Abstract
We aim to evaluate the change in the diagnostic spectrum in dermatology outpatient applications compared to before COVID‐19. All patients were enrolled from the Department of Dermatology between February 12 and May 8, 2020, the duration of 4 weeks before COVID‐19 and 8 weeks after were analyzed in three parts consisting of 4 weeks. Data obtained from the database such as age, gender, diagnoses were anonymized. Repeated applications with the same diagnosis in 10 days after the first presentation were ignored. Compared to the pre‐outbreak, there was a 3.5‐fold decrease in dermatology applications in the first month after COVID‐19 and an 8.8‐fold in the second month. We found a significant increase in the frequency of diagnoses such as generalized pruritus, pityriasis rosea, alopecia areata, bacterial skin/mucosa diseases, and zona zoster after COVID‐19. The frequency declined in diseases such as verruca vulgaris, hyperpigmentation, skin tag, melanocytic nevus, and seborrheic keratosis/solar lentigo. It has been found that the frequencies of most diseases, including acne (⁓25% of patients), did not change. We think that many factors, such as affecting the quality of life, risk perception, increased stress burden may cause a change in the diagnostic distribution of the dermatology applications.
Keywords: clinical dermatology, COVID‐19, dermatologic diseases, diagnostic distribution, epidemiology
1. INTRODUCTION
Pneumonia cases caused by a newly identified coronavirus arose in Wuhan, China, on December 31, 2019. The World Health Organization (WHO) named the disease as the coronavirus disease 2019 (COVID‐19) on February 2020. 1 COVID‐19, which initiated in China, affected many countries in a short time and spread to the whole world. The first case in Turkey was seen on March 11, 2020, the day WHO declared a pandemic. 2 , 3 Although the disease affects all age groups, it is more common in men. 4 Some patients can be defined as a high‐risk group for death because it is more severe in the elderly and those with underlying comorbidity. It was reported that of the patients who died, 42.2% were aged 80 to 89 years, 32.4% were aged 70 to 79 years, 8.4% were aged 60 to 69 years. 5 Thus, we estimate that the risk perception in the population may differ according to age and gender.
After the first confirmed‐case, the government took step‐by‐step a series of radical measures, including curfews, to ensure social isolation due to arising new cases in all cities across the country within a short time. In this context, face‐to‐face education was stopped in schools and universities and distance education was started. Flexible working hours were set at all public institutions, including hospitals. Common areas such as shopping malls, hairdressers, barbershops, restaurants, places of worship, cafes, cinema, and theater were closed indefinitely. Domestic and international flights and intercity trips have been stopped. A curfew was imposed over the age of 65 and fewer than 20 as of the last week of March and the first week of April, respectively. In some cities, curfews were imposed on all age groups only on weekends and public holidays. The call “stay at home” to all citizens has been repeated continuously through all media channels. As a result of all these measures, there were over 167 410 identified cases and 4630 death of COVID‐19 by June 4, 2020, in Turkey per Worldometer, a reference website that provides real‐time world statistics. 6 The case fatality rate (~2.77%) in our country is slightly lower than the overall case fatality rate envisaged as 3% in the article published in Lancet on January 24. 7
During the pandemic, it has been recommended to limit the outpatient services and to develop mechanisms to filter elective applications until the outbreak is controlled. 8 We aim to evaluate the change in the frequency, profile and diagnostic spectrum of the patients who applied to Dermatology department, which may occur due to all these regulations, restrictions and COVID risk perception varying according to the patient. We ask to have a sense about the motivation reasons pushing to seek medical advice in such a period, to understand our patients better.
2. MATERIALS AND METHODS
All patients were enrolled from the Department of Dermatology and Venereology in the Regional Training and Research Hospital between February 12 and May 8, 2020. We evaluated the months within these dates to divide them into 4 weeks of five consecutive working days, excluding weekends and public holidays. Thus, a 3‐month period consisting of 4 weeks before the COVID‐19 outbreak and 8 weeks after the first confirmed case was achieved. Data such as age, gender, application dates, the first three of the ICD‐10 (International Classification of Diseases—10th Revision) codes entered into the system were obtained from the electronic registration database. These data were anonymized on the condition that individual uniqueness was maintained in applications to outpatient clinics. Patients with recurrent presentations for control or follow‐up and their diseases were identified. However, repeating applications with the same diagnosis in 10 days after the first presentation were ignored.
The reasons such as the wide diagnostic spectrum, lack of ICD‐10 code corresponding to each diagnosis, and the unique style of each doctor at the entrance of ICD‐10 codes in the dermatology practice necessitated the standardization of diagnostic data within the framework of certain principles in such a retrospective study. For this purpose, the first three ICD‐10 codes entered into the system for each patient were reassessed by each patient's own doctor, based on the classification of the diseases in Dermatology textbook edited by Bolognia et al 9 For the same patient, separate diagnoses related to each other were attempted to be expressed as a single origin diagnosis, free from sub‐breakings of the disease classification (eg, xerosis + dermatitis = xerotic eczema, pruritus ani + lichen simplex chronicus = lichen simplex chronicus, pruritus + atopic dermatitis = atopic dermatitis, etc.). Patients without an additional dermatological diagnosis other than pruritus were evaluated as the “idiopathic pruritus and dysesthesia.” A small number of patients who applied for consultation only and had no significant complaints were included in the title of “undetermined reason for examination and observation.” We considered only the first ICD‐10 codes for patients with multiple irrelevant diagnoses. Thanks to these principles, 371 different ICD‐10 codes, including sub‐breakings in the raw data, were reduced to 17 main titles and 90 subtitles. Then, the application date and the diagnoses of patients with repeated presentations were compared with each other via the program. In this way, according to the previous presentations of the relevant patient, application types could be classified under the names of normal and control/follow‐up.
This single‐center cross‐sectional retrospective‐study was approved by the Ethics Committee of Regional Training and Research Hospital, Erzurum, Turkey (Decision No: 2020/11‐122) and Ministry of Health Scientific Research Platform (Application form number: 2020‐05‐14T17_30_07). This study was conducted as per the latest version of the “Helsinki Declaration” and the “Guidelines for Good Clinical Practice.” No patient consent was required, provided that the data such as name and citizenship numbers were anonymized by the IT team and with the permission of the ethics committee. Nevertheless, informed consent for the study was obtained from 30 patients who were only interviewed by phone.
All statistical procedures were conducted using IBM SPSS Statistics® 21.0 and MS‐Excel® 2010. Due to studying with massive data, Python™ 3.7.5 program (Python Software Foundation, released in October 2019) was used primarily in determining the control and follow‐up patients and classifying the data.
Pearson's chi‐squared test was used for categorical variables. In chi‐square tests with a degree of freedom >1, pairwise comparisons (post‐hoc) were performed using the z‐test. After checking the normality distribution of scale variables by Kolmogorov‐Smirnov test, independent samples were compared with appropriate significance tests (Kruskal‐Wallis H test or Mann‐Whitney U test). Results were presented as the median (interquartile range) or count of the patients (percentage). The change in application frequency and age distribution by workdays and weeks was displayed with the “scatter with straight lines” graphic. Two‐sided P values of <.05 were considered statistically significant. Correction for alpha inflation (Bonferonni style) was applied as post‐hoc after the Kruskal‐Wallis H and chi‐square tests.
3. RESULTS
It was determined that 9531 (9.2%) of 104 142 applications to all clinical departments, except COVID‐19 outpatient clinic, were made to dermatology outpatient clinics within 12 weeks (12 February to May 8, 2020). As with the dermatology outpatient clinic, there was a gradually significant decrease in hospital‐wide applications (P < .001). Also, the proportion of those who visited the dermatology outpatient clinics, among applicants to the hospital was about 10.3% before COVID‐19 and then decreased statistically significantly to 7.3% and 6.9%, respectively (P < .001). 8905 different patients were evaluated at 9531 visits. 6554 (73.6%) patients with 6820 (71.5%) applications presented in the last 4‐weeks before COVID‐19. In the first and second months after COVID‐19, 1747 (19.6%) patients with 1940 (20.4%) applications and 652 (7.3%) patients with 771 (8.1%) applications attended to our outpatient clinics; respectively (Table 1).
TABLE 1.
Evaluation of age, gender, and frequency of application before and after COVID‐19
| Before COVID‐19 | After COVID‐19 | P value | |||
|---|---|---|---|---|---|
| Last 4‐weeks | 1st 4‐weeks | 2nd 4‐weeks | |||
| Number of applications to the all departments a (n = 104 142) | 66 418 (63.8%) | 26 518 (25.5%) | 11 206 (10.8%) | <.001*,**,*** | |
| Number of applications to the dermatology (n = 9531) | 6820 (71.5%) | 1940 (20.4%) | 771 (8.1%) | <.001*,**,*** | |
| Application type | The first and sole application, normal | 6167 (90.4%) | 1649 (85.0%) | 619 (80.3%) | <.001*,**,*** |
| Repeated application, control or follow‐up b | 653 (9.6%) | 291 (15.0%) | 152 (13.9%) | ||
| Age (year) | 24 (21) | 25 (20) | 29 (23) | <.001**,*** | |
| Gender | Male | 2949 (43.2%) | 895 (46.1%) | 385 (49.9%) | <.001** |
| Female | 3871 (56.8%) | 1045 (53.9%) | 386 (50.1%) | ||
Note: Data are expressed as the number of applications to the outpatient clinic (percentage). Kruskal‐Wallis H and Pearson's chi‐squared tests were used. Bonferroni correction was applied as post‐hoc (Mann‐Whitney U and z‐test, respectively) after Kruskal‐Wallis H and chi‐square tests. Significant values were shown in bold.
Other than COVID‐19 outpatient clinics.
Repeated applications within 10 days after the first application were excluded.
*Adjusted P value <.05 for the difference between “last 4‐weeks before COVID‐19” and “1st 4‐weeks after COVID‐19.”
**Adjusted P value <.05 for the difference between “last 4‐weeks before COVID‐19” and “2nd 4‐weeks after COVID‐19.”
***Adjusted P value <.05 for the difference between “1st 4‐weeks after COVID‐19” and “2nd 4‐weeks after COVID‐19.”
The changes in application frequencies, age, and gender distribution before and after COVID‐19 summarized in Table 1 and Figures 1 and 2. It was observed that after the first‐confirmed COVID‐19 case, the number of patients examined in the daily outpatient clinics has decreased from 350 to 150 in the first week, to 50 and below in the second week. The patient's median age, following a stable curve before COVID‐19, followed a fluctuating course during the outbreak, and the frequency of female‐dominant application turned out to be equal over time. Before and after COVID‐19, the diagnostic distribution of patients applied to the dermatology outpatient clinics for three periods consisting of 4‐weeks was as in Table 2.
FIGURE 1.
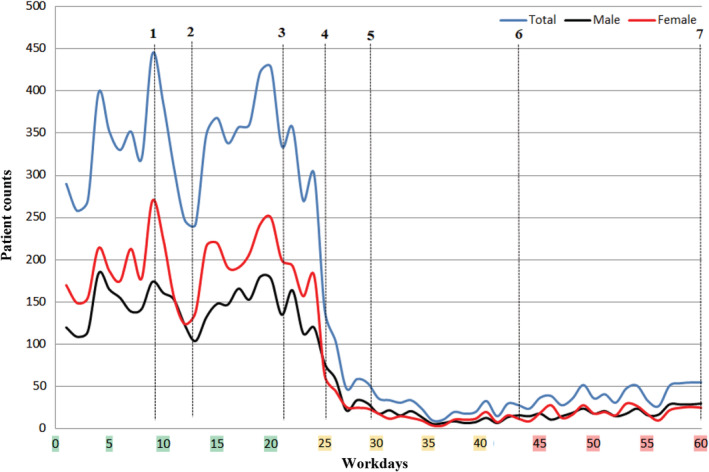
Change in daily patient frequency before and after COVID‐19 outbreak. (1) February 24: There was news that many illegal refugees from Iran, where has been fighting against the COVID‐19 outbreak, quarantined in our city. (2) February 28: It was announced that the refugees' COVID‐19 test results were negative. (3) March 11: The first COVID‐19 case was confirmed in Turkey, and the World Health Organization has declared a pandemic. (4) March 17: The first death due to COVID‐19 was reported in Turkey. (5) March 22: Curfew was declared indefinitely for individuals over the age of 65. (6) April 4: Curfew was declared indefinitely for individuals under the age of 20. (7) May 11: Country‐wide normalization process has been started
FIGURE 2.
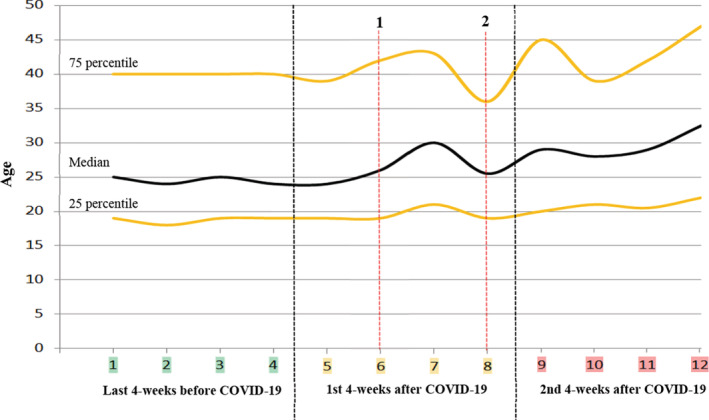
Change of age distribution of patients by the week before and after COVID‐19 outbreak. (1) March 22 (6th week): Curfew was declared indefinitely for individuals over the age of 65. (2) April 4 (8th week): Curfew was declared indefinitely for individuals under the age of 20
TABLE 2.
The diagnoses of patients attended to dermatology outpatient clinic
| Diseases | Before COVID‐19 | After COVID‐19 | P value | |||
|---|---|---|---|---|---|---|
| Last 4‐weeks (n = 6820) | 1st 4‐weeks (n = 1940) | 2nd 4‐weeks (n = 771) | ||||
| 1 | Pruritus and dysesthesia | 228 (3.3%) | 70 (3.6%) | 48 (6.2%) | <.001**,*** | |
| a | Idiopathic generalized pruritus | 225 (3.3%) | 63 (3.2%) | 48 (6.2%) | <.001**,*** | |
| b | Idiopathic pruritus ani, scroti, vulva and paresthesia | 3 (<0.1%) | 7 (0.4%) | 0 (0.0%) | N/A | |
| 2 | Papulosquamous and eczematous diseases | 1714 (25.1%) | 475 (24.5%) | 219 (28.4%) | .095 | |
| a | Psoriasis (all types) | 103 (1.5%) | 37 (1.9%) | 16 (2.1%) | .289 | |
| b | Lichen planus and other lichenoid dermatoses | 36 (0.5%) | 7 (0.4%) | 0 (0.0%) | .093 | |
| c | Lichen simplex chronicus | 49 (0.7%) | 7 (0.4%) | 5 (0.6%) | .219 | |
| d | Pityriasis lichenoides (acute and chronic) | 3 (<0.1%) | 2 (0.1%) | 2 (0.3%) | N/A | |
| e | Pityriasis rosea | 43 (0.6%) | 12 (0.6%) | 12 (1.6%) | .013**,*** | |
| f | Atopic dermatitis | 227 (3.3%) | 69 (3.6%) | 29 (3.8%) | .758 | |
| g | Seborrheic dermatitis | 287 (4.2%) | 88 (4.5%) | 44 (5.7%) | .148 | |
| h | Allergic/Irritant contact dermatitis | 294 (4.3%) | 101 (5.2%) | 33 (4.3%) | .233 | |
| i | Stasis dermatitis | 11 (0.2%) | 3 (0.2%) | 1 (0.1%) | N/A | |
| j | Pompholyx | 6 (0.1%) | 6 (0.3%) | 1 (0.1%) | N/A | |
| k | Diaper dermatitis | 14 (0.2%) | 1 (0.1%) | 1 (0.1%) | N/A | |
| l | Xerosis cutis and xerotic eczema | 363 (5.3%) | 73 (3.8%) | 46 (6.0%) | .011*,*** | |
| m | Other eczematous dermatitis and cheilitis | 278 (4.1%) | 69 (3.6%) | 28 (3.6%) | .527 | |
| 3 | Urticaria, erythema, and purpuras | 339 (5.0%) | 94 (4.8%) | 40 (5.2%) | .932 | |
| a | Urticaria and angioedema | 301 (4.4%) | 85 (4.4%) | 34 (4.4%) | .998 | |
| b | Figurate erythemas | 6 (0.1%) | 0 (0.0%) | 0 (0.0%) | N/A | |
| c | Erythema multiforme (minor and major) | 13 (0.2%) | 1 (0.1%) | 1 (0.1%) | N/A | |
| d | Drug reactions | 6 (0.1%) | 3 (0.2%) | 3 (0.4%) | N/A | |
| e | Vasculitis (small and medium vessel) | 6 (0.1%) | 1 (0.1%) | 1 (0.1%) | N/A | |
| f | Sweet syndrome | 1 (<0.1%) | 1 (0.1%) | 0 (0.0%) | N/A | |
| g | Spontaneous purpura and ecchymosis | 6 (0.1%) | 3 (0.2%) | 1 (0.1%) | N/A | |
| 4 | Autoimmune vesiculobullous diseases | 5 (0.1%) | 1 (0.1%) | 1 (0.1%) | N/A | |
| a | Pemphigus | 1 (<0.1%) | 0 (0.0%) | 0 (0.0%) | N/A | |
| b | Dermatitis herpetiformis | 4 (0.1%) | 1 (0.1%) | 1 (0.1%) | N/A | |
| 5 | Adnexal diseases | 1933 (28.3%) | 612 (31.5%) | 205 (26.6%) | .008*,*** | |
| a | Acne | 1775 (26.0%) | 582 (30.0%) | 194 (25.2%) | .001*,*** | |
| b | Rosacea and associated diseases | 98 (1.4%) | 17 (0.9%) | 6 (0.8%) | .067 | |
| c | Follicular occlusion tetrad | 26 (0.4%) | 7 (0.4%) | 4 (0.5%) | .824 | |
| d | Regional hyperhidrosis | 26 (0.4%) | 4 (0.2%) | 0 (0.0%) | .127 | |
| e | Miliaria | 8 (0.1%) | 2 (0.1%) | 1 (0.1%) | N/A | |
| 6 | Rheumatologic disorders | 22 (0.3%) | 10 (0.5%) | 2 (0.3%) | .406 | |
| a | Behçet's diseases | 20 (0.3%) | 6 (0.3%) | 1 (0.1%) | .700 | |
| b | RA, SLE, Scleroderma and associated diseases | 2 (<0.1%) | 4 (0.2%) | 1 (0.1%) | N/A | |
| 7 | Genodermatosis | 10 (0.1%) | 4 (0.2%) | 0 (0.0%) | N/A | |
| a | Ichthyosis | 5 (0.1%) | 3 (0.2%) | 0 (0.0%) | N/A | |
| b | Neurofibromatosis | 3 (<0.1%) | 0 (0.0%) | 0 (0.0%) | N/A | |
| 8 | Pigmentary disorders | 140 (2.1%) | 26 (1.3%) | 8 (1.0%) | .028** | |
| a | Vitiligo and other hypopigmentation disorders | 31 (0.5%) | 6 (0.3%) | 3 (0.4%) | .677 | |
| b | Hyperpigmentation (Melasma, ephelid, PIH) | 109 (1.6%) | 20 (1.0%) | 5 (0.6%) | .031** | |
| 9 | Hair disorders and nail disorders and mucous membranes | 480 (7.0%) | 115 (5.9%) | 49 (6.4%) | .205 | |
| a | Telogen effluvium | 176 (2.6%) | 38 (2.0%) | 14 (1.8%) | .158 | |
| b | Androgenic alopecia | 37 (0.5%) | 13 (0.7%) | 1 (0.1%) | .217 | |
| c | Alopecia areata | 95 (1.4%) | 29 (1.5%) | 21 (2.7%) | .017** | |
| d | Trichotillomania | 2 (<0.1%) | 0 (0.0%) | 1 (0.1%) | N/A | |
| e | Cicatricial alopecia | 2 (<0.1%) | 0 (0.0%) | 0 (0.0%) | N/A | |
| f | Hirsutism and hypertrichosis | 9 (0.1%) | 3 (0.2%) | 0 (0.0%) | N/A | |
| g | Ingrown toenail | 26 (0.4%) | 7 (0.4%) | 2 (0.3%) | .868 | |
| h | Nail dystrophies and others | 48 (0.7%) | 8 (0.4%) | 1 (0.1%) | .072 | |
| i | Oral candidiasis, glossodynia, and stomatitis | 36 (0.5%) | 8 (0.4%) | 2 (0.3%) | .525 | |
| j | Recurrent aphthous stomatitis | 45 (0.7%) | 9 (0.5%) | 6 (0.8%) | .543 | |
| k | Mucocel | 2 (<0.1%) | 0 (0.0%) | 1 (0.1%) | N/A | |
| 10 | Infections and infestations and bites | 1390 (20.4%) | 402 (20.7%) | 161 (20.9%) | .911 | |
| a | Bacterial skin/mucosa diseases | 184 (2.7%) | 58 (3.0%) | 39 (5.1%) | .001**,*** | |
| b | Lymphangitis and thrombophlebitis | 1 (<0.1%) | 1 (0.1%) | 0 (0.0%) | N/A | |
| c | Tinea capitis and kerion | 34 (0.5%) | 6 (0.3%) | 3 (0.4%) | .479 | |
| d | Pityriasis versicolor | 76 (1.1%) | 27 (1.4%) | 8 (1.0%) | 0.569 | |
| e | Anogenital candidiasis and erythema intertrigo | 49 (0.7%) | 13 (0.7%) | 2 (0.3%) | .335 | |
| f | Other superficial fungal skin//nail infections | 332 (4.9%) | 110 (5.6%) | 38 (4.9%) | .565 | |
| g | Molluscum contagiosum | 22 (0.3%) | 1 (0.1%) | 1 (0.1%) | N/A | |
| h | Herpes simplex infections (genital and non‐genital) | 53 (0.8%) | 12 (0.8%) | 8 (1.0%) | .736 | |
| i | Varicella | 7 (0.1%) | 1 (0.1%) | 2 (0.3%) | N/A | |
| j | Zona zoster and post‐zoster nevralgia | 58 (0.9%) | 22 (1.1%) | 22 (2.9%) | <.001**,*** | |
| k | Verruca vulgaris | 357 (5.2%) | 71 (3.7%) | 12 (1.6%) | <.001*,**,*** | |
| l | Anogenital warts | 47 (0.7%) | 26 (1.3%) | 4 (0.5%) | .012* | |
| m | Gonorrhea | 1 (<0.1%) | 0 (0.0%) | 0 (0.0%) | N/A | |
| n | Other viral diseases (defined and undefined) | 10 (0.1%) | 1 (0.1%) | 0 (0.0%) | N/A | |
| o | Scabies | 138 (2.0%) | 41 (2.1%) | 20 (2.6%) | .574 | |
| p | Pediculosis | 5 (0.1%) | 2 (0.1%) | 0 (0.0%) | N/A | |
| q | Insect bite | 16 (0.2%) | 7 (0.4%) | 2 (0.3%) | .631 | |
| 11 | Physical agents related disorders | 127 (1.9%) | 27 (1.4%) | 9 (1.2%) | .177 | |
| a | Polymorph light eruption | 7 (0.1%) | 4 (0.2%) | 2 (0.3%) | N/A | |
| b | Burns (sun, thermal, etc.) | 9 (0.1%) | 1 (0.1%) | 1 (0.1%) | N/A | |
| c | Erythema ab igne | 5 (0.1%) | 1 (0.1%) | 0 (0.0%) | N/A | |
| d | Corn and callus | 106 (1.6%) | 21 (1.1%) | 6 (0.8%) | .092 | |
| 12 | Langerhans cells and macrophage associated diseases | 5 (0.1%) | 1 (0.1%) | 1 (0.1%) | N/A | |
| a | Xanthomas and xanthelasmas | 4 (0.1%) | 0 (0.0%) | 0 (0.0%) | N/A | |
| b | Non‐infectious granulomas and granulomatous diseases | 1 (<0.1%) | 1 (0.1%) | 1 (0.1%) | N/A | |
| 13 | Atrophies and dermal connective tissue diseases | 95 (1.4%) | 19 (1.0%) | 0 (0.0%) | .002**,*** | |
| a | Morphea and lichen sclerosus et atrophicus | 3 (<0.1%) | 0 (0.0%) | 0 (0.0%) | N/A | |
| b | Hypertrophic scars and keloids | 29 (0.4%) | 6 (0.3%) | 0 (0.0%) | .161 | |
| c | Skin tags | 55 (0.8%) | 10 (0.5%) | 0 (0.0%) | .022** | |
| d | Connective tissue atrophies | 8 (0.1%) | 3 (0.2%) | 0 (0.0%) | N/A | |
| 14 | Panniculitis | 1 (<0.1%) | 0 (0.0%) | 6 (0.8%) | NA | |
| 15 | Vascular disorders | 23 (0.3%) | 9 (0.5%) | 5 (0.6%) | .351 | |
| a | Raynaud's syndrome | 2 (<0.1%) | 0 (0.0%) | 0 (0.0%) | N/A | |
| b | Pernio | 6 (0.1%) | 0 (0.0%) | 1 (0.1%) | N/A | |
| c | Chronic skin ulcers | 2 (<0.1%) | 1 (0.1%) | 0 (0.0%) | N/A | |
| d | Peripheral vascular diseases and lymphedema | 1 (<0.1%) | 3 (0.2%) | 2 (0.3%) | N/A | |
| e | Pyogenic granuloma | 12 (0.2%) | 5 (0.3%) | 2 (0.3%) | NA | |
| 16 | Neoplasms | 210 (3.1%) | 51 (2.6%) | 10 (1.3%) | .015** | |
| a | Melanocytic nevus | 64 (0.9%) | 11 (0.6%) | 1 (0.1%) | .025** | |
| b | Basal cell carcinoma | 10 (0.1%) | 1 (0.1%) | 0 (0.0%) | N/A | |
| c | Squamous cell carcinoma | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | N/A | |
| d | Epidermal cyst | 39 (0.6%) | 10 (0.5%) | 4 (0.5%) | .947 | |
| e | Lipoma | 8 (0.1%) | 2 (0.1%) | 0 (0.0%) | N/A | |
| f | Seborrheic keratosis and solar lentigo | 25 (0.4%) | 14 (0.7%) | 0 (0.0%) | .017** | |
| g | Actinic keratosis | 30 (0.4%) | 5 (0.3%) | 2 (0.3%) | .437 | |
| h | Hemangiomas | 9 (0.1%) | 2 (0.1%) | 1 (0.1%) | N/A | |
| i | Mastocytosis | 1 (<0.1%) | 0 (0.0%) | 0 (0.0%) | N/A | |
| j | Other benign epithelial tumors and proliferations (undefined) | 25 (0.4%) | 6 (0.3%) | 1 (0.1%) | .258 | |
| 17 | Undetermined reason for examination and observation | 97 (1.4%) | 24 (1.2%) | 7 (0.9%) | .452 | |
Abbreviations: N/A, not applicable, PIH, post‐inflammatory hyperpigmentation, RA, rheumatoid arthritis, SLE, systemic lupus erythematosus.
Note: Data are expressed as the number of applications to the outpatient clinic (column percentage). Pearson's chi‐square test was used. Bonferroni correction was applied as post‐hoc (z‐test) after chi‐square tests. Significant values were shown in bold.
*Adjusted P value <.05 for the difference between “last 4‐weeks before COVID‐19” and “1st 4‐weeks after COVID‐19.”
**Adjusted P value <.05 for the difference between “last 4‐weeks before COVID‐19” and “2nd 4‐weeks after COVID‐19.”
***Adjusted P value <.05 for the difference between “1st 4‐weeks after COVID‐19” and “2nd 4‐weeks after COVID‐19.”
The repeated applications in the 3 months belonged to 518 patients, and their number of visits ranged from 2 to 5. Respectively, 234 (45.2%) and 59 (11.4%) of them were diagnosed with acne and verruca vulgaris. Others applied to the outpatient clinics more than once with the following diagnoses: various eczematous dermatitis (28, 5.4%), urticaria/angioedema (n = 20, 3.9%), anogenital warts (19, 3.7%), idiopathic pruritus (17, 3.3%), scabies (17, 3.3%), superficial skin/nail fungal infection (15, 2.9%), atopic dermatitis (14, 2.7%), psoriasis (11, 2.1%), bacterial skin/mucosa diseases (10, 1.9%), and callus (87, 1.4%), respectively. Besides, a total of 67 (12.9%) patients with 30 different diagnoses consisting of 1‐5 patients, applied to the outpatient clinic at least two times. The rate of repeated applications was statistically significantly higher during the outbreak compared to before (P < .001). 10.3% of 2276 acne patients examined in these 3 months had repeated application. Apart from this, the diagnoses of those who applied to the outpatient clinic more than once during this period were as follows in order of frequency: anogenital warts (n = 19/56, 33.9%), verruca vulgaris (59/365, 16.2%), scabies (17/180, 9.4%), psoriasis (11/140,7.9%), idiopathic generalized pruritus (17/315, 5.4%), urticaria/angioedema (20/394, 5.1%), atopic dermatitis (14/308, 4.5%), bacterial skin/mucosa diseases (10/268, 3.7%), and superficial fungal skin/nail infections (15/503, 3.0%).
4. COMMENT
After the COVID‐19 pandemic, which rapidly affected the whole world, there were significant changes in the application to outpatient clinics due to reasons such as measures, call to “stay at home”, and panic in the community. It was understood that the number of patients decreased for the first time in the period when there was news that the refugees from Iran quarantined in our city. However, it increased again quite rapidly with the announcement of favorable developments (Figure 1). It is noteworthy that a slight fluctuation occurred in the median age of patients who applied to outpatient clinics during this period. We have found a logarithmic decline in the frequency of application to our outpatient clinics within about 2 weeks after the first confirmed case was announced. This shows how effective awareness‐raising campaigns are in reducing application to hospitals without any legal restrictions yet. Compared to the pre‐pandemic, there was a 3.5‐fold decrease in applications to dermatology outpatient clinics in the first month after COVID‐19 as a transition period, and an 8.8‐fold decrease in the second month. In general hospital applications, there was a decrease of 2.5 times and then 5.9 times, respectively. So, compared to other departments, there was a more obvious loss in applications to dermatology outpatient clinics. While there was female dominance in dermatology before COVID‐19, the gradual equalization of the female to male ratio indicates that the risk perception does not differ in terms of gender. After the first confirmed case, the median age line slipped up, and the curve became more stable with the introduction of a curfew for <20 years of age. The slight movements of the curve before curfews and the fluctuating course until the curfew for <20 years of age indicate that the risk perception varies by age. However, the young age group exhibits inconsistent behavior in outpatient applications. Although the risk of death from COVID‐19 is lower in young individuals, they can cause the disease to spread rapidly. 5 Considering their inconsistent behavior, we understood that the curfew brought to this age group is crucial.
There were significant changes in the distribution of diagnosis for some dermatologic diseases after the outbreak (Table 3). Considering that the frequency of application to the outpatient clinic captured a stable curve after 2 weeks, we found it appropriate to consider the first month after COVID‐19 as a transition period. When evaluating the change in frequency distributions, we commented on the results between “last 4‐weeks before COVID‐19” and “2nd 4‐weeks after COVID‐19” to reveal the difference more clearly. In our study, we found a significant increase in the frequency of diagnoses such as idiopathic generalized pruritus, pityriasis rosea, alopecia areata, bacterial skin/mucosa diseases, and zona zoster/post‐zoster neuralgia after COVID‐19. In such an analysis to be made at an ordinary time, the frequency changes will suggest that it is due to the seasonal relationship of the disease and the change in its frequency in the community. In such an extraordinary period, it can be assumed that the causes such as the differentiation of personal risk perception due to age, comorbidity, occupation, etc., increased social stress, and changing personal hygiene habits may alter the diagnostic distribution. Various studies have shown that psychological disorders such as anxiety and depression increase in the community during the COVID‐19 outbreak. 10 , 11 It is known that diseases such as idiopathic generalized pruritus, alopecia areata, and zona zoster/post‐zoster neuralgia are associated with stress. 12 , 13 Besides, these diseases can significantly affect the quality of life (QoL). 14 , 15 , 16 Therefore, the needs of patients to seek medical advice may be superior to the risk perception of COVID‐19. The increase in bacterial skin/mucosa diseases may be through the shifts from departments such as Infectious Diseases and Internal Medicine, which have a primary role in outbreak management. Also, it may be secondary to xerosis and eczema, the frequency of which is reported an increase due to changes in personal hygiene behaviors. 17 , 18 The absence of an increase in the frequency of xerotic eczema and contact dermatitis in our study should not be concluded that such complaints do not increase in the community. Because, based on many studies and our experience, we think that the frequency of xerosis cutis and contact dermatitis increases in the community. 18 , 19 The absence of an increase can be explained by the reasons such as predicting the etiology by patients, not associating with COVID‐19, not being experienced for the first time and easily treating the disease with the use of a moisturizer. The increase in the frequency of pityriasis rosea can be explained by the opposite of the reasons listed for contact dermatitis. Because pityriasis rose‐like lesions have been reported among the cutaneous findings of COVID‐19, but it may also cause undue anxiety in patients who experience the disease for the first time. 20 Another reason is that pityriasis rosea increases in the spring seasons, which coincides with the period after COVID‐19. 21 Although a significant increase in the frequency of panniculitis is unusual, statistical analysis could not be performed due to the low number of cases. In 20% of COVID‐19 patients, many skin findings, except panniculitis, such as erythematous rash, petechia, urticaria, vesicular rash, livedo reticularis, and pernio have been reported. 22 , 23 All pityriasis rosea (n = 24) and panniculitis (n = 6) patients diagnosed after COVID‐19 were contacted by phone, and any suspicious condition related to COVID‐19 was questioned. However, no patient had an anamnesis associated with COVID‐19.
TABLE 3.
Change in diagnostic distribution of patients applied to dermatology outpatient clinic
| Change in distribution | Diseases a | Possible remarks/motivations b |
|---|---|---|
| Increase in frequency, significantly | Idiopathic generalized pruritus, pityriasis rosea, alopecia areata, bacterial skin/mucosa diseases, zona zoster/post‐zoster neuralgia |
1. Increasing incidence of the disease in the community. 2. Some diseases cause more serious impairment in quality of life and/or more anxiety or vice versa. 3. It is not desired to be neglected considering that a disease encountered for the first time may be related to COVID‐19. 4. For a disease whose treatment has already begun, the cure is not desired to be left in half. 5. The possible relationship of some diseases with risky age groups or certain comorbidities. 6. Applications to departments such as Infectious Diseases, Internal Medicine playing a primary role in outbreak management may shift to other departments. 7. Treatment of some diseases is not complicated and alternative methods can be applied easily. |
| No significant change in frequency | Psoriasis, atopic dermatitis, seborrheic dermatitis, allergic/irritant contact dermatitis, urticaria/angioedema, acne, rosacea, Behçet's diseases, vitiligo/other hypopigmentation disorders, telogen effluvium, androgenic alopecia, ingrown toenail, oral candidiasis‐glossodynia/stomatitis, recurrent aphthous stomatitis, superficial fungal infections, pityriasis versicolor, herpes simplex infections, varicella, scabies, callus, epidermal cyst, actinic keratosis | |
| Decreases in frequency, significantly | Hyperpigmentation (melasma, ephelides, post‐inflammatory hyperpigmentation), verruca vulgaris, skin tags, melanocytic nevus, seborrheic keratosis/solar lentigo |
Note: It was designed according to diseases with significant differences between “last 4‐weeks before COVID‐19” and “2nd 4‐weeks after COVID‐19.”
See Table 2 for the complete list.
One or more remarks may be appropriate, provided that the nature of the disease is considered.
It was found that the frequencies of most diseases did not change. Acne, which is one‐quarter of the applications among all diseases, was in this group. Various studies have shown that depression, anxiety disorders, and body dysmorphic disorder are higher in acne patients. 24 , 25 Also, it was reported that there was a significant effect on the QoL, depending on the severity of acne. 26 Some diseases (such as psoriasis, urticaria/angioedema and Behçet's disease) requiring follow‐up can seriously impair patients' QoL when they are severe. 27 Nevertheless, the absence of frequency increase in diseases in this group can be explained by one or more of the following reasons: The risk perception of patients in this group is similar, the treatment plan for chronic diseases is pre‐drawn, and some diseases do not cause severe deterioration in their QoL.
The frequency decline in diseases such as verruca vulgaris, hyperpigmentation, skin tag, melanocytic nevus, seborrheic keratosis/solar lentigo suggested that these diseases do not affect the QoL too much or that such diseases can be ignored during the outbreak. It was reported that melasma compared to vitiligo is less associated with depression, anxiety, and somatoform disorders, though it causes more severe impairment of life quality. 28 Unlike hyperpigmentation, the constant frequency of hypopigmented skin diseases suggested that the motivation for outpatient applications is different for each disease.
Acne (45.2%), verruca vulgaris (11.4%), urticaria/angioedema (3.9%), and anogenital warts (3.7%) were the primary ones in the distribution of patients with repeated applications within 3 months. Besides, anogenital warts (33.9%), verruca vulgaris (16.2%), acne (10.3%), and scabies (9.4%) were at the forefront in the frequency of disease‐specific repeated applications. It was understood that the patients who desired treatment (drug or procedure) and experienced treatment failure needed to come to the polyclinic more than once during the pandemic. Indeed, the frequency of repeated application was significantly higher in the post‐COVID‐19 period than before.
The study has certain limitations. Since the study was retrospective, we had no data such as detailed clinical examinations, life quality, anxiety levels, their treatments, and individual risk perception for COVID‐19. Patients often have multiple independent complaints, and sharing their complaints without prioritization may change the order of the diagnoses in the database. Besides, due to the extreme diversity of dermatological diseases, dermatologists work within a narrow framework for the use of ICD‐10 in clinical practice.
This study was carried out to understand the reasons why patients with dermatological diseases with low urgency and mortality come to the hospital in such a worldwide fatal pandemic period. Many factors such as affecting the QoL, risk perception varying according to the individual, increased stress burden, dermatological rashes encountered for the first time caused the change in the diagnostic distribution of the dermatology applications during the pandemic period. However, the motivation for application to dermatology outpatient clinics was not similar for all diseases and related to the nature of the disease.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors have contributed significantly, and all authors agree with the content of the manuscript.
ACKNOWLEDGMENT
We would like to thanks Nuri Binici in the IT team for his support in writing code on Python.
Turan Ç, Metin N, Utlu Z, Öner Ü, Kotan ÖS. Change of the diagnostic distribution in applicants to dermatology after COVID‐19 pandemic: What it whispers to us? Dermatologic Therapy. 2020;33:e13804. 10.1111/dth.13804
REFERENCES
- 1. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak: an update on the status. Mil Med Res. 2020;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singhal T. A review of coronavirus disease‐2019 (COVID‐19). Indian J Pediatr. 2020;87:281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Republic of Turkey Ministry of Health, General Directorate of Public Health . COVID‐19 (SARS‐CoV2 Infection) Guide (Science Board Study): General Information, Epidemiology and Diagnosis. Ankara: Republic of Turkey Ministry of Health Website. 2020. https://covid19bilgi.saglik.gov.tr/depo/rehberler/covid-19-rehberi/COVID-19_REHBERI_GENEL_BILGILER_EPIDEMIYOLOJI_VE_TANI.pdf. Accessed June 1, 2020. [Google Scholar]
- 4. Cheng ZJ, Shan J. 2019 novel coronavirus: where we are and what we know. Infection. 2020;48:155‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Remuzzi A, Remuzzi G. COVID‐19 and Italy: what next? Lancet. 2020;395:1225‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. COVID‐19 Coronavirus Pandemic . Worlometers website. Updated June 4, 2020. Accessed June 4, 2020. https://www.worldometers.info/coronavirus/
- 7. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet. 2020;395:473‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kowalski LP, Sanabria A, Ridge JA, et al. COVID‐19 pandemic: effects and evidence‐based recommendations for otolaryngology and head and neck surgery practice. Head Neck. 2020;42(6):1259‐1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bolognia J, Schaffer J, Cerroni L. Dermatology. Vol 1. 4th ed. China: Elsevier; 2017. [Google Scholar]
- 10. Ni MY, Yang L, Leung CM, et al. Mental health, risk factors, and social media use during the COVID‐19 epidemic and cordon sanitaire among the community and health professionals in Wuhan, China: cross‐sectional survey. JMIR Ment Health. 2020;7:e19009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ozamiz‐Etxebarria N, Dosil‐Santamaria M, Picaza‐Gorrochategui M, Idoiaga‐Mondragon N. Stress, anxiety, and depression levels in the initial stage of the COVID‐19 outbreak in a population sample in the northern Spain. Cad Saude Publica. 2020;36:e00054020. [DOI] [PubMed] [Google Scholar]
- 12. Livengood JM. The role of stress in the development of herpes zoster and postherpetic neuralgia. Curr Rev Pain. 2000;4:7‐11. [DOI] [PubMed] [Google Scholar]
- 13. Osman OT, Mufaddel A, Almugaddam F, Augusterfer EF. The psychiatric aspects of skin disorders. Expet Rev Dermatol. 2011;6:195‐209. [Google Scholar]
- 14. Putri Mellaratna W, Jusuf NK, Yosi A. The impact of pain intensity on quality of life of postherpetic neuralgia patients. Medicinski Glasnik: Official Publication of the Medical Association of Zenica‐doboj Canton, Bosnia and Herzegovina. 2020;17(2). 10.17392/1111-20. [DOI] [PubMed] [Google Scholar]
- 15. Mizukami A, Sato K, Adachi K, et al. Impact of herpes zoster and post‐herpetic neuralgia on health‐related quality of life in Japanese adults aged 60 years or older: results from a prospective, observational cohort study. Clin Drug Invest. 2018;38:29‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Erturk IE, Arican O, Omurlu IK, Sut N. Effect of the pruritus on the quality of life: a preliminary study. Ann Dermatol. 2012;24:406‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haslund P, Bangsgaard N, Jarlov J, et al. Staphylococcus aureus and hand eczema severity. Br J Dermatol. 2009;161:772‐777. [DOI] [PubMed] [Google Scholar]
- 18. Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID‐19) epidemic. J Am Acad Dermatol. 2020;82:1085‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health‐care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;e13310. 10.1111/dth.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020. [Published online ahead of print, 2020 May 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ayanlowo O, Akinkugbe A, Olumide Y. The pityriasis rosea calendar: a 7 year review of seasonal variation, age and sex distribution. Nig Q J Hosp Med. 2010;20:29‐31. [DOI] [PubMed] [Google Scholar]
- 22. Türsen Ü, Türsen B, Lotti T. Coronavirus: days in dermatology. Dermatol Ther. 2020. [Published online ahead of print, 2020 Apr 19]. [Google Scholar]
- 23. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5):e212‐e213. [DOI] [PubMed] [Google Scholar]
- 24. Sereflican B, Tuman TC, Tuman BA, Parlak AH. Type D personality, anxiety sensitivity, social anxiety, and disability in patients with acne: a cross‐sectional controlled study. Postepy Dermatol Alergol. 2019;36:51‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dogruk Kacar S, Ozuguz P, Bagcioglu E, et al. The frequency of body dysmorphic disorder in dermatology and cosmetic dermatology clinics: a study from Turkey. Clin Exp Dermatol. 2014;39:433‐438. [DOI] [PubMed] [Google Scholar]
- 26. Vilar GN, Santos LA, Sobral Filho JF. Quality of life, self‐esteem and psychosocial factors in adolescents with acne vulgaris. An Bras Dermatol. 2015;90:622‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanclemente G, Burgos C, Nova J, et al. The impact of skin diseases on quality of life: a multicenter study. Actas Dermosifiliogr. 2017;108:244‐252. [DOI] [PubMed] [Google Scholar]
- 28. Dabas G, Vinay K, Parsad D, Kumar A, Kumaran MS. Psychological disturbances in patients with pigmentary disorders: a cross‐sectional study. J Eur Acad Dermatol Venereol. 2020;34:392‐399. [DOI] [PubMed] [Google Scholar]


