Abstract
Background
The COVID‐19 pandemic, declared by WHO on March 13, 2020, had a major global impact on the healthcare system and services. In the acute phase, the presence of the SARS‐CoV‐2 virus in the aerodigestive tract limited activities in the gastroenterology clinic and procedures to emergencies only. Motility and function testing was interrupted and as we enter the recovery phase, restarting these procedures requires a safety‐focused approach with adequate infection prevention for patients and healthcare professionals.
Methods
We summarized knowledge on the presence of the SARS‐CoV‐2 virus in the aerodigestive tract and the risk of spread with motility and functional testing. We surveyed 39 European centers documenting how the pandemic affected activities and which measures they are considering for restarting these measurements. We propose recommendations based on current knowledge as applied in our center.
Results
Positioning of catheters for gastrointestinal motility tests carries a concern for aerosol‐borne infection of healthcare workers. The risk is low with breath tests. The surveyed centers stopped almost all motility and function tests from the second half of March. The speed of restarting and the safety measures taken varied highly.
Conclusions and Inferences
Based on these findings, we provided recommendations and practical relevant information for motility and function test procedures in the COVID‐19 pandemic era, to guarantee a high‐quality patient care with adequate infection prevention.
Keywords: breath test, COVID‐19, manometry, pH impedance monitoring
Overview of use of Personal Protective Equipment for motility studies during the COVID‐19 pandemic. Left: Donning procedure. Right: Doffing procedure.
Key Points.
A survey of 39 European centers showed that almost all motility and function tests were stopped from the second half of March 2020 due to the COVID pandemic.
In the recovery phase, restarting these procedures requires a safety‐focused approach.
The speed of restarting and the safety measures taken vary highly across centers.
We provide recommendations and practical relevant information for conducting motility and function test procedures in the recover ypahse of the COVID‐19 pandemic , to guarantee a high‐quality patient care with adequate infection prevention.
1. INTRODUCTION
Since the end of 2019, the world has seen rapidly spreading cases of a pneumonia and acute respiratory distress syndrome, caused by the transmission of a novel coronavirus, named SARS‐CoV‐2 by the World Health Organization (WHO), causing the disease COVID‐19. 1 , 2 Governments throughout the World have tried to contain the spread of this highly contagious disease through strict isolation measures and a focus of healthcare systems, staff, and services on COVID‐19 cases, while suspending care for all but essential and urgent medical conditions. 3 , 4 , 5 , 6 , 7 , 8
Early May 2020, the infection rates have decreased in Europe and a gradual resumption of non‐urgent medical services is proposed. In the gastroenterology specialty, several guidelines have been issued on how to select and safely conduct endoscopic procedures during the phase of urgent‐care only, 6 , 7 , 8 , 9 and early guidance is issued for the recommencing of procedures in the deceleration and early recovery phases of the pandemic. 9 , 10 , 11 This is done with careful precautions, as endoscopies are aerosol generating procedures with considerable risk of infection to other patients and endoscopy staff when performed on a SARS‐CoV‐2–infected individual. 6 , 7 , 8 , 9 , 10 , 11
Besides endoscopy, motility and function test procedures in gastroenterology units were also interrupted with the focus on urgent procedures. As motility and functional disorders are a large part of gastroenterology clinical practice, the question arises when and how to resume diagnostic testing for these conditions. Indeed, breath tests and insertion of upper gastrointestinal manometry and pH‐monitoring probes carry a risk for viral spread through droplet formation when probes pass the nose or mouth and pharynx, or when air is blown into breath test tubes. 12
The aim of this paper is to summarize the current knowledge and recommendations, to describe the impact of the COVID‐19 pandemic on motility and function testing in gastroenterology practice in Europe, and to provide some practical guidance for the protective restoration of motility and function testing.
2. METHODS
We performed a PubMed, Medline, and Embase search between the April 26 and the May 10, 2020, using “SARS‐CoV‐2”, “COVID‐19”, “(esophageal) manometry”, “(esophageal) pH monitoring”, “anorectal manometry” and “breath test” as MeSH terms. We also searched websites of gastroenterology and motility societies for information on procedures and “SARS‐CoV‐2”, “COVID‐19” or “Corona virus”. In addition, we conducted a PubMed, Medline, and Embase search using “gastrointestinal endoscopy”, “endoscopy, digestive system endoscopy” as MeSH terms for general protective measures for staff and patients in endoscopy units. We only used published data, reports, and articles written in the English language. We summarized available literature for content and extrapolated from endoscopy guidance to develop guidance toward performing motility and functional testing procedures in the early recovery phases of the COVID‐19 pandemic.
To assess the impact of the pandemic on motility and function testing in Europe, we generated a questionnaire in QualtricsXM for European clinicians with an interest in this pathology based on their involvement in recent consensus documents and on membership of the European Society for Neurogastroenterology and Motility. The following gastrointestinal activities were included: esophageal manometry, catheter‐based pH monitoring, wireless pH monitoring (Bravo®), anorectal manometry, and breath tests. The questions aimed at evaluating whether the center reduced or stopped motility and functional testing, and if so at what time and to which extent. The questions also assessed estimated timing for restarting these activities and at what capacity. Additionally, the questions also evaluated which protection measures and screening methods the center would be using during the restart of these activities.
3. RESULTS
3.1. Phases of a pandemic
Many countries affected by the pandemic are now entering a postpeak period, where disease levels in countries with adequate surveillance are dropping below the observed peak levels. 2 , 13 This phase may or may not be followed by additional waves of transient rises of infectious activity. 1 , 2 , 13 The British Society of Gastroenterology distinguishes build‐up, peak and deceleration phases, where hospital facilities are repurposed for managing the infected case load besides urgent non‐infection care. 11 In the postpeak period, admissions decrease and requisitioned beds are gradually returned to normal services. This is followed by a phase of late recovery, where the case load falls and hospital configuration and activity is close to normal, although localized reactivations may still occur. 11 Hence, there is a persisting risk of patients carrying the infection even after the peak and recovery episodes, which may be of low prevalence, but needs to be taken into account when planning procedures.
3.2. COVID‐19 presentation and virus presence in the gastrointestinal tract
Symptoms of COVID‐19 infection are often those of a common cold (runny nose, sneezing, fatigue, cough), a body temperature of 37.5℃ or higher and dysgeusia and dysosmia, without apparent cause. 9 , 14 , 15 In addition, there may also be digestive symptoms (see below). The highest viral loads of SARS‐CoV‐2 are found in the nasopharynx, and the virus mainly spreads directly via droplets and aerosols, and indirectly by contact with contaminated surfaces. 14 , 15 Transmission by infected persons may already occur in the presymptomatic phase. 14 SARS‐CoV‐2 enters cells via the angiotensin‐converting enzyme 2 (ACE2) receptor, which is expressed not only in the lungs but also on blood vessels, in the brain, the skin, and the digestive system. 15
Angiotensin‐converting enzyme 2 is highly expressed in esophageal epithelial cells, on gastric glandular cells and on enterocytes in the small bowel and the colon, which explains gastrointestinal manifestations of the infection. 15 , 16 , 17 Gastrointestinal symptoms in COVID‐19 patients include decreased appetite, loss of taste, nausea, vomiting, abdominal pain, diarrhea, and possibly lower gastrointestinal bleeding. 16 , 17 , 18 Positive stool (real time) reverse transcription polymerase chain reaction ((RT‐)PCR) tests for SARS‐CoV‐2 have been reported and fecal tests may be positive when a respiratory test is or has become negative. 16 , 19 These observations support the possibility of fecal‐oral transmission. 16 , 19 , 20
3.3. Motility and functional disorder‐related procedures
See supplementary file (Appendix S1).
3.3.1. Survey on motility and functional disorder‐related procedures during the peak period and early recovery phase of the COVID‐19 pandemic in Europe
Impact of the build‐up and peak period of the COVID‐19 pandemic
We conducted a survey to evaluate the impact of COVID‐19 on motility testing in hospital setting. The survey was sent out to 46 gastroenterologists/motility experts in Europe, of whom 39 replied. The centers represent the following countries (1 center unless otherwise stated): Belgium; France (n = 3), Germany (n = 8), Spain (n = 3), Israel (n = 2), Portugal (n = 3), Denmark, Turkey (n = 2), Italy (n = 3), UK (n = 2), Ireland, Poland, Romania (n = 3), Croatia, Russia, Switzerland, Norway, the Netherlands, and Sweden. Unless otherwise specified, data are represented as median (range).
Esophageal manometry was performed by 38 out of 39 centers before the start of the COVID‐19 pandemic. A significant impact of COVID‐19 on performing esophageal manometry was found, as 35/38 performing centers majorly reduced or stopped their capacity, from the 16th of March 2020 (range 29th of February 2020‐6th of April 2020). These 35 centers reduced their capacity with a median of 100% (50%‐100%). Fifteen centers stopped their activities immediately and nine centers gradually reduced activities before completely stopping this type of investigations. Eleven centers reduced their activities with 50% (n = 1), 80% (n = 4), and 90% (n = 6), as they still were performing some urgent esophageal manometries (eg, presurgery).
Furthermore, COVID‐19 majorly impacted catheter‐based pH‐monitoring investigations, as 36/39 centers reduced or stopped these activities on the 16th of March 2020 (range 29th of February 2020‐6th of April 2020). These centers reduced their capacity with a median of 100% (80%‐100%). Twenty‐two centers stopped their activities immediately, while six centers gradually reduced activities before completely stopping this type of investigations and five centers reduced their activities with 80% (n = 2) and 90% (n = 3).
In addition, there was a significant impact of COVID‐19 on the wireless pH capsule testing. Nineteen centers did not perform this type of test, and 13 out of 15 centers who offered it reduced or stopped performing wireless pH testing on the 16th of March 2020 (9th of March 2020‐1st of April 2020). The capacity was reduced with 100% (range 80%‐100%). Twelve centers stopped their activities immediately and only one center did not completely halt Bravo® pH capsule investigations (80%).
Anal manometry was performed by 31 centers before the start of the COVID‐19 pandemic. Twenty‐eight centers diminished their activity with 100% (50%‐100%). Three centers did not reduce their capacity for anal manometry testing for COVID‐19 reasons. Testing for anal manometry was immediately stopped in 22 centers, while two centers gradually reduced before completely stopping all activities. Four centers reduced their activities with 50% (n = 1) and 90% (n = 3). Median time of reduction or stopping anal manometry was on 15th of March 2020 (16th of February 2020‐6th of April 2020).
Thirty centers performed breath tests before the start of the COVID‐19 pandemic. Twenty‐six centers reduced their capacity with a median of 100% (40%‐100%) from the 16th of March 2020 (26th of February 2020‐6th of April 2020) onwards. Five centers reduced their activities with only 40% (n = 1) and 90% (n = 4).Three centers did not change their capacity for performing breath tests due to COVID‐19. Breath tests were immediately reduced in 23 centers, while five centers gradually reduced before complete stopping all activities. Two centers did not clarify if activities were stopped gradually or not.
Plans for restoration of motility and function testing during the early recovery phase of the COVID‐19 pandemic
The 14 centers that already restarted with at least one of the discussed investigations concerning gastrointestinal motility testing were able to restart after a median of 49 days (34‐71 days), including all motility tests that were discussed earlier. However, centers that have not been able to restart yet estimated to restart after a median of 79 days, on the 1st of June (42‐171 days). Three centers did not have a perspective on when to restart activities in general and three centers provide no estimate a time point to restart performing breath tests. All centers that already started or still have to restart activities, estimate to restart at 55% of initial capacity for esophageal manometry (20%‐100%), 50% for pH monitoring (20%‐100%), 60% for the Bravo® pH capsule (10%‐100%), 50% of initial capacity (30%‐100%) for anal manometry, and 50% for breath tests (10%‐100%).
Plans for personal protective measures for motility and function testing during the early recovery phase of the COVID‐19 pandemic
Currently, the most frequently used protection measures when performing motility testing included an FFP2 mask, a face shield, a hairnet, a water‐resistant gown, and standard gloves (Table 1). For anorectal manometry, a surgical mask was regularly used as well.
Table 1.
Personal protective equipment for different gastrointestinal motility investigations
| Protection mechanism | Esophageal manometry (n = 38) | Catheter‐based pH monitoring (n = 39) | Bravo® pH capsule (n = 16) | Anal manometry (n = 32) | Breath tests (n = 30) |
|---|---|---|---|---|---|
| None (%) | 0 | 0 | 0 | 0 | 3 |
| Negative pressure room (%) | 3 | 3 | 6 | 3 | 0 |
| Surgical mask (%) | 29 | 29 | 13 | 53 | 33 |
| FFP2‐mask (%) | 61 | 59 | 69 | 50 | 37 |
| FFP 3‐mask (%) | 18 | 21 | 25 | 9 | 17 |
| Goggles (%) | 39 | 41 | 31 | 31 | 37 |
| Face shield (%) | 63 | 69 | 63 | 47 | 50 |
| Hairnet (%) | 58 | 64 | 56 | 59 | 47 |
| Water‐resistant gown (%) | 58 | 64 | 75 | 59 | 40 |
| Non–water‐resistant gown (%) | 21 | 21 | 19 | 28 | 20 |
| Long sleeved gloves (%) | 16 | 18 | 25 | 19 | 0 |
| Standard gloves (%) | 71 | 77 | 63 | 81 | 70 |
| Overshoe covers (%) | 3 | 3 | 0 | 3 | 0 |
Abbreviation: FFP, filtering face piece.
The most common screening procedures, currently or expected to be used by the contacted centers to detect possible COVID‐19 infection prior to performing investigations, were anamnestic risk assessment and temperature check (Table 2). Before performing esophageal manometry, catheter‐based pH monitoring or tests with the Bravo® pH capsule, a PCR swab for acute COVID‐19 infection diagnosis was also frequently applied. One center did not decide yet which screening procedures will be used in the near future.
Table 2.
Screening procedures used prior to performing gastrointestinal motility investigations
| Screening procedure | Esophageal manometry (n = 38) | Catheter‐based pH monitoring (n = 39) | Bravo® pH capsule (n = 16) | Anal manometry (n = 32) | Breath tests (n = 30) |
|---|---|---|---|---|---|
| None (%) | 0 | 0 | 0 | 0 | 3 |
| Anamnestic risk assessment (%) | 3 | 3 | 6 | 3 | 0 |
| Temperature check (%) | 29 | 29 | 13 | 53 | 33 |
| Nasopharyngeal PCR swab (%) | 61 | 59 | 69 | 50 | 37 |
| CT‐scan (%) | 18 | 21 | 25 | 9 | 17 |
| Serology test (%) | 39 | 41 | 31 | 31 | 37 |
| Saturation O2 (%) | 63 | 69 | 63 | 47 | 50 |
Abbreviations: CT, computed tomography; PCR, polymerase chain reaction.
3.4. Recommendations for restoration of motility and function testing during the early recovery phase
3.4.1. General assessment
Prior to performing any type of endoluminal procedure of the gastrointestinal tract, a general assessment of the urgency and need of the procedure, as well as the associated risk for patients as well as healthcare workers, is strongly recommended. We outline the approach based on the literature review, as is being implemented in our center.
Urgency of the procedure
The vast majority of motility and functional disorders are chronic and non‐urgent, without life‐threatening complications. 33 Diagnostic endoscopy is likely to precede any upper gastrointestinal motility and functional testing to rule out organic disease. Moreover, empirical treatment is available for several conditions, such as PPIs for GERD and anti‐emetics for nausea/vomiting disorders. Most procedures therefore can be postponed and only need to be considered in the late recovery phases of the pandemic. Exceptions are dysphagia where only fluids can be managed, dysphagia either oropharyngeal or esophageal, that is associated with aspiration and aspiration pneumonia, and intractable nausea and vomiting with electrolyte imbalances or weight loss. In these conditions, further exploration is recommended within the first 2 to 4 weeks, and this may include (pharyngo‐) esophageal manometry and breath testing (mainly gastric emptying).
Assessment of the patient’s risk of infection
All endoluminal procedures, especially in the upper gastrointestinal tract, should be considered high‐risk procedures if the patient is infected, even if asymptomatic. 14 , 15 , 16 , 17 However, considering all patients potentially infected places high demand on supplies of high‐level personal protective equipment (PPE) and slows down procedures because of the time required for preparation, more comprehensive room cleaning and air circulation between procedures. 7
An alternative approach is to determine the presence of active infection by a combination of the clinical presentation, RT‐PCR‐test (nasopharyngeal swab and/or in rare cases bronchoalveolar lavage) and/or multi‐sliced chest computed tomography scan although the value of the latter in screening is questionable. 34 , 35 , 36 This information can then be combined to determine the level of protective measures needed. Detection of viral RNA by PCR, which has moderate to high sensitivity depending on timing and type of test, has become a mainstay of COVID‐19 disease detection, also in asymptomatic subjects. 10 , 34 , 35 This method has been recommended for identifying patients with active infection prior to elective endoscopy, and we recommend the same approach for risk management with broad testing prior to function testing procedures involving upper gastrointestinal tract intubation. 10 However, this technique has several limitations including the need for technical expertise, the occurrence of false‐negative results, and an inability to detect individuals who may be immune. 3 , 37 , 38 It has been recommended to consider a negative PCR test valid for 48 hours. 10 Anal swabs have also been explored but seem to be inconsistent and, at best, positive in later stages of the infection. 4 , 15 , 16 Antibody testing probably has the potential to play a supplementary role to PCR in diagnosis, screening of contacts and possibly in the determination of population immunity. However, there is a lack of standardized, reliable tests, and sensitivity varies with the stage of infection. 40
Figure 1 visualizes the flowchart to assess the patient’s risk of infection and the allocated procedures. Before arrival, patients who need to undergo a motility or function test should be questioned about: (a) fever, (b) occupational exposure (including healthcare workers or laboratory staff handling COVID‐19 specimens), (c) contact history with confirmed cases (in the last 14 days), (d) clustering and (e) in some areas of low prevalence, travel history (especially to all countries with a high incidence in COVID‐19 transmission within the past 14 days). In case of presence of one of these five risk factors, the need for the test needs to be reconsidered and the procedure should be postponed if possible. If the test is considered necessary and urgent, PCR testing as described above should be performed. 10 , 34 , 35 In case no testing is available, the patient needs to be considered as potentially infected and high‐level PPE and room handling procedures need to be used (Figure 1).
Figure 1.
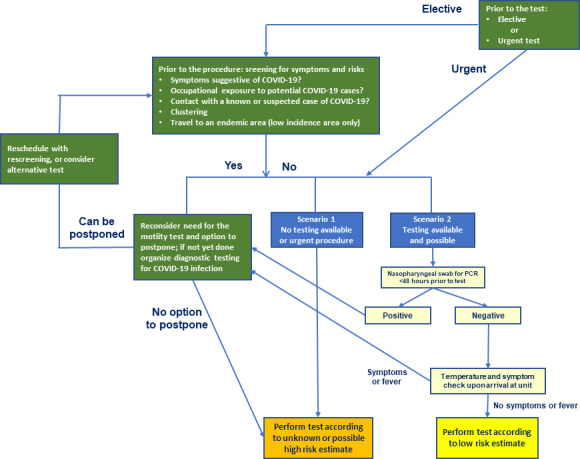
Flowchart to assess the patient’s risk of infection and the allocated procedure
In case of a positive or inconclusive RT‐PCR test, the patient’s profile is high‐risk and the procedure should be postponed until the window of possible transmission has passed. In the exceptional case that this would not be possible, for example, dysphagia with important weight loss, the test should be performed under high‐level PPE (Figure 1). PCR positivity disappears after a median of 20 days, but may continue for up to 46 days. 39 Hence, a postponement of 8 weeks should be considered for the motility test. Patients with a positive RT‐PCR test should not undergo repeated screening after 8 weeks; patients with inconclusive RT‐PCR should be offered repeated risk stratification and screening.
In the absence of one of the five risk factors, symptoms should be questioned. When symptoms are present, the profile is considered intermediate‐risk. Suggestive symptoms include cough, dyspnea, rhinitis, new onset of nausea, dysosmia or dysgeusia, new onset of abdominal discomfort, and diarrhea, the latter of which can be considered as suspect for (entero)colitis, especially when fever is also present. Additionally, body temperature can easily be measured before entering the function testing unit or room. Procedures in patients with intermediate‐risk profile should also preferably be postponed. If not possible, the motility tests should be performed respecting all the protective measures (see below). Low‐risk patients are those without risk factor and a negative laboratory RT‐PCR test. 10 In case of 1 risk factor and negative PCR, the patient is still considered low‐risk. For low‐risk patients, a COVID‐minimized track can be followed, with more targeted use of PPE and lower‐intensity procedures.
3.4.2. General protective measures for the unit and for staff
Given the variable reliability of possible tests and their results in combination with the possible spectrum of symptoms, there is an inherent lingering uncertainty about the patient’s COVID‐19 status. 5 , 9 Therefore, systematic general protective measures and the use of different levels of PPE are recommended for all motility procedures. 5 On the other hand, in the Leuven University Hospitals, systematic PCR tests were positive in only 2% of asymptomatic patients screened prior to elective endoscopy procedures. 9 Hence, low‐risk assessment, based on a combination of absence of risk factors, symptoms, and a negative nasopharyngeal swab seems to hold a low risk for contamination during the procedure and justifies less stringent measures to save on limited protective resources. Specific recommendations for management of motility function units are summarized in Table 3. Table 4 summarizes the protective measures for staff in case of low‐risk or unknown‐risk status of the patient.
Table 3.
Management of motility function units
| 1. Individual workstations for center staff |
| 2. Appropriate spacing of waiting room chairs to keep appropriate social distancing of patients. Separation of COVID‐positive subjects from the others |
| 3. Linear patient and staff flow through the unit (no crossing of COVID‐positive and negative pathways, separate entrance and exit) |
| 4. Similar separate in‐ and outflow for material used in procedures |
| 5. Preferential use of single use and disposable material |
| 6. Frequent cleaning and disinfecting of objects and surfaces in the units |
| 7. Required masks for patients for respiratory hygiene |
| 8. Restriction of accompanying visitors |
| 9. Organization of workflow patterns and job descriptions to minimize cross‐contamination |
| 10. Adequate time for air exchanges in rooms and deep cleaning between procedures, especially in unknown‐ or high‐risk procedures. If possible, the flow of the air (air pressure differential) should be graded toward the high‐risk area |
| 11. Building a platform for all employees to quickly communicate and sending important messages to every staff member |
Table 4.
Protective measures for function testing staff in case of procedure in low‐risk (1‐6) or unknown‐ or high‐risk (7) patient
| 1. All medical staff should properly receive relevant training on infection control of COVID‐19, including potential contaminated sources, measures, risk factors, and epidemiology of COVID‐19 |
| 2. Staffs should be screened daily with a temperature check and surveyed for COVID‐19 exposure and symptoms |
| 3. Diligent hand hygiene for at least 20 s, before and after patient contact. The same before and after material contact |
| 4. Avoiding touching the face (in particular eyes, nose, and mouth) |
| 5. Appropriate PPE should be available for each type of functional test for all staff and patients involved |
| 6. In patients classified as low risk, PPE should include gloves, a hairnet, protective eyewear (goggles or face shield), gowns, and surgical masks |
| 7. In patients with unknown or high‐risk COVID‐19 status, PPE should include waterproof gowns, booties/shoe covers, a hairnet, protective eyewear (goggles or face shield), and a level 2 PPE with FFP2/FFP3‐mask, and two pairs of gloves |
3.4.3. Dressing and undressing
Personnel wears dedicated hospital clothing when performing clinical tasks. The sequence of dressing and undressing with these PPE is specific and should be followed in the correct order at all times to avoid patient to healthcare worker transmission. The dressing procedure is called “the donning” and the undressing‐procedure is called “the doffing.” Illustrative videos are available on www.uzleuven.be/nl/covid-19-voor-woonzorgcentra/omkleedprocedure.
The donning procedure consists of eight steps (Figure 2A, Table 5). The doffing procedure consists of the same eight steps but in an altered sequence and every step is separated from another by disinfecting your hands with alcohol. Steps 1 to 3 are inside of the room, or in a separate dedicated room, for the removal of disposable PPE, steps 4 to 6 are outside of the room for collection of recyclable face shield, goggles, and mask. Due to its scarcity, specialized cleaning and sterilization programs have been implemented for these items after recollection (Figure 2B, Table 6). Since contamination is most likely to happen because of errors during the “undressing/doffing” procedure, leading to accidental contact with the contaminated mask, goggles, or front of the gown, extra awareness and supervised training for this procedure is advisable.
Figure 2.
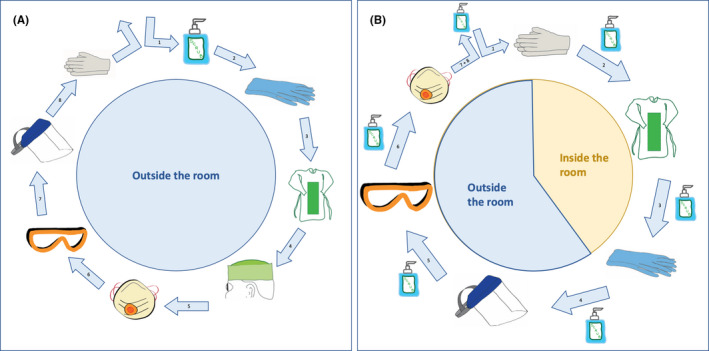
Overview of use of personal protective equipment for motility studies during the COVID‐19 pandemic. A, Donning procedure. B, Doffing procedure
Table 5.
Donning procedure
| 1. Disinfect hands with alcohol |
| 2. Put on long nitrile gloves (second skin) |
| 3. Put on an impermeable gown |
| 4. Shoe covers if preferred |
| 5. Put on a surgical hat or hairnet |
| 6. Put on a surgical or FFP2/3‐mask (adjust correctly around the nose and beneath the chin) |
| 7. Put on the goggles over the surgical or FFP2/3‐mask |
| 8. Put on the face shield if required |
| 9. Put on a second pair of (short) nitrile gloves if required. In some centers, an additional apron is worn |
Table 6.
Doffing procedure
| 1. Remove the second pair of nitrile gloves |
| 2. Remove the impermeable gown |
| 3. Remove the long nitrile gloves |
| 4. Take off the face shield and put in a recycle bin for collection |
| 5. Take of the goggles (from behind–over the head, do not touch the front or glasses) and put them in the same recycle bin as the face shield for collection |
| 6. Take of the FFP2/3‐mask (from behind–over the head, do not touch the front) into a second recycle bin for collection |
The face shield, goggles, and FFP2/3‐mask should be taken off after putting on a new pair of nitrile gloves outside of the room, to minimize possible transmission to the healthcare worker’s skin while taking off these protection items, which can be highly contaminated. Removal of the surgical hat and disinfection of the hand with alcohol as final step are considered standard of care (steps 7 and 8).
3.4.4. Precautions for specific tests
(Pharyngo‐)esophageal manometry
Most of the upper gastrointestinal function tests, including (pharyngo‐)esophageal manometry and pH(‐MII) monitoring, involve the positioning of a catheter through the nasopharynx which is the region containing the highest viral load in COVID‐19‐positive patients. 40 During the insertion of the catheter, aerosols can be produced, especially when the patient starts coughing or sneezing resulting from local irritation. Although no specific data are available in the literature related to the SARS‐CoV‐2 transmission risk during placement of nasogastric tubes or manometry devices, this procedure should be considered high‐risk based on the localization (nasopharynx) and the risk of aerosol formation. Based on the assumed high risk of the procedure, combined with the possibility to postpone the procedure for at least 2 weeks, manometry and pH‐MII monitoring should preferably only be performed in low‐risk patients, that is with a negative RT‐PCR test and without any signs (fever) or symptoms suggestive of COVID‐19 (cf. supra). In case testing cannot be performed, the patient needs to be considered uncertain risk with more extensive PPE measures.
The height of the bed should be adjusted in a way that the upper part of the head of the patient is under the chin of the nurse or technician. We recommend applying lubricating gel containing a local anesthetic on the catheter and to avoid the use of sprays with local anesthetic because of the risk of aerosol formation. 41 During the insertion of the catheter, the mask should still be worn over the mouth, exposing the nose only.
The monitor and manometry set‐up should be positioned at the maximal distance of the patient and protection with a plastic removable cover should be considered. Prior to the start of the measurement, the patient’s mask can be removed. We recommend that the boluses are still administered using a syringe to protect the quality of the test, but this should be done while maintaining a maximal distance. During assessment of patients with oropharyngeal dysphagia, boluses that are known to trigger coughing in the patient should be avoided. Overall, the time devoted to the assessment procedures should be restricted as much as possible. Before removal of the catheter at the end of the measurement, the mask should be repositioned over the mouth.
Regular disinfection procedures of the manometry probe suffice since the standard biocidal solutions and wipes demonstrate an effective inactivation of coronaviruses. 42 Additionally, the manometry set‐up, keyboard, computer screen, desk, and headboard of the bed should be wiped with standard biocidal wipes between two patients. Regular cleaning of the floor is also needed as this is where many aerosol droplets may end.
pH, pH‐MII, and wireless pH capsule monitoring
The main risk of pH‐MII measurement procedure lies in the positioning of the catheter for which the same precautionary measures, including patient selection, apply as for esophageal manometry. After each use, the portable registration device should be wiped with biocidal wipes. As an alternative, the portable registration device can be wrapped in transparent plastic which is sealed with tape, eliminating direct contact with body and body fluids, while allowing screen checking and use of buttons. Moreover, we recommend using single‐use or washable holders and shoulder straps for the recorder. Since virtually all pH‐MII probes are single‐use catheters, specific disinfection protocols do not apply.
The catheter‐free pH‐monitoring system can be used as an alternative, although there is no clear preference for one or the other in the current pandemic. The wireless pH capsule is positioned by the gastroenterologist, using the delivery system, usually preceded by a gastroscopy with the general safety procedures for endoscopy. 7 , 9 An additional limitation is that the capsule only quantifies acid reflux.
Anorectal manometry
Investigation of dyschezia or fecal incontinence is hardly ever urgent and should be restricted to low‐risk patients. Although no oropharyngeal manipulations are performed, close proximity to the patient is required and therefore patients should keep wearing a mask throughout the test.
Prior to anorectal manometry, a water enema can be given in case of fecal loading of the rectum. As defecation is considered an aerogenic process and SARS‐CoV‐2 particles potentially can be shed via feces, a toilet in a separate room is preferred over in‐room commode seat. In all cases, toilet or commode seats should be disinfected between patients.
During measurement of resting pressure, but especially during measurement of squeezing pressure and simulated defecation, seepage of fecal content can occur. Therefore, staff should wear PPE throughout the entire procedure, based on the above‐mentioned risk stratification.
Similar to esophageal manometry, reusable anorectal manometry catheters should be disinfected with standard biocidal solutions, as well as set‐up, computer, keyboard, bed/stretcher, and toilet/commode.
Breath tests
Although breath tests do not require pharyngeal passage with a catheter, there is a theoretical risk of virus particle dispersion through aerosolized breaths.
For 13C and for H2‐based breath tests, the patient blows a breath sample via a straw into a tube that is subsequently sealed. 43 , 44 , 45 While one cannot exclude minor aerosol production from saliva during this repetitive sample collection where the subject has to blow out alveolar air, this is likely to be minimal. 30 , 46 The patient should remain in the dedicated test room during the test. As the patient can perform this test independently, it is probably sufficient for staff to maintain a distance of 1.5 m, for the patient to wash their hands before and after the test with soap or disinfectant, and to clean the table before and after the test with disinfectant wipes. Sample handling and storing should be done wearing protective gloves, and the tubes should be carried in isolation plastic bags. If required, storage for further analysis should be in dedicated shelve sections.
With 14C breath tests, the risk of aerosol generation is greater as the patient blows via a straw into a liquid‐filled vial until color change occurs. 43 However, as the liquid consists of 70% EtOH, which is a disinfectant in its own right, the risk seems contained. The same hygienic and disinfectant measures as outlined for 13C and for H2‐based breath tests can also be applied.
The isotope ratio mass spectrometer used to measure 13CO2 enrichment has a syringe with a needle that sucks the air into the system. The needle and syringe should be regularly disinfected after analysis of the suspect/positive patient samples. A filter can be positioned at the outlet section of the spectrometer and regularly changed, avoiding operator contamination. Personnel involved in the analysis should wear appropriate PPE while handling sample tubes.
H2‐based tests are usually analyzed with either a gas‐chromatography with thermal conductivity detection or portable instruments based on an electrochemical cell (344. As gas chromatographs are particularly sensitive to the humidity transferred with the breath sample, they often contain a chemical‐based water trap that needs periodical replacement. 44 A particular attention has to be paid for protection of staff when changing this filter after analyzing samples of suspect/positive COVID‐19 patients, and personnel involved in the analysis should wear appropriate PPE when changing the filter as well as when handling sample tubes. Portable H2‐analyzers in which the patient directly blows via a mouthpiece are protected by a dedicated filter that traps airborne bacteria and viruses. Similar precautions as above are needed when removing the disposable mouthpiece and when replacing this filter.
4. DISCUSSION
The outbreak of the COVID‐19 pandemic has had a major impact on healthcare delivery across the world. In the build‐up, peak and deceleration phase of the pandemic, the healthcare system focused on management of patients with COVID‐19 infection and urgent other care. 2 , 7 , 8 Motility and functional disorder care shifted to the background because of the non‐urgent and chronic nature of these pathologies. However, these are highly prevalent conditions with a major impact on patients and society, and hence both diagnostic and therapeutic care of these patients needs to be restored in the postpeak period. 33 , 47 This needs to be done with awareness of the persisting risk of infection being present in and spreading from a small group of patients. 5 , 6 , 11
Several guidelines have been published on patient selection and staff and patient protection when performing endoscopies during the different phases of the pandemic, but such guidance has been lacking for functional and motility disorders and has mostly been limited to acknowledging their non‐urgent status. 5 , 6 , 7 , 8 , 9 , 11 Based on a survey, we conducted in 31 centers in Europe, motility and function testing was practically abandoned throughout the continent during the peak phase of the pandemic, starting second half of March and continuing well into May 2020. In almost all centers, the number of procedures was diminished by 100% or at least by 90%. We also documented that most centers are (preparing to be) starting up again by June, although some centers do not have a formal start‐up date determined at this point. All centers plan precautions to screen for infected patients, and to protect staff and other patients from infection related to motility and function testing, but the type and scope of measures show tremendous variation. For protection of medical personnel, different types of masks, facial shield, gloves and gowns will be applied. There is occasional use of negative pressure rooms and shoe covers. For detection of acute infection, there is a high, though not uniform, use of anamnestic evaluation and temperature check. Approximately half of the centers will use PCR on throat swabs to screen patients.
Next, we proposed a practical guide for clinical practice based on the precedent for endoscopy and the currently limited understanding of the SARS‐CoV‐2 infection. The PPE recommendations are adapted to the possible COVID‐19 status of patients and the available diagnostic measures in the center. By combining both symptoms and test results, we seek rational use of PPE and procedure times. In the absence of validated data, prospective follow‐up of this recommendation scheme, as well as improved understanding of SARS‐CoV‐2 will clarify its merits in the coming months. As a measure of extra caution, we categorized doubtful or unknown patients under the same approach as the COVID‐19 positive patients, with a preferred postponement of the procedure until a possible infection has subsided.
Guidelines regarding COVID‐19 diagnosis and treatment are still evolving. The current guidelines reflect a pragmatic interpretation of the current knowledge and have not been subjected to a rigorous scientific evaluation. In the future, it is conceivable that optimized widespread screening and availability of reliable serological tests may alter the current recommendations and facilitate the procedures. Finally, the most important asset in this stage of the pandemic is a careful attitude of well‐trained and well‐informed medical staff performing motility and function testing.
5. SUMMARY AND CONCLUSIONS
Sars‐CoV‐2 infection is a highly contagious new disease which primarily spreads via droplets from the naso‐oropharynx, but may also be present in the lower gastrointestinal tract. Hence, the risk of transmission needs to be taken into account when planning and performing motility and function testing. Screening for infection in patients and protection of the medical staff, using targeted PPE measures, are the key factor to avoid further spreading and procedure‐related extra infections.
AUTHOR CONTRIBUTIONS
JT: initiated the process, wrote manuscript draft, created figures, revised manuscript and approved final version. JS: conducted the survey and summarized data, wrote manuscript section, revised manuscript and approved final version. AG: conducted the survey and summarized data, wrote manuscript section, revised manuscript and approved final version. IHH: wrote manuscript section (infection and transmission), revised manuscript and approved final version. HM: designed the survey, wrote manuscript section (risk assessment), revised manuscript and approved final version. ES: wrote manuscript section (personal protection, breath test), revised manuscript and approved final version. PS: wrote manuscript section (dressing, undressing), generated figure, revised manuscript and approved final version. FC: wrote manuscript section (protective measures for unit and staff; general measures), revised manuscript and approved final version. HG, LT: wrote manuscript section (pH monitoring), revised manuscript and approved final version. KVH: wrote manuscript section (manometry), revised manuscript and approved final version. PR: wrote manuscript section (anorectal manometry), revised manuscript and approved final version. HS: co‐developed survey, wrote manuscript section (risk assessment), revised manuscript and approved final version. KV: wrote manuscript section (breath tests and analysis), revised manuscript and approved final version. TV: wrote manuscript section (procedures overview), revised manuscript and approved final version. EC, SJ, AM, JP, WV, LW, RB, IH, NR, MS, HT: revised manuscript and approved final version.
Supporting information
Appendix S1
Tack J, Schol J, Geeraerts A, et al. A survey on the impact of the COVID‐19 pandemic on motility and functional investigations in Europe and considerations for recommencing activities in the early recovery phase. Neurogastroenterology & Motility. 2020;32:e13926. 10.1111/nmo.13926
REFERENCES
- 1. Harapan H, Itoh N, Yufika A, et al. Coronavirus disease 2019 (COVID‐19): a literature review. J Infect Public Health. 2020;13(5):667‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO: coronavirus disease 2019 (COVID‐19) situation report – 110. Geneva, Switzerland: World Health Organization; 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200509covid-19-sitrep-110.pdf?sfvrsn=3b92992c_4. Accessed May 10, 2020. [Google Scholar]
- 3. Xia L, Wu K Gastroenterology Practice in COVID‐19 Pandemic. https://www.worldgastroenterology.org/publications/e-wgn/gastroenterology-practice-in-covid-19-pandemic. Accessed May 6th, 2020.
- 4. Magro F, Abreu C, Rahier JF. The daily impact of COVID‐19 in gastroenterology. United European Gastroenterol J. 2020;8(5):520‐527. 10.1177/2050640620920157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang L, Lin G, Tang L, Yu L, Zhou Z. Special attention to nurses’ protection during the COVID‐19 epidemic. Crit Care. 2020;24(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gralnek IM, Hassan C, Beilenhoff U, et al. ESGE and ESGENA Position Statement on gastrointestinal endoscopy and the COVID‐19 pandemic. Endoscopy. 2020;52(06):483‐490. 10.1055/a-1155-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sultan S, Lim JK, Altayar O, et al. AGA Institute Rapid Recommendations for Gastrointestinal Procedures During the COVID‐19 Pandemic. Gastroenterology. 2020. pii: S0016‐5085(20):30458‐3 (epub ahred of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bilal M, Simons M, Rahman AU, et al. What constitutes urgent endoscopy? A social media snapshot of gastroenterologists’ views during the COVID‐19 pandemic. Endosc Int Open. 2020;8(5):E693‐E698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sinonquel P, Roelandt P, Demedts I, et al. COVID‐19 and gastrointestinal endoscopy: what should be taken into account? Dig Endosc. 2020. 10.1111/den.13706 (epub ahred of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corral JE, Hoogenboom SA, Kröner PT, et al. COVID‐19 polymerase chain reaction testing before endoscopy: an economic analysis. Gastrointest Endosc. 2020. pii: S0016‐5107(20)34248‐6 (epub ahred of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Penman I, Edwards C, McKinlay A.BSG Guidance on recommencing GI Endoscopy in the deceleration & early recovery phases of the COVID‐19 pandemic. https://www.bsg.org.uk/covid-19-advice/bsg-guidance-on-recommencing-gi-endoscopy-in-the-deceleration-early-recovery-phases-of-the-covid-19-pandemic. Accessed May 10, 2020. [DOI] [PMC free article] [PubMed]
- 12. Keller J, Bassotti G, Clarke J, et al. Expert consensus document: advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat Rev Gastroenterol Hepatol. 2018;15(5):291‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pandemic Influenza Preparedness and Response: A WHO Guidance Document. https://www.ncbi.nlm.nih.gov/books/NBK143061. Accessed May 6th, 2020. [PubMed]
- 14. Furukawa NW, Brooks JT, Sobel J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis. 2020;26(7) (epub ahred of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li M‐Y, Li L, Zhang Y, Wang X‐S. Expression of the SARS‐CoV‐2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. Published online 2020 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID‐19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51(9):843‐851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carvalho A, Alqusairi R, Adams A, et al. Sars‐CoV‐2 gastrointestinal infection causing hemorrhagic colitis: implications for detection and transmission of COVID‐19 disease. Am J Gastroenterol. 2020;115(6):942‐946. 10.14309/ajg.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin L, Jian X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS‐CoV‐2 infection. Gut. 2020;69(6):997‐1001. [DOI] [PubMed] [Google Scholar]
- 19. Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS‐CoV‐2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta‐analysis. Gastroenterology. 2020; 10.1053/j.gastro.2020.03.065 (epub ahred of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS‐CoV‐2. Gastroenterology. 2020;158(6):1831‐1833.e3. Published online Mar 3. 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scarpellini E, Ang D, Pauwels A, De Santis A, Vanuytsel T, Tack J. Management of refractory typical GERD symptoms. Nat Rev Gastroenterol Hepatol. 2016;13(5):281‐294. [DOI] [PubMed] [Google Scholar]
- 22. Pauwels A, Boecxstaens V, Andrews CN, et al. How to select patients for antireflux surgery? The ICARUS guidelines (International consensus regarding preoperative examinations and clinical characteristics assessment to select adult patients for antireflux surgery). Gut. 2019;68(11):1928‐1941. [DOI] [PubMed] [Google Scholar]
- 23. Aziz Q, Fass R, Gyawali CP, Miwa H, Pandolfino JE, Zerbib F. Functional esophageal disorders. Gastroenterology. 2016:1368‐1379. pii:S0016‐5085(16):00178‐5. [DOI] [PubMed] [Google Scholar]
- 24. Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018;67(7):1351‐1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kessels SJM, Newton SS, Morona JK, Merlin TL. Safety and efficacy of wireless pH monitoring in patients suspected of gastroesophageal reflux disease: a systematic review. J Clin Gastroenterol. 2017;51(9):777‐788. [DOI] [PubMed] [Google Scholar]
- 26. Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27(2):160‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ang D, Pannemans J, Vanuytsel T, Tack J. A single‐center audit of the indications and clinical impact of prolonged ambulatory small intestinal manometry. Neurogastroenterol Motil. 2018;30(9):e13357. [DOI] [PubMed] [Google Scholar]
- 28. Kröner PT, Tolaymat OA, Bowman AW, Abril A, Lacy BE. Gastrointestinal manifestations of rheumatological diseases. Am J Gastroenterol. 2019;114(9):1441‐1454. [DOI] [PubMed] [Google Scholar]
- 29. Malagelada C, Malagelada JR. Small bowel motility. Curr Gastroenterol Rep. 2017;19(6):26. [DOI] [PubMed] [Google Scholar]
- 30. Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane‐based breath testing in gastrointestinal disorders: the North American Consensus. Am J Gastroenterol. 2017;112(5):775‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carrington EV, Heinrich H, Knowles CH, et al. The International Anorectal Physiology Working Group (IAPWG) recommendations: standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil. 2020;32(1):e13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Corsetti M, Costa M, Bassotti G, et al. First translational consensus on terminology and definitions of colonic motility in animals and humans studied by manometric and other techniques. Nat Rev Gastroenterol Hepatol. 2019;16(9):559‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation Global Study. Gastroenterology. 2020; pii: S0016‐5085(20)30487‐X. 10.1053/j.gastro.2020.04.014 (epub ahred of print). [DOI] [PubMed] [Google Scholar]
- 34. Chu DKW, Pan Y, Cheng SMS, et al. Molecular diagnosis of a novel coronavirus (2019‐nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66(4):549‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li B, Li X, Wang Y, et al. Diagnostic value and key features of computed tomography in Coronavirus Disease 2019. Emerg Microbes Infect. 2020;9(1):787‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang Y, Cheng W, Zhao N, Qu H, Tian J. CT screening for early diagnosis of SARS‐CoV‐2 infection. Lancet Infect Dis. 2020. 10.1016/S1473-3099(20)30241-3 (epub ahred of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xiao AT, Tong YX, Gao C, Zhu L, Zhang YJ, Zhang S. Dynamic profile of RT‐PCR findings from 301 COVID‐19 patients in Wuhan, China: a descriptive study. J Clin Virol. 2020;127:104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xiao AT, Tong YX, Zhang S. Profile of RT‐PCR for SARS‐CoV‐ 2: a preliminary study from 56 COVID‐19 patients. Clin Infect Dis. 2020. pii: ciaa460 (epub ahred of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Loeffelholz MJ, Tang YW. Laboratory diagnosis of emerging human coronavirus infections – the state of the art. Emerg Microbes Infect. 2020;9(1):747‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kamming D, Gardam M, Chung F. Anaesthesia and SARS. Br J Anaesth. 2003;90(6):715‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Braden B. Methods and functions: breath tests. Best Pract Res Clin Gastroenterol. 2009;23:337‐352. [DOI] [PubMed] [Google Scholar]
- 44. Gasbarrini A, Corazza GR, Gasbarrini G, et al. Methodology and indications of H2‐breath testing in gastrointestinal diseases: the Rome Consensus Conference. Aliment Pharmacol Ther. 2009;29:1‐49. [DOI] [PubMed] [Google Scholar]
- 45. Scarpellini E, Abenavoli L, Balsano C, Gabrielli M, Luzza F, Tack J. Breath tests for the assessment of the orocecal transit time. Eur Rev Med Pharmacol Sci. 2013;17(Suppl 2):39‐44. [PubMed] [Google Scholar]
- 46. Modak A. Breath tests with (13)C substrates. J Breath Res. 2009;3: 040201. [DOI] [PubMed] [Google Scholar]
- 47. Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil. 2008;20(Suppl 1):121‐129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


