 milliseconds and/or absolute QTc > 500 milliseconds. 6 Secondary outcomes included ventricular tachyarrhythmias (TdP, polymorphic ventricular tachycardia [VT] or ventricular fibrillation [VF]).
Demographic, clinical, and laboratory characteristics were compared between patients who developed QTc prolongation compared to those who did not. Continuous variables are expressed as mean ± SD. Proportions were compared using the χ 2 test. Continuous variables were compared using t test for normally distributed data or the Mann‐Whitney U test if non‐normal. Statistical significance was defined as P < .05. Unadjusted odds ratios (OR) were calculated separately for each potential predictor of significant QT prolongation using univariable logistic regression. Significant predictors (P < .05) from univariable analysis were then used as covariates in a multiple logistic regression model to calculate adjusted odds ratios (aOR).
3. RESULTS
The cohort consisted of 91 patients (age 62.7 ± 15.1 years, 44% females,). Excessive QTc prolongation occurred in 23% of patients receiving hydroxychloroquine/azithromycin, increasing from 437 ± 37 to 504 ± 41 milliseconds. In 14% of the cohort, the QTc exceeded 500 milliseconds. The baseline characteristics of those with and without significant prolongation are shown in Table 1. Patients with excessive prolongation were generally older (age 70.0 ± 15.1 vs 60.5 ± 14.5; P = .016), with more hypertension (58% vs 40%; P = .045), renal insufficiency (52% vs 20%; P = .006), coronary artery (33% vs 8%; P = .01), and cerebrovascular disease (24% vs 3%, P = .01). In addition, they appeared to develop greater severity of disease with 10 out of 21 (48%) requiring mechanical ventilation compared to 15 out of 70 (21%) without significant QT prolongation (P = .03). Nine out of 21 (43%) patients with excessive QT prolongation had baseline renal insufficiency or developed acute renal failure. An additional concurrent QT‐prolonging medication was administered in 42% of patients. Among patients with excessive QT prolongation, a concurrent QT‐prolonging drug was used in 67% of patients vs only 34% in patients without excessive QT prolongation (P = .01). Most was due to intravenous propofol, which was used in 48% of patients with excessive QT prolongation compared to only 19% of patients without (P = .01).
Table 1.
Baseline characteristics
| QTc prolongation (>60 ms from baseline and/or >500 ms) | ||||
|---|---|---|---|---|
| All patients (n = 91) | QTc prolongation (n = 21) | No QTc prolongation (n = 70) | P‐value | |
| Age, mean ± SD (range), y | 62.7 ± 15.1 (29‐93) | 70.0 ± 15 (41‐93) | 60.5 ± 14.5 (29‐91) | .016 |
| Demographics, n (%) | ||||
| Female | 40 (44) | 9 (43) | 31 (44) | 1.00 |
| Hypertension | 42 (46) | 14 (58) | 28 (40) | .05 |
| Diabetes mellitus | 26 (29) | 8 (38) | 18 (26) | .28 |
| Coronary artery disease | 13 (14) | 7 (33) | 6 (8) | .01 |
| Cerebrovascular disease/stroke | 7 (8) | 5 (24) | 2 (3) | .01 |
| Chronic lung disease | 6 (7) | 0 (0) | 6 (9) | .33 |
| Laboratory, mean (SD) | ||||
| Leukocytes, ×1000/μL | 8.0 (4.0) | 7.1 (2.4) | 8.3 (4.3) | .13 |
| Lymphocytes, % | 14 (9) | 16.8 (11.5) | 13.2 (8.3) | .20 |
| Platelets, ×1000/μL | 210 (83) | 188.3 (74.1) | 216.7 (84.3) | .14 |
| Hgb, g/dL | 13.4 (1.9) | 13.3 (1.8) | 13.5 (2.0) | .82 |
| CRP, mg/dL | 12.8 (8.6) | 11.1 (7.3) | 13.3 (9.0) | .26 |
| Ferritin, mg/mL | 1170 (1574) | 813 (696) | 1279 (1747) | .08 |
| Procalcitonin, ng/mL | 0.4 (1.4) | 0.2 (0.3) | 0.4 (1.6) | .35 |
| Tn, ng/mL | 0.04 (0.19) | 0.06 (0.14) | 0.04 (0.20) | .82 |
| D‐dimer, mg/L | 2.5 (5.1) | 3.9 (6.3) | 2 (4.7) | .22 |
| AST, U/L | 77 (83) | 76.7 (61.1) | 77.4 (88.8) | .97 |
| ALT, U/L | 67 (69) | 54.4 (55.2) | 70.5 (72.3) | .28 |
| Bilirubin, mg/dL | 0.59 (0.27) | 0.51 (0.16) | 0.61 (0.29) | .3 |
| Albumin, g/dL | 3.0 (0.6) | 3 (1.0) | 3 (0.4) | .87 |
| K, mmol/L | 4.0 (0.5) | 4.1 (0.6) | 4 (0.4) | .21 |
| Creatinine, mg/dL | 1.09 (0.62) | 1.3 (0.9) | 1 (0.5) | .22 |
| BUN, mg/dL | 18.3 (9.9) | 24 (11.1) | 16.6 (8.9) | .009 |
| GFR < 60 mL/min, n (%) | 25 (27) | 11 (52) | 14 (20) | .006 |
| QTc interval, ms | ||||
| Baseline QTc, mean (SD) | 437 (25) | 437 (37) | 437(21) | .98 |
| Maximal QTc, mean (SD) | 473 (31) | 504 (41) | 464 (19) | <.001 |
| Concurrent high‐risk drug, n (%) | 38 (42) | 14 (67) | 24 (34) | .012 |
| Mechanical ventilation, n (%) | 25 (27) | 10 (48) | 15 (21) | .03 |
| Outcomes, n (%) | ||||
| Bradyarrhythmia | 9 (10) | 4 (19) | 5 (7) | .2 |
| TdP/VF | 2 (2) | 2 (10) | 0 (0) | .05 |
| Death | 8 (9) | 6 (29) | 2 (3) | .002 |
| Hospital length of stay, d | 10.8 ± 6.3 | 11.4 ± 5.1 | 10.6 ± 6.7 | .55 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CRP, C‐reactive protein; GFR, glomerular filtration rate; Hgb, hemoglobin; K, serum potassium; QTc, corrected QT interval; TdP, torsades de pointes; Tn, troponin T; VF, ventricular fibrillation.
Significant ventricular arrhythmias occurred in two patients. One patient had classic TdP (Figure 1) and the second patient developed polymorphic VT that degenerated into VF in the setting of severe multisystem disease. Table 2 shows the results of the univariable and multiple logistic regression analysis. Older age (>75 years), prolonged baseline QTc (>460 milliseconds), impaired renal function (GFR < 60 mL/minute), and concurrent use of a high‐risk QTc‐prolonging drug were each associated with excessive QTc prolongation. Multiple regression demonstrated the use of additional QT‐prolonging agents (especially propofol) to be independently associated with QTc prolongation (any drug: adjusted OR, 3.69; CI [1.22, 11.20]; P = .02 and propofol: adjusted OR, 3.28; CI [1.06, 10.17]; P = .04).
Figure 1.
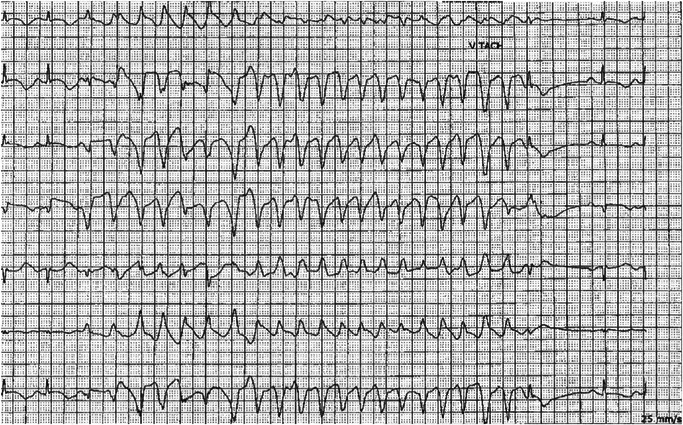
Telemetry rhythm strip demonstrating significant QTc prolongation during sinus rhythm followed by a self‐terminating run of torsades de pointes. QTc, corrected QT interval
Table 2.
Univariable and multiple logistic regression analysis
| Univariable regression analysis | |||
|---|---|---|---|
| OR | 95% CI | P‐value | |
| Age > 75 y | 2.97 | (1.01‐8.70) | .047 |
| Female | 1.03 | (0.38‐2.80) | .955 |
| Baseline QTc > 460 ms | 3.30 | (1.11‐9.83) | .032 |
| Concurrent high‐risk drug | 3.83 | (1.36‐10.77) | .011 |
| Propofol use | 3.99 | (1.4‐11.36) | .01 |
| Leukocytes, ×1000/μL | 0.92 | (0.79‐1.07) | .26 |
| CRP, mg/dL | 0.97 | (0.91‐1.03) | .359 |
| Tn, ng/mL | 1.27 | (0.11‐14.37) | .846 |
| D‐dimer, mg/L | 1.06 | (0.98‐1.15) | .164 |
| K, mmol/L | 2.17 | (0.77‐6.16) | .145 |
| GFR < 60 mL/min | 4.40 | (1.56‐12.42) | .005 |
| Multiple regression analysis | |||
|---|---|---|---|
| Adjusted OR | 95% CI | P‐value | |
| Concurrent high‐risk drug | 3.69 | (1.22‐11.20) | .021 |
| Propofol use | 3.28 | (1.06‐10.17) | .04 |
| GFR < 60 mL/min | 3.11 | (0.95‐10.20) | .061 |
| Baseline QTc > 460 ms | 2.32 | (0.68‐7.97) | .181 |
| Age > 75 y | 1.93 | (0.57‐6.60) | .292 |
Abbreviations: CRP, C‐reactive protein; GFR, glomerular filtration rate; K, serum potassium; OR, odds ratio; QTc, corrected QT interval; Tn, troponin T.
4. DISCUSSION
We found that combined hydroxychloroquine/azithromycin results in significant QTc prolongation in approximately one in four hospitalized patients. The degree of QTc prolongation is severe, exceeding 500 milliseconds in 14% of patients, and resulting in a case of TdP. Thus, combined hydroxychloroquine/azithromycin should not be administered without continuous telemetry monitoring, even in the setting of a clinical trial. Determinants of QTc prolongation include older age, impaired renal function, baseline QTc > 460 milliseconds, and concurrent use of other high‐risk QTc‐prolonging drugs. Care is required to minimize the concurrent administration of these medications. Vigilance in QTc and arrhythmia monitoring is required for patients in whom the concurrent use of high‐risk QTc drugs is necessary, with particular attention to intravenous propofol given the frequency of its use in severe infection. Propofol, which is often used as a sole agent without clinical sequelae, may increase proarrhythmic risk when administered with hydroxychloroquine/azithromycin in patients with severe COVID‐19 infection.
5. LIMITATIONS
Although there is a high degree of collinearity between severity of illness, mechanical ventilation, and propofol use, this only highlights the importance of considering the determinants of QTc prolongation in this very sick population.
6. CONCLUSIONS
Despite equipoise regarding the benefit of hydroxychloroquine/azithromycin treatment for COVID‐19 infection, these medications continue to be used in large clinical trials both in the United States and across the world. This study demonstrates that the risk of QTc prolongation by these medications is enhanced in the setting of renal failure, older age, baseline QTc prolongation, and the concomitant use of other QT‐prolonging medications in patients with severe COVID‐19 infection. A better understanding of these factors associated with QTc prolongation is important for safe administration of these medications, especially in light of data showing low efficacy in COVID‐19 treatment.
Maraj I, Hummel JP, Taoutel R, et al. Incidence and determinants of QT interval prolongation in COVID‐19 patients treated with hydroxychloroquine and azithromycin. J Cardiovasc Electrophysiol. 2020;31:1904–1907. 10.1111/jce.14594
Ilir Maraj and James P. Hummel contributed equally to this study.
Disclosures: No relationships with industry to be disclosed related to this specific work. Joseph Akar reports consulting for Biosense Webster.
REFERENCES
- 1. Gollob MH. COVID‐19, clinical trials and QT‐prolonging prophylactic therapy in healthy subjects: first, do no harm. J Am Coll Cardiol. 2020;20:79. 10.1016/j.jacc.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020:e201834. 10.1001/jamacardio.2020.1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID‐19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020:e201787. 10.1001/jamacardio.2020.1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saleh M, Gabriels J, Chang D, et al. The effect of chloroquine, hydroxychloroquine and azithromycin on the corrected QT interval in patients with SARS‐CoV‐2 infection. Circ Arrhythm Electrophysiol, 2020. 10.1161/CIRCEP.120.008662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in‐hospital mortality in patients with COVID‐19 in New York State. JAMA. 2020:e208630. 10.1001/jama.2020.8630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drew BJ, Ackerman MJ, Funk M, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121:1047‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]


