Abstract
Few reports described the outcome of kidney transplanted patients (KTs) affected by COVID‐19 treated with interleukin‐6 receptor inhibitor tocilizumab (TCZ). We report our case series of 6 KTs with COVID‐19 pneumonia who received TCZ: All were of male gender, with a mean age of 55.5 ± 8.4 years, a median time from transplantation of 3611 days (1465‐5757); 5/6 had cardiovascular comorbidities, 1/6 had diabetes, and 3/6 have one or more previous KTs. Four out of six patients died, at an average time of 9.75 ± 2.4 days after tocilizumab administration, 3/6 due to a coexistent septic shock. Two patients improved after TCZ and were discharged at 20 and 21 days, respectively; in both patient, a significant increase of total lymphocyte count was observed. In conclusion, KTs, where the role of peculiar factors such as chronic immunosuppression is still undetermined, represent a high‐risk group with significant COVID‐19‐associated mortality. The evaluation of the TCZ effect in COVID‐19 pneumonia requires controlled studies (ideally RCTs) in this specific population.
Keywords: COVID‐19, kidney transplant, Tocilizumab
1. BACKGROUND
Severe acute respiratory syndrome coronavirus 2 (named SARS CoV‐2/COVID‐19) is a novel pandemic infectious disease, which emerged in Wuhan, China, in late December 2019 and spread rapidly worldwide. 1 , 2 At the time we are writing (05/10/2020), in Italy there are about 219 000 patients infected, 28 600 only in Piedmont, our region. 3 Clinically, the disease is characterized with fever, cough, dyspnea, diarrhea, and eventually respiratory failure. 4 , 5 According to their intrinsic frailty and comorbidities, transplanted patients were considered a high‐risk population. 6 , 7
Tocilizumab (TCZ), a humanized monoclonal antibody against interleukin‐6 (IL‐6) receptor widely adopted in adult rheumatoid arthritis and also used as rescue therapy for chronic antibody‐mediated rejection in kidney transplantation, 8 has been recently registered for the treatment of severe or life‐threatening chimeric antigen receptor T–cell induced cytokine release syndrome (CRS) in adult and pediatric patients. 9 In this context, because the development of acute respiratory distress syndrome (ARDS) in COVID‐19 pneumonia has been associated with activation of the immune system and consequent cytokine storm with high levels of IL‐6, some initial reports suggested a beneficial role of this drug, 10 , 11 also in solid organ transplanted patients. 12 Herein, we reported our experience in 6 kidney transplanted patients treated with TCZ after occurrence of COVID‐19 infection.
2. CASE SERIES
Clinical characteristics and laboratory data are shown in Tables 1 and 2. Figure 1 reported the timeline of maintenance immunosuppression, COVID‐19‐specific treatments, and outcome. In all patients, diagnosis was performed by nasopharyngeal swab test (PCR) and chest radiography or high‐resolution computed tomography (HRCT). TCZ was administered once daily for two consecutive days (dose 8 mg/kg) after a consultation with infectious disease specialist in patients with contemporary evidence of pulmonary involvement (oxygen saturation—Sa02—<93% if patients breath ambient air, or a ratio of the partial pressure of oxygen—PaO2—to the fraction of inspired oxygen—FiO2—of less than 300 mm Hg) and pro‐inflammatory profile (C‐reactive protein and/or IL‐6 > × 10 normal values). All patients gave written informed consent for TCZ off‐label use.
Table 1.
Clinical characteristics, comorbidities, and symptoms at presentation in our COVID‐19‐positive kidney transplant recipients
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Gender | Male | Male | Male | Male | Male | Male |
| Age, years | 41 | 65 | 54 | 62 | 49 | 62 |
| Previous KT (n) | No | Yes (1) | Yes (2) | Yes (1) | No | No |
| Time from last KT, days | 5354 | 8 | 2053 | 4681 | 6411 | 3163 |
| Comorbidities | ||||||
| Hypertension | Yes | Yes | No | Yes | No | Yes |
| Diabetes mellitus | No | Yes | No | No | No | No |
| Cardiovascular disease | No | Yes | Yes | Yes | Yes | Yes |
| HCV infection a | No | No | No | Yes | Yes | No |
| Immunosuppressive therapy | ||||||
| TAC | Yes | Yes | Yes | Yes | Yes | Yes |
| MMF | No | Yes | No | Yes | Yes | No |
| Steroids | Yes | Yes | Yes | Yes | Yes | Yes |
| Symptoms at presentation | ||||||
| Fever | Yes | Yes | Yes | Yes | Yes | Yes |
| Cough | Yes | Yes | No | No | Yes | No |
| Dyspnea | No | No | No | Yes | No | Yes |
| Diarrhea | No | No | Yes | Yes | No | No |
Abbreviations: KT, kidney transplant; MMF, mycophenolate mofetil; TAC, tacrolimus.
Negative HCV‐RNA in both cases after eradication.
Table 2.
Laboratory and pulmonary functional tests before and after TCZ adoption in our COVID‐19‐positive kidney transplant recipients
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| C‐reactive protein (mg/L) | ||||||
| Before TCZ | 170.4 | 90.2 | 154.7 | 32 | 49.8 | 71.4 |
| Day 3 after TCZ | 35.4 | 20.3 | 44.6 | 12 | 23.7 | 8.6 |
| At last f/up | 3.6 | 34.9 | 2.24 | 10 | 0.6 | 2.4 |
| Procalcitonin (ng/mL) | ||||||
| Before TCZ | 24.1 | 0.19 | 0.18 | 0.12 | 0.43 | 0.4 |
| Day 3 after TCZ | 1.02 | 0.1 | 0.3 | 0.13 | 0.29 | NA |
| At last f/up | 0.17 | 0.73 | 0.07 | 0.15 | 0.06 | 0.45 |
| Whyte‐cell count [lymphocyte] (per mm 3 ) | ||||||
| Before TCZ | 9930 [400] | 7240 [370] | 4380 [610] | 13 660 [230] | 4130 [300] | 7890 [480] |
| Day 3 after TCZ | 11 300 [230] | 6270 [310] | 4020 [800] | 21 790 [400] | 4370 [330] | 4110 [140] |
| At last f/up | 6390 [850] | 11 720 [410] | 3770 [950] | 36 890 [800] | 6440 [1030] | 10 420 [380] |
| Lactate dehydrogenase (UI/L) | ||||||
| Before TCZ | 771 | 518 | 652 | 704 | 573 | 1068 |
| Day 3 after TCZ | 496 | 792 | 765 | 565 | 762 | 816 |
| At last f/up | 480 | 660 | 603 | 814 | 779 | 739 |
| D‐Dimer (ng/mL) | ||||||
| Before TCZ | 12 552 | 707 | 730 | 3188 | 876 | 351 |
| Day 3 after TCZ | 18 111 | 1497 | 840 | 2873 | 2020 | <215 |
| At last f/up | 1565 | 2032 | 500 | 2842 | 1468 | 281 |
| Ferritin (ng/mL) | ||||||
| Before TCZ | 1754 | 523 | NA | 830 | 1567 | 955 |
| Day 3 after TCZ | 1108 | 847 | NA | 637 | 1065 | 767 |
| At last f/up | 1310 | 906 | NA | 840 | 371 | NA |
| IL‐6 (pg/mL) | ||||||
| Before TCZ | NA | NA | 24.9 a | 465 b | NA | 312.5 b |
| Day 3 after TCZ | NA | NA | 188.7 a | 564.5 b | NA | 282.7 b |
| At last f/up | NA | NA | 63.3 a | 1077.6 b | NA | NA |
| Pa02/Fi02 (mm Hg) | ||||||
| Before TCZ | 115 | 291 | 333 | 300 | 83 | 182 |
| Day 3 after TCZ | 135 | 203 | 256 | 111 | 291 | 265 |
| At last f/up | 92 | 87 | 493 | 170 | 347 | 187 |
Abbreviations: FiO2, fraction of inspired oxygen; PaO2, partial pressure of oxygen; TCZ, tocilizumab.
Normal range 0‐4.4.
Normal range 2‐29.
FIGURE 1.
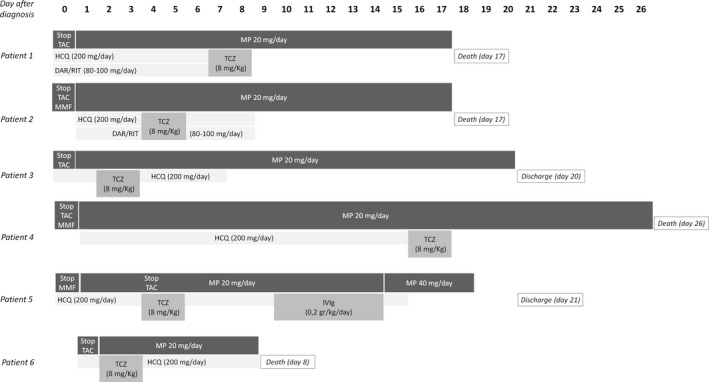
Therapeutic timeline for anti‐COVID‐19 and immunosuppressant medications in our case series. DAR/RIT, darunavir/ritonavir; HCQ, hydroxychloroquine; IVIg, intravenous immunoglobulin; MMF, mycophenolate mofetil; MP, methylprednisolone; TAC, tacrolimus; TCZ, tocilizumab
2.1. Patient 1
41‐year‐old patient. He received kidney transplant from deceased donor 15 years ago. Ongoing immunosuppressive therapy was composed by tacrolimus (TAC) and prednisone.
After few days of fever and cough, patient was tested positive for COVID‐19 on 03/25/2020. TAC was immediately discontinued while glucocorticoids (methylprednisolone 20 mg daily) were maintained. In the same day of the diagnosis, hydroxycloroquine and antiviral treatment (darunavir/ritonavir 800/100 mg) were started, in association with antibiotic therapy (cefepime). On day 7 after diagnosis, because of rapid worsening of respiratory status, TCZ was administered and repeated in day 8; concomitantly, according to progressive deterioration of kidney function and difficult management of fluid overload, continuous renal‐replacement therapy (CRRT) was initiated. Despite the antibiotic therapy implementation with azithromycin, respiratory failure occurred needing mechanical ventilation from day 15; patient died two days after.
2.2. Patient 2
65‐year‐old patient. He received kidney transplant from a deceased donor on March 2020. A negative COVID‐19 test was obtained before kidney transplant, and no positive contact with COVID‐19 subjects was reported in the days before KT.
Induction immunosuppressive therapy was composed by thymoglobulin (for three days) and methylprednisolone, and subsequent maintenance therapy included TAC (levels 10‐12 ng/mL), mycophenolate mofetil (MMF) and prednisone. Patient also received ganciclovir for CMV prophylaxis (CMV IgG‐/donor CMV IgG+).
Eight days after KT patient experienced fever and cough; swab test was subsequently repeated and resulted positive. Immunosuppressive medications (TAC, MMF) were immediately stopped, except of glucocorticoids (methylprednisolone 20 mg/d). Patient started hydroxycloroquine (200 mg daily) and darunavir/ritonavir (800/100 mg daily) the day after and remained hemodynamically stable with only slow reduction of peripheral oxygen saturation (94%‐95% with low‐flow oxygen through nasal cannula); TCZ was administered on day 4 and 5.
On day 6, arterial pO2 decreased and patient started non‐invasive ventilation (NIV); after a further deterioration of pulmonary status, mechanical ventilation was needed from day 7.
On day 8, blood and urinary cultures were both positive for multi‐sensitive Klebsiella Pneumoniae (urinary culture was also positive for Enterococcus Faecalis), and antibiotic treatment was shifted from amoxicillin/clavulanic acid to cefepime.
Despite progressive modifications on antibiotic therapy (with at least adoption of gentamicin, piperacillin/tazobactam, and daptomycin), fluid and medication support patient became oliguric (starting CRRT from day 12) and died on day 17.
2.3. Patient 3
54‐year‐old patient. He received his third kidney transplant in 2014. Ongoing immunosuppressive therapy was composed by TAC and glucocorticoids. On 04/03/2020, patient experienced fever, emesis, and diarrhea and tested positive for COVID‐19. TAC was immediately discontinued, and only methylprednisolone 20 mg/daily was maintained in association of hydroxycloroquine (200 mg/d). On day 2, hemophtysis and worsening of respiratory status occurred. Oral anticoagulant therapy (in patient with mechanical aortic valve) was stopped and replaced by low‐molecular‐weight heparin, and non‐invasive ventilation (NIV) was started. TCZ was administered on day 2 and 3. On day 12, after an improvement in respiratory condition, NIV was suspended; patient was discharged on day 20. Currently (26 days after discharge), he recovered to normal pulmonary functional status, keeping only steroids (prednisone 25 mg/d) as immunosuppressive therapy.
2.4. Patient 4
62‐year‐old patient. He performed his second KT on 2007. Immunosuppressive therapy was composed by TAC, MMF, and steroids. On 03/22/2020, patient experienced fever, dyspnea, and diarrhea and then tested positive for COVID‐19. TAC and MMF were immediately discontinued. Hydroxycloroquine 200 mg daily, methylprednisolone 20 mg daily, and piperacillin‐tazobactam, in association to fluconazole, were started. A rapid worsening of respiratory status was observed from day 1 after diagnosis, needing mechanical ventilation. TCZ was administered (8 mg/kg) on day 16; respiratory status showed no improvement and patient deceased on day 26.
2.5. Patient 5
49‐year‐old patient. He received kidney transplant 18 years ago. Ongoing immunosuppressive therapy was TAC, MMF, and glucocorticoids. On 03/24/2020, he was admitted to the Emergency Department because fever and cough from two days. After positive swab test, TAC level was reduced, MMF was stopped, and hydroxycloroquine (200 mg/d) plus antibiotic therapy (ceftaroline) was started.
On day 4, after progressive worsening of respiratory status, TAC was discontinued and NIV plus TCZ were started. On day 11, IVIg (0.2 g/kg/d) was also administered for four consecutive days. On day 16, glucocorticoid therapy was increased with methylprednisolone 40 mg twice daily. NIV was discontinued on day 17 and the patient was discharged on day 21 after diagnosis.
Currently (12 days after discharge), pulmonary function recovered to basal patient's status and TAC target through level are progressively increased to 4‐5 ng/mL with glucocorticoids maintained.
2.6. Patient 6
62‐year‐old patient. He received KT in 2011. Ongoing immunosuppressive therapy was composed by TAC and glucocorticoids. Patient was admitted to Emergency Department on 04/03/2020 because of fever and dyspnea. After diagnosis of COVID‐19 pneumonia, TAC was stopped on day 1 and treatment with hydroxycloroquine (200 mg daily) and amoxicillin‐clavulanic acid (1 g twice daily) were started, in association with methylprednisolone 20 mg daily. Mechanical ventilation was also initiated on day 1; TCZ was administered on day 2 and 3. Despite all supportive therapies, pulmonary function rapidly declined and patient deceased on day 8 after diagnosis.
3. DISCUSSION
COVID‐19 is now considered as the most prominent health‐care problem around the world. 13 Emerging data highlight the role of comorbid conditions (hypertension, diabetes, cardiovascular disease) and older age as major determinants of negative outcome. 10 Recently, kidney disease has also been associated with increased in‐hospital mortality. 14
Transplant patients, due to the combination of this frail profile associated with immunosuppressive medications, are considered a high‐risk population 15 ; despite preventive strategies and medication protocols are urgently needed, definite guidelines for KT are not currently established and different and specular approaches have so far been reported.
Some group suggest the immediately withdrawn of immunosuppressive drugs with only steroid maintenance, 1 , 16 despite steroid use is controversial and associated with reduced viral clearance. 17 Other authors report a possible antiviral effect of CNI inhibitors 2 , 4 , 6 , 10 , 18 suggesting per contrast their maintenance in solid organ transplanted patients, especially in liver transplants. This theory is also supported by the evidence that ARDS occurred in patients with important inflammatory activation (the so‐called cytokine storm) and in this context immunosuppressive medications and anti‐inflammatory drug (ie, TCZ) may mitigate the damage. All these data are partially questioned by a recent US report 19 were mortality in KT patients is extremely high compared to normal population, despite a recent Spanish case series detailed a more favorable picture 20 .
To date, few reports included TCZ‐treated kidney transplanted patients with COVID‐19 pneumonia 12 , 19 , 20 , 21 , 22 , 23 (Table 3): In all these available experiences, inclusion criteria, dose, f/up, and outcome indicators were different, and a significant number of cases was still inpatients.
Table 3.
Kidney transplant recipients with COVID‐19 treated with tocilizumab (PubMed update on 05/09/2020)
| Author | Patients, n (studied population) | Criteria for tocilizumab adoption | Dose, mg (n) | Pulmonary outcome | Patients outcome at last f/up |
|---|---|---|---|---|---|
| Ferandez‐Ruiz et al 21 | 1 (1) | Progressive respiratory failure + increasing inflammatory parameters | 600 mg iv (one dose) | Mild radiological improvement | Inpatient |
| Alberici et al 22 | 6 (20) | Worsening of respiratory infection | 8 mg/kg iv, max 800 mg (two doses, intervals 12‐24 h) |
3/6 reduced oxygen requirement 2/6 radiological improvement |
3/6 inpatient 2/6 death 1/6 discharged |
| Akalin et al 19 | 2 (28) a | NA | NA | NA | NA (10/28 discharged) |
| Fontana et al 12 | 1 (1) |
PFT deterioration + elevated IL‐6 |
324 mg sc (one dose) | Recovery | Discharged |
| Montagud‐Marrahi et al 20 | 13 (26) a | NA | NA | NA | NA (21/26 discharged) |
| Pereira et al 23 | 14 (90) b | Rapid pulmonary decompensation due to high and deleterious cytokine activity | 400 mg sc or 8 mg/kg iv, max 800 mg (9/14 one dose, 4/14 two doses, 1/14 three doses) | NA |
3/14 death 4/14 inpatient (ICU) 5/14 inpatient 2/14 discharged |
Abbreviations: ICU, intensive care unit; iv, intravenous; NA, not available; sc, subcutaneous.
Inpatient group.
46/90 kidney tranplants.
Despite different timing, in our case series TCZ was administered at the same dosage after deterioration of pulmonary status and contemporary evidence of significant inflammatory activation (increase C‐reactive protein and/or IL‐6, if available). The adoption of TCZ determined a C‐reactive protein reduction in all patients without significant modification in LDH or D‐Dimer; also IL‐6 decreased in the 2/3 patients with available test, albeit levels remain significantly upper the normal range. However, four out of six patients died; in the 2/6 who recovered a significant increase in lymphocyte count was observed, confirming that a restoration of lymphocyte activity is crucial for obtaining a favorable outcome. 24
We need to underline that some factors may be confounding in the analysis of the direct effect of TCZ: For example, sepsis may have concurred to death in patients 1, 4, and 6; however, as reported by other Authors, the virus caused a profound immunosuppression 5 , 11 and, in this context, the result of combined inhibition of IL‐6 receptor in predisposing to bacterial infections is not well understood. We also pointed out that we treated with TCZ only kidney transplanted patients who required hospitalization with significant reduction of pulmonary function and increase of inflammatory markers. At the time we are writing, 20 patients who received KTs in our Center were tested positive for COVID‐19, and 8/20 (including 4/8 who received TCZ) died. Because all patients with COVID‐19 who do not need hospitalization were tested and remained at home, we do not have laboratory/pulmonary functional markers to compare this cohort to TCZ‐treated patients.
In conclusion, despite these data should be evaluated with extremely caution and have some limitations (selection bias, availability of IL‐6 dosage in all patients, absence of CD4+/CD8 + count), our study confirmed that kidney transplanted patients are a high‐risk group with significant COVID‐19‐associated mortality. The evaluation of the TCZ effect in COVID‐19 pneumonia requires controlled studies (ideally RCTs) in this specific population.
CONFLICT OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose as described by the Transplant Infectious Disease Journal.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
AUTHORS CONTRIBUTION
AM, SM and LB wrote the main manuscript text; AM, SM, EG, AL, MR, RC, OR, SM, MS, DI, and LB contributed to the conception, design, and analysis of the data; all authors also contributed to the revision and approval of the final manuscript.
Mella A, Mingozzi S, Gallo E, et al. Case series of six kidney transplanted patients with COVID‐19 pneumonia treated with tocilizumab. Transpl Infect Dis. 2020;22:e13348. 10.1111/tid.13348
REFERENCES
- 1. Zhu L, Xu X, Ma K, et al. Successful recovery of COVID‐19 pneumonia in a renal transplant recipient with long‐term immunosuppression. Am J Transplant. 2020. 10.1111/ajt.15869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang H, Chen Y, Yuan Q, et al. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. 2020;77(6):742‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coronavirus (COVID‐19) Data in Italy, Italian Health Department. http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?lingua=italiano&id=5351&area=nuovoCoronavirus&menu=vuoto, May 10 2020.
- 4. Wang J, Li X, Cao G, et al. COVID‐19 in a Kidney Transplant Patient. Eur Urol. 2020;77(6):769‐770. 10.1016/j.eururo.2020.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Romanelli A, Mascolo S. Immunosuppression drug‐related and clinical manifestation of Coronavirus disease 2019: A therapeutical hypothesis. Am J Transplant. 2020. 10.1111/ajt.15905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID‐19 in a kidney transplant recipient: Does immunosuppression alter the clinical presentation? Am J Transplant. 2020. 10.1111/ajt.15874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi J, Aubert O, Vo A, et al. Assessment of Tocilizumab (Anti‐Interleukin‐6 Receptor Monoclonal) as a Potential Treatment for Chronic Antibody‐Mediated Rejection and Transplant Glomerulopathy in HLA‐Sensitized Renal Allograft Recipients. Am J Transplant. 2017;17(9):2381‐2389. 10.1111/ajt.14228 [DOI] [PubMed] [Google Scholar]
- 9. Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell‐induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15(8):813‐822. 10.1080/1744666X.2019.1629904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmadpoor P, Rostaing L. Why the immune system fails to mount an adaptive immune response to a COVID‐19 infection. Transplant Int. 2020. 10.1111/tri.13611. [DOI] [PubMed] [Google Scholar]
- 11. Luo P, Liu Y, Qiu L, et al. Tocilizumab treatment in COVID‐19: A single center experience. J Med Virol. 2020. 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fontana F, Alfano G, Mori G, et al. COVID‐19 pneumonia in a kidney transplant recipient successfully treated with tocilizumab and hydroxychloroquine. Am J Transplant. 2020. 10.1111/ajt.15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019. Apr 27 2020.
- 14. Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in‐hospital death of patients with COVID‐19. Kidney Int. 2020;97(5):829‐838. 10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alberici F, Delbarba E, Manenti C, et al. Management Of Patients On Dialysis And With Kidney Transplant During SARS‐COV‐2 (COVID‐19) Pandemic In Brescia, Italy [published online ahead of print, 2020 Apr 4]. Kidney Int Rep. 2020. 10.1016/j.ekir.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gandolfini I, Delsante M, Fiaccadori E, et al. COVID‐19 in kidney transplant recipients. Am J Transplant. 2020. 10.1111/ajt.15891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet. 2020;395(10223):473‐475. 10.1016/S0140-6736(20)30317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Columbia University Kidney Transplant Program . Early Description of Coronavirus 2019 Disease in Kidney Transplant Recipients in New York [published online ahead of print, 2020 Apr 21]. J Am Soc Nephrol. 2020. ASN.2020030375. 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and Kidney Transplantation. N Engl J Med. 2020. 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montagud‐Marrahi E, Cofan F, Torregrosa JV, et al. Preliminary data on outcomes of SARS‐CoV‐2 infection in a Spanish single centre cohort of kidney recipients [published online ahead of print, 2020 May 5]. Am J Transplant. 2020. 10.1111/ajt.15970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fernández‐Ruiz M, Andrés A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: A single‐center case series from Spain. Am J Transplant. 2020. 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short‐term outcome of 20 kidney transplant patients admitted for SARS‐CoV2 pneumonia. Kidney Int. 2020;97(6):1083‐1088. 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020. 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


