1. INTRODUCTION
According to data from the World Health Organization, Italy has been particularly affected by the COVID‐19 pandemic. On 1 April 2020, Italy gained, at a world level, the highest number of total confirmed cases (n = 110 574) and deaths (n = 13 155) since the beginning of the outbreak. 1 The number of cases raised exponentially, reaching a total of 227 364 infected subjects and 32 330 deaths on May 20. The distribution of infected subjects and deaths, however, was not homogeneous, being respectively about 7 times and 12 times higher in northern than in southern regions. 2
Besides monitoring the outbreak, epidemiologic data should be analysed to test the adequacy of strategies adopted to contain the pandemic, to better face the rising cases of COVID‐19 in other countries and to use lessons from this pandemic for future possible outbreaks. From a clinical point of view, evidence points to a multi‐organ involvement secondary to the SARS‐CoV‐2 infection. 3 In this rapidly changing and novel context, the role of the specialist in internal medicine represents a winning factor for optimal management of critically ill adults with suspected or diagnosed COVID‐19. The role of the internist provides a precious support to specialists working in different settings, namely emergency room (ER), infective disease, respiratory and intensive care units (ICU).
Here, we analyse the main phases of the COVID‐19 outbreak in Italy. In addition, while discussing some pathophysiological implications of this complex disease, we briefly describe the events that we experienced—following the COVID‐19 outbreak—at our academic division of internal medicine in the largest regional hospital of Apulia.
2. MATERIAL AND METHODS
Epidemiologic data were searched from public available databases from the World Health Organization 4 and the Italian Ministry of Health. 5 The rates of infected subjects and deaths in Italy (whole country and regional level) per 100 000 residents were calculated considering the official number of residents derived from the National Institute of Statistics (ISTAT). Correlations were tested using the Pearson coefficient and plotted using linear regression. Data updated to 20 May, 2020.
3. RESULTS
The spread of the COVID‐19 pandemic is not homogenous worldwide. Most rapid and extensive outbreak occurs in a few countries (i.e. USA, Italy, Spain, China, Germany, France, Iran). 4 In Italy, the first cases of infection (a couple of Chinese tourists) occurred on 30 January 2020 at the Italian Experimental Institute 'Lazzaro Spallanzani' in Rome. Figure 1 summarizes the daily progression in the cumulative number of COVID‐19‐positive subjects and in the incidence of deaths related to COVID‐19 in southern and northern Italy, since the start of the outbreak. Table 1 lists the containment strategies adopted by the Central Government. The spread of infection followed divergent trends between northern and southern Italy.
Figure 1.
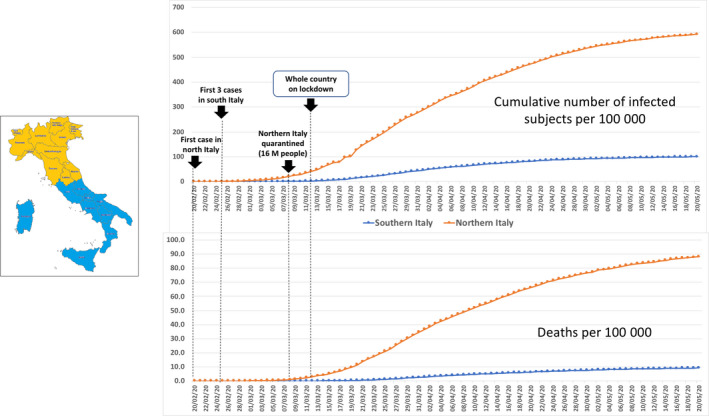
The upper graph shows the cumulative number of COVID‐19‐infected patients in Italy from 20 February to 20 May 2020. The lower graph shows the time progression in the absolute number of deaths. 2 Dots and lines indicate the incidence of cases per 100 000 residents in southern and northern Italian regions
Table 1.
Time progression of containment strategies adopted by the Italian Government
| January 30: first case in Italy (Chinese couple in Rome) |
| January 30: air traffic embargo for flights coming to Italy from any Chinese city, including the autonomous regions of Hong Kong and Macau |
| February 20: first case hospitalized in Codogno (Lombardy, northern Italy). Start of a local outbreak, with strict quarantine for 14 d starting from February 23 |
| February 25: first 3 cases in Sicily |
| March 8: 7365 cases (91% in northern Italy). Northern Italy quarantined (16 million people) |
| March 12: 15 113 official cases in Italy (90.5% in northern Italy). The whole country is on lockdown, and a massive communication campaign starts |
| May 4: lockdown was stopped |
Since the average incubation period for COVID‐19 is 5‐6 days and, in its early stages, the epidemic doubles in size every 7.4 days, 6 the initial outcome of the country lockdown was expected by two‐three weeks. As shown in Figure 2, this hypothesis was confirmed by the analysis of the absolute daily number of new cases of infection in northern and southern Italy. Figure 3 depicts the reduction trend.
Figure 2.
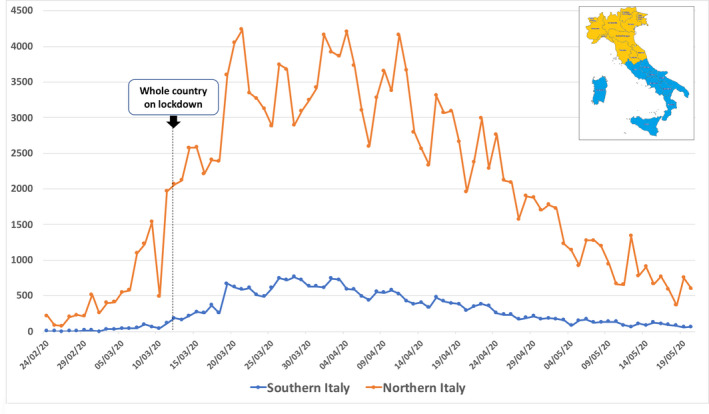
Time changes in the absolute daily number of new cases of infection in northern and southern Italy from 24 February to 20 May 2020. 2 Data show a progressive increase before the lockdown and a plateau with a following decrease about 2 wk after
Figure 3.
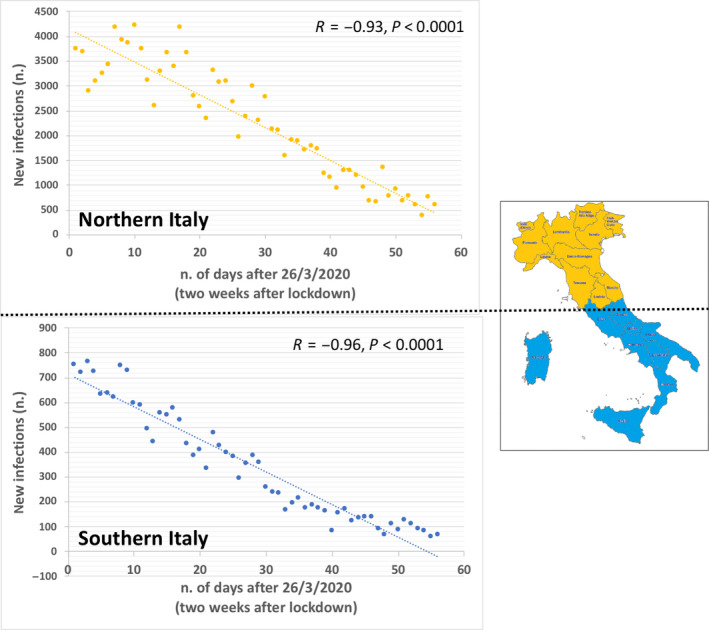
Linear regression analysis between the number of days starting from March 26 (2 wk after the day of the lockdown, 12 March 2020) and the daily number of new cases of infection in northern and southern Italy. A significant, negative correlation was present in both southern and northern Italian regions
3.1. COVID‐19 outbreak in northern Italy
A total of 33 883 040 subjects live in northern Italian regions. The first COVID‐19 case in a northern region was noticed in Codogno, a small town in Lombardy, 60 km south‐east from Milan. In Codogno a local quarantine started on February 23. After 2 weeks (March 8), the whole northern Italy was quarantined. On the national 'lockdown' day (March 12), in northern Italy there were a total of 14 335 infected patients and 997 COVID‐19‐related deaths. On May 20, infected subjects were 200 768 and the number of deaths increased to 29 876.
3.2. COVID‐19 outbreak in southern Italy and the experience in Bari (Apulia)
A total of 26 476 506 subjects live in southern Italian regions. The first three cases in this area occurred only five days after the first case in northern Italy. However, on the 'lockdown' day, the total number of infected patients was 778, about 18‐fold lower than in northern Italy. The COVID‐19‐related deaths were 19, about 52‐folds lower than in northern Italian regions. On May 20, infected subjects were 26 596 and COVID‐19‐related deaths were 2454, about 7‐ and 12‐fold lower, respectively, than in northern Italy.
In most southern regions, travellers were obliged to inform the local authorities upon their arrival to track possible newly diagnosed infections. Thus, before the whole country was on lockdown, the containment measures in southern Italy were only on a voluntary basis (social isolation was advised).
In Apulia (southern Italy, about 4 million inhabitants), the regional authorities estimated that at least 16 545 subjects travelled from northern Italy from February 29 to March 14. In this region, the incidence of infected subjects increased from 0.1 (February 29) to 109.4 per 100 000 residents (May 20) being, on the same day, slightly higher than the rate observed in southern Italy (100.5 per 100 000 residents). This value, however, remained about 6‐fold lower than the incidence recorded in northern Italy (ie 592.5 per 100 000 residents). On May 20, a total of 4407 confirmed cases and 478 COVID‐19‐related deaths were recorded in Apulia. 7 The median age of infected subjects was 56 years, and about 80% of deaths were recorded in subjects with 70 or more years of age. At a regional level, a total of 1535 patients were isolated at home, and 25% of cases were admitted in COVID‐19‐dedicated hospitals. 7 Of note, only a minority of patients had a severe (16%) or critical (3%) clinical presentation. 7 The majority of patients remained asymptomatic (37%) or had a noncritical clinical presentation (44%) requiring, after an initial triage, home isolation or clinical management in nonintensive care units.
Following the COVID‐19 outbreak, the local Apulian government firstly increased the number of available beds in the units of intensive care, pneumology and infectious diseases across the region. Afterwards, the local healthcare system was re‐modulated by creating 'COVID‐19' and 'non‐COVID‐19' dedicated hospitals. The Policlinico, the second largest Hospital in southern Italy, hosts about 1550 beds, with all medical specialties. It is the main hospital in Bari (capital city of Apulia), with a population of 320 257 inhabitants, raising to 750 000 inhabitants in the urban area and to 1.3 million inhabitants in the metropolitan area. Before the outbreak, the Clinica Medica 'A. Murri' was a typical academic division of internal medicine in the large referral hospital (i.e. 20 beds with 850 admissions/year, 900 day services/year, and about 6000 outpatient visits/year, including an active ultrasound service). All nonurgent outpatient activities, however, had been suspended on the first week of March across the whole region. Inpatient activities were regularly carried out until March 16. At that time, a total of 383 new infected subjects and 19 COVID‐19‐related deaths were recorded in the Apulia region. Following the urgent request of the General Director and the Sanitary Direction at the Policlinico on the same day, all inpatients (without symptoms or an oropharyngeal swab suggestive of COVID‐19 infection) were discharged between March 17 and 18 or transferred to other medical wards. At the same time, we merged two divisions of internal medicine (one academic and one nonacademic, total of 40 beds). Within 48 hours, the medical and paramedical staff from both divisions (including several residents in geriatrics, internal medicine and sport medicine) were properly trained in the correct use of personal protection devices. The staff was therefore re‐located in the so‐called 'grey zone' equipped with up to 64 beds, occupying the whole 5th floor of the 'Asclepios' block, a big five‐storey building entirely dedicated to COVID‐19 patients (Figure 4). As internists, our specific mandate was the screening of a wide range of (noncritical, nonsurgical) patients received from the ER, and suspected of having COVID‐19 infection. The division was quickly arranged to accommodate COVID‐19 positive, negative or suspected subjects, with clear separate pathways (dedicated rooms, corridors, elevators, doors, routes). After admission, patients not requiring ICU support are assessed and immediately transferred to a room with single bed, where they undergo the nasopharyngeal swab to obtain a specimen for testing by RT‐PCR. If negative, a second test is repeated 24 hours later, at the local laboratory of public health. 8 Confirmed negative patients are either transferred to specific COVID‐19‐negative wards or discharged, with a nasopharyngeal swab repeated (outpatient basis) 2 weeks later. Persistently positive patients are managed accordingly. One option is the transfer to other COVID‐19‐positive wards in the 'Asclepios' block, depending on the clinical evolution. A second option is the transfer to post‐acute facilities in the territory. A third option (if asymptomatic) is to be placed in quarantine at home, after being fully informed about isolation requirements. Two weeks later, a special hospital unit performs two nasopharyngeal swabs at home, with intervals of 24 hours. This organization facilitates the initial management of non‐COVID‐19‐ and COVID‐19‐related clinical presentations, while decreasing the burden of patients entering other COVID‐19‐dedicated wards. On June 5, a total of up to 700 patients had already been screened at our unit.
Figure 4.
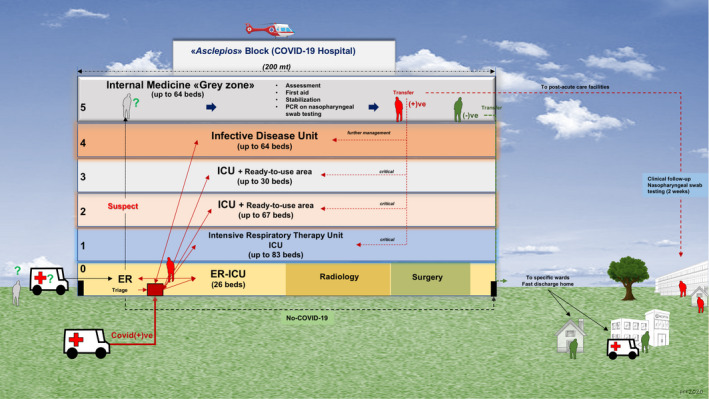
Graphical abstract depicting the Asclepios COVID‐19 Hospital and the flow chart adopted for the management of patients at the Policlinico Hospital in Bari, Italy, during the COVID‐19 pandemic. The building also housed the emergency room, radiology, and the operating theatre (ground floor) and other COVID‐19‐dedicated wards along the other floors, that is intensive respiratory therapy unit, intensive care unit and infective disease unit, with an overall capacity of 334 beds (see text for details)
3.3. The clinical features of COVID‐19 and the preeminent role for the internist
COVID‐19 infection may remain asymptomatic 9 in a still undefined number of patients. Otherwise, a 3‐stage classification system of increasing severity has been proposed for COVID‐19 infection, namely stage I (early infection), stage II (pulmonary phase) and stage III (hyperinflammation phase). 10 Thus, the clinical symptoms range from fever, dry cough, headache to dyspnoea, fatigue, to ARDS, shock and cardiac failure.10, 11 The multi‐organ involvement secondary to SARS‐CoV2 infection is therefore a possibility.
Gastrointestinal symptoms (i.e. diarrhoea, nausea, vomiting and abdominal pain) may be observed early.12, 13 About half of the patients show elevation of liver tests, ALT, AST and GGT during disease progression, and liver injury is possible in severe cases of COVID‐19, possibly due to a direct viral infection of vascular endothelium, cholangiocytes, to immune‐mediated inflammation or to drug hepatotoxicity.14, 15 Diabetes mellitus can increase the risk linked with COVID‐19 infection, negatively affecting the progression and prognosis of the disease.16, 17 Furthermore, relationships exist between COVID‐19 and myocardial injury, 18 heart failure, 18 vascular inflammation, myocarditis, cardiac arrhythmias 19 and hypoxic encephalopathy. 20
Thus, the final clinical presentation of COVID‐19 infection can be composite and ranges from asymptomatic disease to death. Pathogenic mechanisms act at a local and systemic level, and play a critical role in the evolution of the disease:
Inoculation and multiplication: the SARS‐CoV‐2 binds to angiotensin‐converting enzyme 2 (ACE2) receptors21, 22, 23 (abundantly present in epithelia of the lung, gastrointestinal tract and vascular endothelium). This early period can evolve to the second stage of a viral pneumonia;
Extra‐pulmonary systemic hyperinflammation syndrome (in the minority of infected patients): the 'cytokine storm' is associated with increased cytokine levels (IL‐2, IL‐6, IL‐7, IL‐10 and TNF‐α), granulocyte colony‐stimulating factor, interferon‐γ inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1‐α, tumour necrosis factor‐α, lymphopenia (in CD4 + and CD8 + T cells) and decreased IFN‐γ expression in CD4 + T cells.24, 25, 26 D‐dimer, Troponin and N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) can also increase, together with altered coagulation function27, 28, 29 in those patients with more severe disease. 10
Available epidemiologic evidence also points to more severe clinical presentations and higher risk of death in the elderly and/or in fragile patients with several comorbidities.30, 31, 32, 33
In this context, the internist is typically trained to manage the complexity of systemic diseases and to coordinate a multidisciplinary approach in the case of severe presentation.
4. DISCUSSION
The spread of COVID‐19 has rapidly reached a pandemic dimension within few weeks, imposing a rapid remodulation of health systems to contain and adequately face the outbreak. The management of the disease also requires the transmission of adequate evidence‐based information and optimal decision‐making. 34
Italy was the first European country extensively affected by the outbreak, with national control measures (air traffic embargo) adopted the same day of the first diagnosed case in Rome, and with strict control measures (lockdown) about 2 weeks after the first local outbreak was noticed in northern Italy. The critical analysis of the ongoing Italian scenario will help to manage the spread of the pandemic in other countries and also to face the outbreak by optimizing the locally available resources.
Italy extends for a length of approximately 1300 km, and a different pandemic burden occurred in southern vs. northern Italy, as also observed in other geographical areas at a global level. 4 The difference in population density between northern and southern Italy (210.3 and 187.8 residents/km2, respectively) seems too small to fully explain the divergent epidemiologic burden of COVID‐19 in these two areas. Thus, geographical differences in the incidence of infected subjects and COVID‐19‐related deaths point to the existence of possible specific and local environmental factors which need to be urgently identified and that can play a major role in determining the spread and the outcomes of the infection. Besides economic, sociocultural and lifestyle‐related factors, a role is possible for climate change variations,35, 36 air pollution,37, 38, 39 and the effects of endocrine disrupting chemicals on the onset and progression of several comorbidities 40 able to influence the individual vulnerability to COVID‐19.30, 31, 32, 33
At a world level, a 1°C temperature rise above pre‐industrial levels is linked with new and emerging infectious diseases. 35 There might be relationships between climate change, marked reduction in biodiversity and transfer by species crossing (from wild species to humans) of several viruses, namely Ebola, SARS coronavirus‐1, Middle East respiratory syndrome (MERS) coronavirus, Nipah virus and Hendra virus.41, 42 Climate changes are also associated with increased incidence of respiratory infections.35, 36 A study evaluating the effects of temperature on mortality in 306 communities from 12 country/regions described Italy as one of the countries with the highest temperature‐related mortality risk. 43
An analysis on 66 administrative regions in Italy, Spain, France and Germany showed that long‐term exposure to nitrogen dioxide, a common air pollutant mainly produced by anthropogenic processes, may be one of the most important contributors to fatality caused by SARS‐CoV‐2 infection. This effect occurs mainly when the air concentrations of this pollutant are combined with downwards airflow which prevent efficient dispersion. 37 Some regions in northern Italy (mainly Lombardy and Emilia Romagna) are among the most polluted European geographical areas and, at the same time, have the highest world level of virus lethality. 38 Positive associations have been also described between newly COVID‐19 confirmed cases and short‐term exposure to particulate matter (ie PM2.5, PM10), nitrogen dioxide and ozone. 39
Lastly, individual outcomes from COVID‐19 are strongly dependent on the presence of comorbidities.30, 31, 32, 33 Chronic exposure to both air pollutants and endocrine disruptors introduced with contaminated food and beverages or through dermal contact might play a role in increasing individual vulnerability and frailty, mainly in elderly subjects.40, 44
Further analyses are therefore required to adequately identify the major environmental determinants regulating the spread of SARS‐CoV‐2 infection and the prognosis of COVID‐19.
Italian epidemiological data confirm the efficacy of early and stringent measures of primary prevention, as observed in China. 45 About two weeks after the drastic containment measures legally imposed by the lockdown, a trend towards a reduction of new cases clearly emerged in the whole country.
In the absence of vaccines or specific therapeutics of confirmed efficacy, the only useful tool to govern and limit the epidemic remains the social distancing, quarantine, community containment and all other public health measures aimed at the primary prevention.46, 47 In addition, we should be aware that rapid and aggressive outbreaks can easily originate from care homes for elderly people. A worrying aspect emerges from a last study showing that rapid and widespread transmission of SARS‐CoV‐2 occurred in skilled nursing facility, where more than half of the residents with positive test results were asymptomatic at the time of testing and most likely contributed to transmission.48, 49 The problem involves the residents, their family members and medical, nurse personnel if protection protocols are poorly implemented. 50
The Italian National Health System was established with the Law N. 833, on 23 December 1978. This law was based on the equity of care and underlined the precious role of primary prevention, parallel to adequate health assistance and rehabilitation. As clinicians working in the public healthcare system, we argue that the system should be prepared to limit everywhere morbidity and mortality deriving from this and future pandemics. In the most affected regions (northern Italy, mainly Lombardy), serious concerns existed about the effective capacity of the national health system to adequately face the burden of disease. This capacity was challenged by the total and simultaneous number of cases, in particular of those requiring intensive care. In this respect, systemic resources required to adequately manage such a critical situation might be insufficient (i.e. personnel, number of available beds, overcrowding, intensive care facilities).51, 52 The structural limits deriving from insufficient resources have also generated ethical concerns regarding the prioritization of patients to be treated (younger patients prioritized over the elderly) and the equity of care. 53 A prospective data collection of critical care bed number in European countries from July 2010 to July 2011 showed a marked variability between countries. Italy had 12.5 critical care beds per 100 000 capita of population, a number slightly over the continental average (11.5) but about half than that of Germany, the European country with the highest value (29.2). Of note, several EU countries downsized their curative bed capacity in the past 10 years, 52 and on March 2020, approximately 5200 beds in intensive care units were present in Italy. 51 As suggested, the most negative outcomes recorded in limited Italian areas (northern regions) can derive, at least in part, from financial cuts in National Healthcare Service (more than 37 billion euros over the period 2010‐2019), and from a progressive privatization of health services. 54 Thus, policymakers should optimize the allocation of resources and the healthcare capacity. This policy will contribute to limit or avoid the hazards secondary to possible further overloads and to adequately face the public health needs. Moreover, the whole chain of COVID‐19 management should be further optimized by adequate involvement of family medicine. 55
From a clinical point of view, although the main negative outcomes derive from lung involvement, available scientific evidence clearly depicts COVID‐19 as a systemic disease with complex pathogenic pathways acting at multi‐organ and systemic level.
Furthermore, evidence shows that outcomes are strongly dependent on age (i.e. worst outcome in the elderly), on the extent of fragile patients and on the presence of comorbidities.30, 31, 32, 33 In the next future, severe health damage from further similar outbreaks could be strongly prevented by measures focusing on successful ageing and healthspan, rather than merely on increasing lifespan. This approach should include a better management of all the environmental factors affecting noncommunicable diseases, thus contributing to primary prevention of diseases and to decreased frailty, as well as to increased number of years lived without diseases or disability. 44
A further aspect in this national (and international as well) scenario is the central role of the internist, that is a specialist in internal medicine or related disciplines. A specific definition of the intrinsic mission of internal medicine, according to the Ministry of University is summarized as follows (http://www.miur.it/UserFiles/116.htm).
The discipline of Internal Medicine is involved in scientific and educational activity, as well as dedicated health care in the field of medical pathophysiology, instrumental and functional medical semeiology, medical methodology, evidence‐based medicine, general clinical medicine and medical therapy, with specific aspects of emergency medicine, geriatrics and gerontology, allergology and clinical immunology; further fields of interests include clinical and metabolic aspects of vascular diseases, clinical nutrition, sport medicine, and thermal medicine.
In this complex scenario, and according to our experience, internists are called to serve prevention measures, front‐line selection of patients, management of the disease and complications, tight coordination with other specialties, according to specific requirements. In our opinion, this is an outstanding example of professional resilience to sudden and unexpected events which endanger the public health.
5. SUMMARY AND TAKE‐HOME MESSAGES
The SARS‐CoV‐2 infection became an unprecedented clinical challenge. The rapid pandemic spread has strongly stressed the national policies and health systems worldwide, highlighting critical issues and capacity of resilience.
The dramatic experience deriving from the COVID‐19 pandemic should be maximized to adequately manage the present outbreak in other countries, but also to provide useful tools for future potential pandemics. An additional goal should be the improvement of the overall health systems, oriented to adequately fulfil public needs.
The best approach derives from a combination of public health measures aimed at primary prevention, and an adequate allocation of financial resources needed to efficiently face public health needs (in terms of personnel, processes and tools) and an appropriate clinical management of affected individuals. This last goal, in particular, relies on the evidence that the infection by SARS‐CoV‐2 generates a complex and systemic disease, with multi‐organ involvement. In this context, specialists in internal medicine play an undisputed pivotal role.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
ADC and PP designed the study, arranged graph and figure drawings, collected data and wrote the first draft of the paper. VOP, MM and GM gave critical comments on manuscript draft. The IMC‐19 group was actively involved in technical advice, design of routes and paths, data collection and discussion about clinical topics and organization. All authors had full access to all the study data and approved the final version. The corresponding author had final responsibility for the decision to submit for publication.
ACKNOWLEDGEMENTS
We would like to thank Dr M. De Luca (Training Unit), Dr N. Serrone (Hospital Information Office), V. Diceglie (Secretary) and chief nurses Mrs A. Ventrella and M. Bruscella, for dedicated work.
APPENDIX A.
IMC‐19 (Internal Medicine COVID‐19) Group. Internal Medicine Staff: A. M. Aquilino, A. Belfiore, M. Binetti, G. Bruno, F. D'Alitto, F. D'Onofrio, A. Gesualdo, L. Grimaldi, A. Lella, C. Mallardi, E. M. Modeo, R. Moretti, M. Noviello, F. Passerini, F. Prigigallo, S. Pugliese, O. Simone; Residents: C. Appice, G. Campanale, S. Cataldi, A. Cezza, M. Ciannarella, L. Cicala, A. Dell'Acqua, M. Diaferia, G. Erroi, F. Fiermonte, G. Giovannetti, G. D. Mazelli, G. Mersini, M. Messina, A. Montesano, A. Noto, M. V. Palma, F. Perez, C. Piro, A. Polito, C. Stasi, M. Zizzari; Hospital Sanitary Direction: M. Marra, G. Calabrese, A. Daleno, F. Lisena, D. Losacco, L. Melpignano, S. Soldano
REFERENCES
- 1. World Health Organization . Coronavirus disease 2019 (COVID 19). Situation Report ‐ 72. Geneva, Switzerland: World Health Organization; 2020. [Google Scholar]
- 2. Italian Government and Istituto Superiore di Sanità . COVID‐19 monitoring in Italy, 2020.
- 3. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Coronavirus disease (COVID‐19) Pandemic, vol. 2020. Geneva, Switzerland: World Health Organization; 2020. [Google Scholar]
- 5. Italian Ministry of Health . COVID‐10 Italia ‐ Monitoraggio della situazione. Rome, Italy: Italian Ministry of Health and National Civil Protection Department; 2020. [Google Scholar]
- 6. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382:1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Regional Health Agency (AReSS) ‐ Apulia . Epidemia COVID‐19. 15 aprile. Bari, Italy: Bollettino Epidemiologico Regione Puglia; 2020. [Google Scholar]
- 8. Loeffelholz MJ, Tang YW. Laboratory diagnosis of emerging human coronavirus infections ‐ the state of the art. Emerg Microbes Infect. 2020;9:747‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Albano D, Bertagna F, Bertolia M, et al. Incidental findings suggestive of Covid‐19 in asymptomatic patients undergoing nuclear medicine procedures in a high prevalence region. J Nucl Med. 2020;61(5):632‐636. [DOI] [PubMed] [Google Scholar]
- 10. Siddiqi HK, Mehra MR. COVID‐19 illness in native and immunosuppressed states: a clinical‐therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jamil S, Mark N, Carlos G, Dela Cruz CS, Gross JE, Pasnick S. Diagnosis and management of COVID‐19 disease. Am J Respir Crit Care Med. 2020;201:P19‐P20. [DOI] [PubMed] [Google Scholar]
- 12. Gu J, Han B, Wang J. COVID‐19: gastrointestinal manifestations and potential fecal‐oral transmission. Gastroenterology. 2020;158:1518‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao QY, Chen YX, Fang JY. 2019 Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21:125‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang C, Shi L, Wang FS. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Portincasa P, Krawczyk M, Machill A, Lammert F, Ciaula AD. Hepatic consequences of COVID‐19 infection. Lapping or biting? Eur J Intern Med. 2020. 10.1016/j.ejim.2020.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maddaloni E, Buzzetti R. Covid‐19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020:e3321. 10.1002/dmrr.3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab Res Rev. 2020;e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Madjid M, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system. A review. JAMA Cardiol. 2020. 10.1001/jamacardio.2020.1286 [DOI] [PubMed] [Google Scholar]
- 20. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med. 2020;14:185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pedersen SF, Ho YC. SARS‐CoV‐2: a storm is raging. J Clin Invest. 2020;130:2202‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS‐CoV‐2 infection. Clin Chem Lab Med. 2020. 10.1515/cclm-2020-0188 [DOI] [PubMed] [Google Scholar]
- 28. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lillicrap D. Disseminated intravascular coagulation in patients with 2019‐nCoV pneumonia. J Thromb Haemost. 2020;18:786‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4‐week follow‐up. J Infect. 2020;80(6):639‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model‐based analysis. Lancet Infect Dis. 2020;20(6):669‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID‐19: a systematic review and meta‐analysis. Arch Acad Emerg Med. 2020;8:e35. [PMC free article] [PubMed] [Google Scholar]
- 33. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid‐19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ioannidis JPA. Coronavirus disease the harms of exaggerated information and non‐evidence‐based measures. Eur J Clin Invest. 2019;2020:e13223. [DOI] [PubMed] [Google Scholar]
- 35. Watts N, Amann M, Arnell N, et al. The 2019 report of The Lancet Countdown on health and climate change: ensuring that the health of a child born today is not defined by a changing climate. Lancet. 2019;394:1836‐1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mirsaeidi M, Motahari H, Taghizadeh Khamesi M, Sharifi A, Campos M, Schraufnagel DE. Climate change and respiratory infections. Ann Am Thorac Soc. 2016;13:1223‐1230. [DOI] [PubMed] [Google Scholar]
- 37. Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to the coronavirus (COVID‐19) fatality rate. Sci Total Environ. 2020;726:138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Conticini E, Frediani B, Caro D. Can atmospheric pollution be considered a co‐factor in extremely high level of SARS‐CoV‐2 lethality in Northern Italy? Environ Pollut. 2020;261:114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu Y, Xie J, Huang F, Cao L. Association between short‐term exposure to air pollution and COVID‐19 infection: evidence from China. Sci Total Environ. 2020;727:138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Di Ciaula A, Portincasa P. Diet and contaminants: driving the rise to obesity epidemics? Curr Med Chem. 2019;26:3471‐3482. [DOI] [PubMed] [Google Scholar]
- 41. Lorentzen HF, Benfield T, Stisen S, Rahbek C. COVID‐19 is possibly a consequence of the anthropogenic biodiversity crisis and climate changes. Dan Med J. 2020;67(5):A205025. [PubMed] [Google Scholar]
- 42. Keesing F, Belden LK, Daszak P, et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468:647‐652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guo Y, Gasparrini A, Armstrong B, et al. Global variation in the effects of ambient temperature on mortality: a systematic evaluation. Epidemiology. 2014;25:781‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Di Ciaula A, Portincasa P. The environment as a determinant of successful aging or frailty. Mech Ageing Dev. 2020;188:111244;in press. [DOI] [PubMed] [Google Scholar]
- 45. Zhang J, Litvinova M, Wang W, et al. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: a descriptive and modelling study. Lancet Infect Dis. 2020. 10.1016/S1473-3099(20)30230-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cetron M, Landwirth J. Public health and ethical considerations in planning for quarantine. Yale J Biol Med. 2005;78:329‐334. [PMC free article] [PubMed] [Google Scholar]
- 47. Koo JR, Cook AR, Park M, et al. Interventions to mitigate early spread of SARS‐CoV‐2 in Singapore: a modelling study. Lancet Infect Dis. 2020;20(6):678‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS‐CoV‐2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081‐2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the Achilles' heel of current strategies to control Covid‐19. N Engl J Med. 2020;382(22):2158‐2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roxby AC, Greninger AL, Hatfield KM, et al. Detection of SARS‐CoV‐2 among residents and staff members of an independent and assisted living community for older adults ‐ Seattle, Washington, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:416‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Remuzzi A, Remuzzi G. COVID‐19 and Italy: what next? The Lancet. 2020;395(10231):1225‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Verelst F, Kuylen E, Beutels P. Indications for healthcare surge capacity in European countries facing an exponential increase in coronavirus disease (COVID‐19) cases, March 2020. Euro Surveill. 2020;25. 10.2807/1560-7917.ES.2020.25.13.2000323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mannelli C. Whose life to save? Scarce resources allocation in the COVID‐19 outbreak. J Med Ethics. 2020;46(6):364‐366. [DOI] [PubMed] [Google Scholar]
- 54. Armocida B, Formenti B, Ussai S. Palestra F and Missoni E. The Italian health system and the COVID‐19 challenge. Lancet . Public Health. 2020;5(5):e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kamerow D. Covid‐19: Don't forget the impact on US family physicians. BMJ. 2020;368:m1260. [DOI] [PubMed] [Google Scholar]


