Abstract
Time to improvement is a crucial characteristic for effective treatments of chronic inflammatory conditions, such as psoriasis. Apremilast is a recently approved drug, belonging to the small molecule phosphodiesterase 4 inhibitors, whose optimal safety and efficacy profile is somewhat affected by slow activity rate in clinical trials. Real world case series are suggesting a more consistent improvement, and with this additional personal investigation on 48 patients, we signal that 58% of patients achieved Psoriasis Area and Severity Index (PASI) 50, and 19% PASI 75 improvement in the first 8 weeks of treatment. Results at 16‐week are remarkable, with overall 55% of patients achieving PASI 75, 21% PASI 90 and 14% PASI 100. Only 8 patients (18, 6%) had slightly improved, although satisfied with the regimen, and determined to continue. Noteworthy, our population was rather problematic in terms of comorbidities (86%), and resistance to other treatments, with only 28% naïve to systemics, including biologics. Moreover, the observation period includes the Italian outbreak of COVID‐19 epidemic, and further information on apremilast safety are provided, no one of the patients having stopped treatment. In such a critical period, the apremilast satisfactory speed of therapeutic response in a real‐world setting has further strengthens patient's compliance to remain safely at home, which is the best strategy to limit contagion.
Keywords: apremilast, COVID‐19, efficacy, psoriasis, safety
1.
Apremilast is an innovative small‐molecule phosphodiesterase 4 inhibitor that has been approved for the treatment of moderate‐to‐severe plaque psoriasis in adult patients; it has a remarkable efficacy and safety profile and does not require particular screening or follow‐up blood tests. 1 The main criticism seems to be its slower activity, in terms of the time required for improvement, with a low rate of Psoriasis Area and Severity Index (PASI) 75 achievement at week 16 in clinical trials: 33.1% of patients in ESTEEM1 and 28% of patients in ESTEEM2. 2 , 3 However, in daily practice, the results were much more satisfactory from the first follow‐up, even in very difficult patients. Thus, a study was conducted in a real‐world setting at the Dermatology Clinic of the University of Cagliari to investigate the short‐term clinical rate of response: at 8 and 16 weeks of treatment. The secondary endpoints were efficacy and safety for difficult‐to‐treat areas and challenging comorbidities. Data collection was initiated in December 2019, and 2 months later, the novel coronavirus disease (COVID‐19) outbreak emerged, spreading across Italy and raising concerns about the risk of contagion and severity of COVID‐19 in immunosuppressed patients. 4 Thus, enhanced surveillance has been arranged in this cohort of patients, advising them to continue the treatment and contact the psoriasis service on noticing the occurrence of any symptoms.
Forty‐eight patients were recruited after they provided informed consent to participate in the study, comprising 25 men and 23 women, with a mean age of 59 years (range, 20‐82). Each patient was administered apremilast (30 mg, twice a day) as monotherapy, after initial titration to minimize side effects. A wide range of different subtypes of psoriasis was included, with 56% of patients affected by difficult‐to‐treat conditions, including palmoplantar psoriasis, scalp psoriasis, and three sub‐erythrodermic forms (Table 1). Comorbidities with contraindications to other treatments were present in 86% of patients: hypertension and dysmetabolic syndrome, a history of malignancies (mammary tumor, bladder cancer, or colorectal cancer), severe viral infections (human immunodeficiency virus, hepatitis C virus, hepatitis B virus), latent tuberculosis, psychiatric disorders, and advanced‐stage liver disease. Only 14 (29%) patients were naïve to systemic therapies, while several courses of traditional systemic treatment were used in 19 (39%) patients, and 11 patients (23%) were required to discontinue biologics because of cancer occurrence or hepatic impairment. Four patients were treated with narrow‐band ultraviolet B‐rays without consistent improvement (Table 2).
TABLE 1.
Baseline patient characteristics concerning type of psoriasis, previous treatments and comorbidities
| No of patients | Percentage | |
|---|---|---|
| Type of psoriasis | ||
| Chronic plaque psoriasis | 20/48 | 42 |
| Palmoplantar psoriasis | 10/48 | 21 |
| Psoriasis of the scalp | 13/48 | 27 |
| Sub‐erythrodermic psoriasis | 3/48 | 6 |
| Facial psoriasis | 2/48 | 4 |
| Previous treatments | ||
| Totally naive to systemics | 14/48 | 29.1 |
| Cyclosporine | 10/48 | 20.8 |
| Methotrexate | 7/48 | 14.5 |
| Acitretin | 2/48 | 4.1 |
| Etanercept | 1/48 | 2.1 |
| Infliximab | 1/48 | 2.1 |
| Adalimumab | 6/48 | 12.5 |
| Ustekinumab | 2/48 | 4.1 |
| UVB narrow band therapy | 4/48 | 8.3 |
| Comorbidities | ||
| None | 7/48 | 14.5 |
| 1‐2 | 23/48 | 47.9 |
| >2 | 18/48 | 37.5 |
| Type of comorbidities | ||
| Hypertension | 13/48 | 27 |
| Hyperlipidemia | 11/48 | 23 |
| Diabetes mellitus | 7/48 | 16 |
| Malignancy in personal history | 13/48 | 27 |
| Psychiatric disorders | 6/48 | 12 |
| Severe infections | 7/48 | 15 |
| Liver disease | 3/48 | 6 |
| Latent tuberculosis | 2/48 | 4 |
| Other | 7/48 | 15 |
TABLE 2.
Clinical response to apremilast
| No of patients | Percentage | ||
|---|---|---|---|
| Primary endpoints at 8 wk | |||
| PASI 50 | 28/48 | 58.3 | |
| PASI 75 | 9/48 | 18.7 | |
| Drug discontinuation for AE | 4/48 | 8.3 | |
| Mild AEs | 16/48 | 33.3 | |
| Primary endpoints at 16 wk | |||
| PASI 50 | 34/43 | 79 | |
| PASI 75 | 24/43 | 55 | |
| PASI 90 | 9/43 | 20.9 | |
| PASI 100 | 6/43 | 13.9 | |
| Treatment failure | 1/48 | 2 | |
| Mean PASI | T0 | T8 | T16 |
| PASI | 10.44 | 5 | 3.3 |
Abbreviation: PASI, Psoriasis Area and Severity Index.
At the 8‐week follow‐up (Table 2), the primary efficacy endpoint of consistent improvement, quantified as at least a 50% reduction (Figure 1) in baseline signs and symptoms (PASI 50), was achieved in 58.3% of patients (28/48), 9 of whom (18.7%) achieved PASI 75 (Figure 2). Four patients discontinued treatment because of the development of gastrointestinal adverse events (AEs). The remaining 17 patients showed a less consistent improvement, although considering the absolute PASI, there was a mean reduction from 9.2 to 6.4, and patients were satisfied with the treatment.
FIGURE 1.
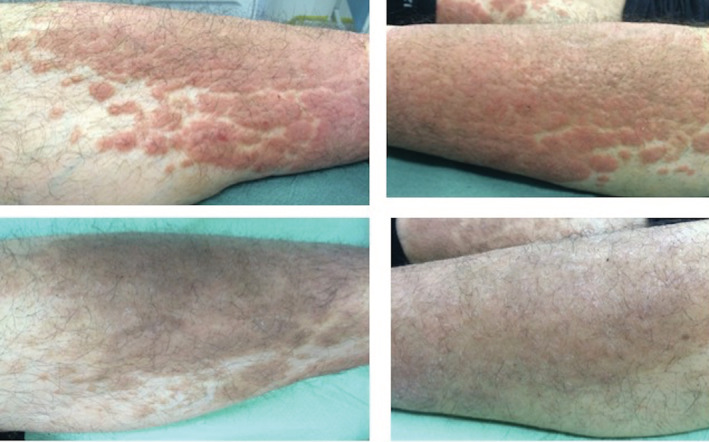
Improvement on infiltrated plaque psoriasis of the legs, the patient overall achieving PASI 50 after 8 weeks of apremilast monotherapy. PASI, Psoriasis Area and Severity Index
FIGURE 2.
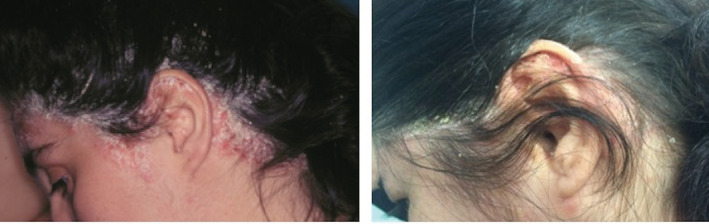
Improvement on severe scalp psoriasis, the patient overall achieving PASI 75 after 8 weeks of apremilast monotherapy. PASI, Psoriasis Area and Severity Index
At the 16‐week follow‐up, 43 patients were assumed to be taking apremilast, and another patient had discontinued taking apremilast because of treatment failure. The improvement was remarkable, with an additional 6 patients achieving PASI 50. Overall, 55% of patients achieved PASI 75, 21% achieved PASI 90, and 14% achieved PASI 100 (Figure 3), with a mean absolute PASI score of 3.29 (Table 2). Only 8 patients (18.6%) showed slight improvements in symptoms, although they were satisfied with the regimen and were determined to continue.
FIGURE 3.
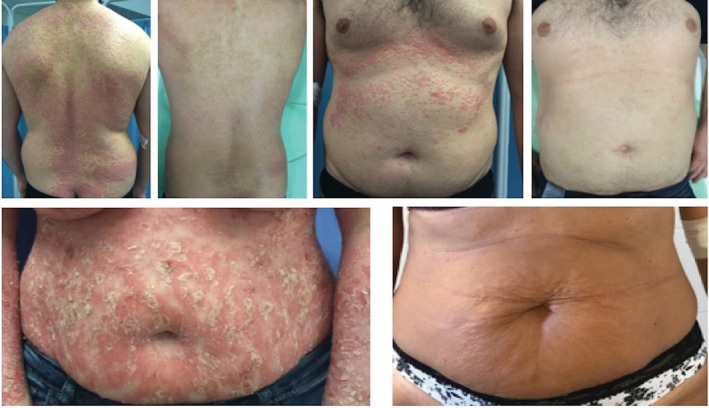
PASI 100 achievement in two different patients, at week‐16 of apremilast monotherapy. PASI, Psoriasis Area and Severity Index
Few real‐world studies have investigated the profile of apremilast 5 , 6 , 7 , 8 , 9 ; there have been no reports of efficacy after 8 weeks of treatment, which we valued as a PASI 50 achievement in 58% of patients and PASI 75 in 19%. Outcomes at 16 weeks were comparable with those of previous case series, even with respect to AEs and the rate of discontinuation. 9 Notably, our population was problematic in terms of comorbidities (86%), and only 28% of the patients were naïve to systemic treatments, including biologics. Overall, only 1 patient was considered a non‐responder, and 8 patients experienced a less remarkable but progressive improvement. The latter might represent a subset of slow responders. The achievements of PASI 90 and PASI 100 were unexpected after only 4 months of treatment. The main limitation to apremilast adherence was the intensity of AEs during the first weeks (41.6%), leading to discontinuation in 4 patients (8.3%), and reports of mild, bearable, and healed with treatment prosecution in the remaining 16 patients. As expected, diarrhea was the most frequent AE (28%), followed by abdominal discomfort, nausea, vomiting, and headache. Unlike other reports, no weight loss or worsened psychiatric disease was observed. 5 , 6 None of the patients developed symptoms requiring assessment for COVID‐19.
During the observation period, the Italian outpatients' services were required to suspend all visits because of the COVID‐19 pandemic outbreak in Italy and the recommendation to prevent patient access to hospitals (Decree of the President of the Council of Ministers, 03/09/2020). Thus, an extraordinary effort was made to implement monitoring of and support for this cohort of patients by distance. Regular telephone recalls were performed to assess whether patients had discontinued taking the drug or had contracted COVID‐19. We followed current experts' opinion 9 that apremilast is one of the safer options for moderate‐to‐severe psoriasis management due to its very specific, non‐immunosuppressive mechanism of action. Recent experience in a COVID‐19 disease affected patient, who maintained apremilast treatment during the bilateral pneumonia course and recovered completely, confirms safety and compatibility with critical patient's management. 10 Exacerbation of psoriasis might have occurred as a consequence of stress burden, with the COVID‐19 pandemic conferring severely limited wellness and opportunities to relieve anxiety and mood disorders through social activities. Of course, COVID‐19, as well as certain medications currently used to control the disease, might exacerbate psoriasis, as recently reported. 11
In this very critical period, the satisfactory speed of the therapeutic response to apremilast in a real‐world setting has further strengthened patient compliance to remain safe at home and led to patients eventually enduring the initial side effects, although further studies are warranted to explore this topic.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Melis D, Mugheddu C, Sanna S, Atzori L, Rongioletti F. Clinical efficacy, speed of improvement and safety of apremilast for the treatment of adult Psoriasis during COVID‐19 pandemic. Dermatologic Therapy. 2020;33:e13722. 10.1111/dth.13722
The authors certify that the manuscript is original, never submitted to other journal for publication before. All authors contributed equally to the manuscript and had the opportunity to revise and approve the final text.
REFERENCES
- 1. Zerilli T, Ocheretyaner E. Apremilast (Otezla): a new oral treatment for adults with psoriasis and psoriatic arthritis. Pharm Ther. 2015;40:459‐500. [PMC free article] [PubMed] [Google Scholar]
- 2. Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37‐49. [DOI] [PubMed] [Google Scholar]
- 3. Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387‐1399. [DOI] [PubMed] [Google Scholar]
- 4. Brownstone ND, Thibodeaux QG, Reddy VD, et al. Novel coronavirus disease (COVID‐19) and biologic therapy in psoriasis: infection risk and patient counseling in uncertain times. Dermatol Ther (Heidelb). 2020;10:339‐349. 10.1007/s13555-020-00377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ighani A, Georgakopoulos JR, Shear NH, Walsh S, Yeung J. Short‐term reasons for withdrawal and adverse events associated with apremilast therapy for psoriasis in real‐world practice compared to clinical trials: a multicenter retrospective study. J Am Acad Dermatol. 2018;78:801‐803. [DOI] [PubMed] [Google Scholar]
- 6. Mayba JN, Gooderham MJ. Real‐world experience with apremilast in treating psoriasis. J Cutan Med Surg. 2017;21:145‐151. [DOI] [PubMed] [Google Scholar]
- 7. Vujic I, Herman R, Sanlorenzo M, et al. Apremilast in psoriasis—a prospective real‐world study. J Eur Acad Dermatol Venereol. 2017;32:254‐259. [DOI] [PubMed] [Google Scholar]
- 8. Wong TH, Sinclair S, Smith B, et al. Real‐world, single‐centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42:674‐685. [DOI] [PubMed] [Google Scholar]
- 9. Papadavid E, Rompoti N, Theodoropoulos K, Kokkalis G, Rigopoulos D. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1173‐1179. [DOI] [PubMed] [Google Scholar]
- 10. Mugheddu C, Pizzatti L, Sanna S, et al. COVID‐19 pulmonary infection in erythrodermic psoriatic patient with oligodendroglioma: safety and compatibility of apremilast with critical intensive care management. JEADV. 2020. [Epub ahead of print]. 10.1111/JDV.16625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kutlu Ö, Metin A. A case of exacerbation of psoriasis after oseltamivir and hydroxychloroquine in a patient with COVID‐19: will cases of psoriasis increase after COVID‐19 pandemic? Dermatol Ther. 2020;e13383 [Epub ahead of print]. 10.1111/dth.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]


