Dear Editor,
Pandemic coronavirus disease 2019 (COVID‐19), caused by SARS‐CoV‐2 virus, mainly affects adult or aging individuals with fever, dry cough, and dyspnea of variable severity. In approximately 20% of patients, a widespread alveolar‐interstitial pneumonia develops with acute respiratory distress syndrome. With progression of the disease, when immune response and/or antiviral therapy fail to get rid of the virus, an excess release of pro‐inflammatory cytokines takes place (e.g., TNF‐α, IL‐1β, IL‐6, IFN‐γ, CXCL10, MCP‐1), the so called “cytokine storm,” eliciting extensive vasculitis, hyper‐coagulability, and multi‐organ damage that, together with associated respiratory failure, represent the most common cause of death (Zhang et al., 2020). It appears that in COVID‐19 there are two consecutive, partially overwhelming, pathological situations: one is due to the spreading of the viral infection, the second to the harsh inflammatory host response, against which anti‐inflammatory drugs should be preferentially employed. Indeed, both classical anti‐inflammatory agents, such as glucocorticoids, and selective molecular drugs targeting a specific cytokine or pathway, such as anti‐IL‐6 and anti‐TNF‐α monoclonal antibodies, antagonists of IL‐1β receptor, JAK inhibitors, are now being tested in clinical trials (Zhang et al., 2020). Major flaws of these treatments are the adverse effects and/or the inability to aim at diverse molecular targets. Despite much effort, no treatment has been found really effective and safe so far.
Actually, from our experimental studies on natural compounds able to protect pancreatic β‐cells against cytokine‐induced damage and death (Menegazzi et al., 2008), we became aware that Hypericum perforatum (St. John's Wort, SJW) extract, as well as its main polyphenol component hyperforin (HPF), can potently counteract the pro‐inflammatory effects of various cytokines. Indeed, both SJW extract and HPF alone prevented cytokine effects not only in β‐cell lines but also in isolated rat and human pancreatic islets (Novelli et al., 2014). The mechanism of action of SJW and HPF is based on the simultaneous blockade of multiple phosphorylation steps, induced by a mixture of IFN‐γ/IL‐1β/TNF‐α (in some way mimicking a cytokine storm), along the JAK/STAT, NF‐κB, and MAPK signaling pathways, with the result of avoiding or limiting the transcriptional activation of dysfunctional, apoptotic, and inflammatory target genes, including iNOS, COX‐2, CXCL9 and CXCL10 chemokines, ICAM‐1 adhesion molecule and others. In particular, SJW and HPF inhibited STAT‐1 and NF‐κB phosphorylation and DNA binding, as well as the activation of a number of kinases, including IKK, ERK1/2, JNK, and Akt (Menegazzi et al., 2008; Novelli et al., 2016). Furthermore, we got evidence that these natural compounds undergo an efficient intracellular uptake and confer to the cells a long‐lasting state of “cytokine resistance” (Novelli et al., 2019). Of note, although SJW extract usually contains other active ingredients at much lower concentrations than HPF (e.g., hypericin, rutin, the flavonoids quercetin and myricetin), no component other than HPF was found to be effective in inhibiting cytokine effects in the 1.0 micromolar range (Menegazzi et al., 2008).
We also showed that SJW extract exerted protective effects in various animal models of acute inflammation. Actually, SJW attenuated carrageenan‐induced inflammatory lung injury in mice by inhibiting NF‐kB and STAT‐3 activation, TNF‐α and IL‐1β production, ICAM‐1 expression, neutrophil lung infiltration, and cellular proteins nitration (Menegazzi et al., 2006). Interestingly, SJW extract was also able to counteract a zymosan‐induced multiple organ dysfunction syndrome in mouse (a kind of model of what may occur in shock, sepsis, and presently in severe COVID‐19), by reducing peritoneal exudation and migration of neutrophils, as well as pulmonary, intestinal and pancreatic injury, renal dysfunction and myeloperoxidase reaction in lung and intestine (Di Paola et al., 2007). Other authors have likewise documented the anti‐inflammatory properties of SJW or HPF, including inhibition of COX and 5‐LO activities (Albert et al., 2002), reduction of IL‐6 release (Gobbi et al., 2004), decrease of neutrophil activation of matrix metalloproteinase‐9, and enhanced resolution of bleomycin‐induced pulmonary inflammation model, with consequent reduction of lung fibrosis (Dell'Aica et al., 2007). Thus, there is clear evidence that SJW extract and HPF efficaciously prevent inflammatory damage in various cell types and tissues. In addition, it is worthwhile to consider that SJW extract is largely employed in Europe and USA as antidepressant and recognized to have a remarkable safety profile, confirmed by extensive clinical trials (Cui & Zheng, 2016).
Hence, we believe that orally administered SJW extract, containing suitable amounts of HPF, could be tested in clinical trials in COVID‐19 patients as a multitasker and well tolerated anti‐inflammatory agent. Taking into account the pharmacokinetics data established for antidepressive SJW therapeutic regimens (900–1,200 mg/day of SJW containing about 5% HPF; Biber, Fischer, Römer, & Chatterjee, 1998), we would suggest a similar or slightly increased daily dosage in COVID‐19 patients, in order to reach circulating maximal and steady state HPF concentrations within the range of those proved to inhibit cytokine effects in vitro. As for other anti‐inflammatory agents, a timely administration of SJW/HPF to COVID‐19 patients is probably crucial. We propose to administer HPF‐containing SJW extract as soon as mild initial symptoms get worse and/or blood inflammatory markers (e.g., PCR, ferritin, LDH, D‐dimer) increase. If so, SJW is expected to prevent clinical aggravation, including cytokine‐dependent thrombotic events (Levi & van der Poll, 2017), halt further rise in biochemical parameters and expedite recover. We would also underscore that the large experience in SJW using for other indications might considerably facilitate and speed up required procedures and protocol settling for controlled clinical research in COVID‐19.
It should be reminded that SJW, mainly due to HPF binding to the PXR receptor, is able to induce the liver P‐450 drug metabolizing system and in particular the CYP3A4 isoenzyme, possibly leading to accelerated clearance and reduced effect of a number of co‐administered drugs (Moore et al., 2000). Thus, upon SJW treatment, drug interaction should be carefully monitored, including appropriate dosage adjustments. Very interestingly, we should also be aware that the increase in circulating IL‐6 and other cytokines levels occurring in COVID‐19 patients suppresses the activity of the P‐450 system (Febvre‐James, Bruyère, Le Vée, & Fardel, 2018), so that SJW could advantageously counterbalance such a decline and avoid the risk of over‐exposure of patients to other drugs required for co‐morbidities management.
In conclusion, we firmly believe that the anti‐inflammatory SJW/HPF treatment deserves evaluation in COVID‐19 patients. Such a treatment, that offers the additional advantages of being orally administrable, well tolerated, and inexpensive, holds considerable promise to prevent or limit the effects of the cytokine storm through the simultaneous inhibition of NF‐κB, JAK/STAT, and MAPK pathways, that is, the three majors signaling and transduction pathways involved in cytokine‐induced local and systemic inflammatory changes (Figure 1). As such, SJW/HPF therapy appears to be compatible with other clinically pertinent treatments, for example, administration of selected antiviral agents, including chloroquine/hydroxychloroquine and/or intravenous supply of human immunoglobulins and LMW heparin.
FIGURE 1.
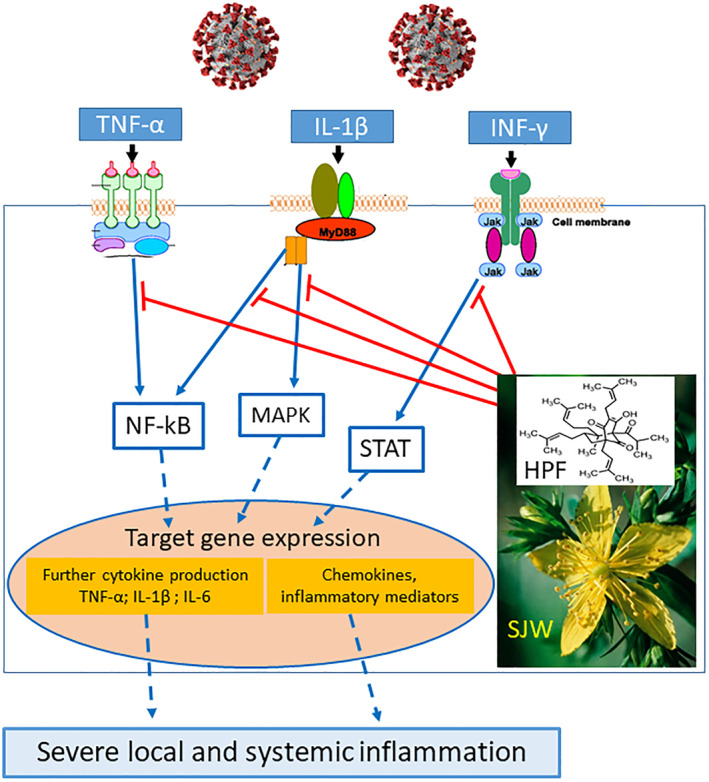
Mechanisms of the potential beneficial effects of St. John's wort (SJW) extract and hyperforin (HPF) in COVID‐19 patients [Colour figure can be viewed at wileyonlinelibrary.com]
Lastly, it is proper to state that all the studies on either SJW preparations or pure HPF quoted in this letter have actually been carried out according to the scientific qualitative standards highlighted in more recent guidelines for best practice in phytopharmacological research (Heinrich et al., 2020). In particular, the concentration or dose range of SJW extract or pure HPF tested in vitro or in vivo in the quoted studies was pharmacologically relevant and largely lower than the upper limits indicated in such guidelines.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- Albert, D. , Zündorf, I. , Dingermann, T. , Müller, W. E. , Steinhilber, D. , & Werz, O. (2002). Hyperforin is a dual inhibitor of cyclooxygenase‐1 and 5‐lipoxygenase. Biochemical Pharmacology, 64(12), 1767–1775. 10.1016/s0006-2952(02)01387-4 [DOI] [PubMed] [Google Scholar]
- Biber, A. , Fischer, H. , Römer, A. , & Chatterjee, S. S. (1998). Oral bioavailability of hyperforin from hypericum extracts in rats and human volunteers. Pharmacopsychiatry, 31(Suppl 1), 36–43. 10.1055/s-2007-979344 [DOI] [PubMed] [Google Scholar]
- Cui, Y.‐H. , & Zheng, Y. (2016). A meta‐analysis on the efficacy and safety of St John's wort extract in depression therapy in comparison with selective serotonin reuptake inhibitors in adults. Neuropsychiatric Disease and Treatment, 12, 1715–1723. 10.2147/NDT.S106752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Aica, I. , Niero, R. , Piazza, F. , Cabrelle, A. , Sartor, L. , Colalto, C. , … Garbisa, S. (2007). Hyperforin blocks neutrophil activation of matrix metalloproteinase‐9, motility and recruitment, and restrains inflammation‐triggered angiogenesis and lung fibrosis. The Journal of Pharmacology and Experimental Therapeutics, 321(2), 492–500. 10.1124/jpet.106.116459 [DOI] [PubMed] [Google Scholar]
- Di Paola, R. , Mazzon, E. , Muià, C. , Crisafulli, C. , Genovese, T. , Di Bella, P. , … Cuzzocrea, S. (2007). Protective effect of Hypericum perforatum in zymosan‐induced multiple organ dysfunction syndrome: Relationship to its inhibitory effect on nitric oxide production and its peroxynitrite scavenging activity. Nitric Oxide: Biology and Chemistry, 16(1), 118–130. 10.1016/j.niox.2006.05.006 [DOI] [PubMed] [Google Scholar]
- Febvre‐James, M. , Bruyère, A. , Le Vée, M. , & Fardel, O. (2018). The JAK1/2 inhibitor Ruxolitinib reverses Interleukin‐6‐mediated suppression of drug‐detoxifying proteins in cultured human hepatocytes. Drug Metabolism and Disposition: The Biological Fate of Chemicals, 46(2), 131–140. 10.1124/dmd.117.078048 [DOI] [PubMed] [Google Scholar]
- Gobbi, M. , Moia, M. , Funicello, M. , Riva, A. , Morazzoni, P. , & Mennini, T. (2004). In vitro effects of the dicyclohexylammonium salt of hyperforin on interleukin‐6 release in different experimental models. Planta Medica, 70(7), 680–682. 10.1055/s-2004-827194 [DOI] [PubMed] [Google Scholar]
- Heinrich, M. , Appendino, G. , Efferth, T. , Fürst, R. , Izzo, A. A. , Kayser, O. , … Viljoen, A. (2020). Best practice in research—Overcoming common challenges in phytopharmacological research. Journal of Ethnopharmacology, 246, 112230. 10.1016/j.jep.2019.112230 [DOI] [PubMed] [Google Scholar]
- Levi, M. , & van der Poll, T. (2017). Coagulation and sepsis. Thrombosis Research, 149, 38–44. 10.1016/j.thromres.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Menegazzi, M. , Di Paola, R. , Mazzon, E. , Muià, C. , Genovese, T. , Crisafulli, C. , … Cuzzocrea, S. (2006). Hypericum perforatum attenuates the development of carrageenan‐induced lung injury in mice. Free Radical Biology & Medicine, 40(5), 740–753. 10.1016/j.freeradbiomed.2005.08.034 [DOI] [PubMed] [Google Scholar]
- Menegazzi, M. , Novelli, M. , Beffy, P. , D'Aleo, V. , Tedeschi, E. , Lupi, R. , … Masiello, P. (2008). Protective effects of St. John's wort extract and its component hyperforin against cytokine‐induced cytotoxicity in a pancreatic beta‐cell line. The International Journal of Biochemistry & Cell Biology, 40(8), 1509–1521. 10.1016/j.biocel.2007.11.019 [DOI] [PubMed] [Google Scholar]
- Moore, L. B. , Goodwin, B. , Jones, S. A. , Wisely, G. B. , Serabjit‐Singh, C. J. , Willson, T. M. , … Kliewer, S. A. (2000). St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proceedings of the National Academy of Sciences of the United States of America, 97(13), 7500–7502. 10.1073/pnas.130155097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli, M. , Beffy, P. , Gregorelli, A. , Porozov, S. , Mascia, F. , Vantaggiato, C. , … Menegazzi, M. (2019). Persistence of STAT‐1 inhibition and induction of cytokine resistance in pancreatic β cells treated with St John's wort and its component hyperforin. The Journal of Pharmacy and Pharmacology, 71(1), 93–103. 10.1111/jphp.12823 [DOI] [PubMed] [Google Scholar]
- Novelli, M. , Beffy, P. , Menegazzi, M. , De Tata, V. , Martino, L. , Sgarbossa, A. , … Masiello, P. (2014). St. John's wort extract and hyperforin protect rat and human pancreatic islets against cytokine toxicity. Acta Diabetologica, 51(1), 113–121. 10.1007/s00592-013-0518-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli, M. , Menegazzi, M. , Beffy, P. , Porozov, S. , Gregorelli, A. , Giacopelli, D. , … Masiello, P. (2016). St. John's wort extract and hyperforin inhibit multiple phosphorylation steps of cytokine signaling and prevent inflammatory and apoptotic gene induction in pancreatic β cells. The International Journal of Biochemistry & Cell Biology, 81(Pt A), 92–104. 10.1016/j.biocel.2016.10.017 [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Zhao, Y. , Zhang, F. , Wang, Q. , Li, T. , Liu, Z. , … Zhang, S. (2020). The use of anti‐inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID‐19): The perspectives of clinical immunologists from China. Clinical Immunology (Orlando, Fla.), 214, 108393. 10.1016/j.clim.2020.108393 [DOI] [PMC free article] [PubMed] [Google Scholar]


