1.
To The Editor,
In late December 2019, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has been identified as a novel pathogen causing coronavirus disease 2019 (COVID‐19) in Wuhan, China, subsequently spread to the rest of China and has been demonstrating a rapid global spread. 1 Nucleic acid testing (NAT, tested by real‐time polymerase chain reaction) of SARS‐CoV‐2 virus in oropharyngeal/nasal swab samples has been described to be extremely sensitive for the diagnose of SARS‐CoV‐2 infection, 2 but false‐negative results have been reported. 3 Recent months, researches demonstrated the importance of IgM/IgG antibody detecting due to the unsatisfied positive rate of NAT, and the increasement IgM/IgG antibody was considered as a confirmed criterion of diagnosis in the official guides of the diagnosis and treatment of COVID‐19 in China (7th Edition). In general population, the IgM/IgG antibody presents positive in 4‐7 days after the onset of disease and peaks at 10‐14 days. IgM and IgG appeared and peaked in different times, and IgG had 100% sensitivity within 2 months with dramatic increased titer. 4 , 5 In this report, we present a case of renal transplant recipient with SARS‐CoV‐2 virus infected confirmed by computed tomography (CT) scan and NAT, who failed to response to the virus with IgM/IgG negative for more than 2 months.
A 63‐year‐old woman with end‐stage renal disease (ESRD) was underwent renal transplantation on December 31, 2019. The immunosuppressive therapy consisted of oral tacrolimus, mycophenolate mofetil (MMF), and methylprednisolone (with gradually decreased dose). The urine volume after transplantation was limited, and the delayed graft function (DGF) was confirmed. On the tenth day after transplantation (January 10, 2020), she developed shortness of breath with low fever, SpO2 decreased to 90%, acute pulmonary edema and infection were considered, she was admitted to intensive care unit (ICU), and CT scan showed pulmonary infection on January 17. Because of her sustained low fever and the outbreak of COVID‐19 in Wuhan, NAT (against SARS‐CoV‐2 ORF Lab and N gene) was performed on nasopharyngeal aspirate on January 28 and was confirmed as a positive result. The NAT results of January 31 and February 2 were negative, and she was admitted to the isolation ward for the following treatment. The renal function and the general condition were gradually recovered.
Two pan‐drug‐resistant strains of klebsiella pneumoniae and pseudomonas aeruginosa were identified in urine samples from March 4 and were detected in blood from March 22. She succumbed to the multiple organ failure caused by the refractory drug‐resistant infection finally. The body temperature, WBC, NEUT%, LYMPH%, LYMPH#, FK506 concentration, CREA, and IL‐6 were presented in Figure 1. The NAT and antibody test (IgM, IgG) for SARS‐CoV‐2 virus were performed on March 15(by chemiluminescent immunoassay (CLIA)), March 27(by chemiluminescent immunoassay (CLIA)), and April 5 (by gold immunochromatography assay (GICA)), and all of the results were negative.
FIGURE 1.
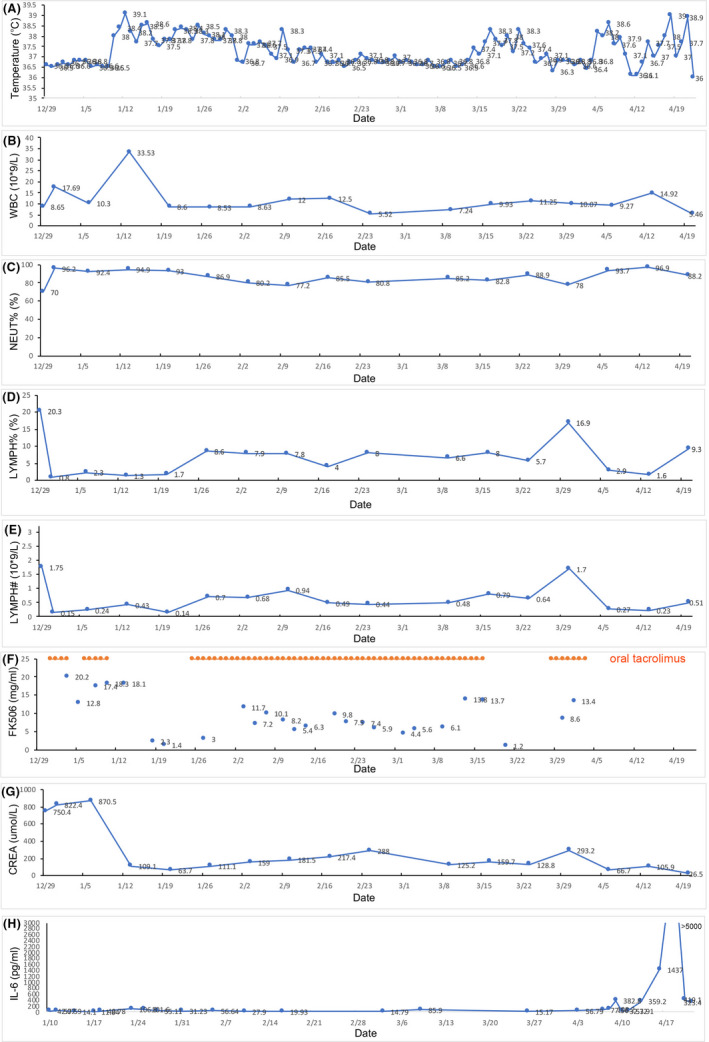
The body temperature, WBC, NEUT%, LYMPH%, LYMPH#, FK506 concentration, CREA, and IL‐6 in different times
In general population, both cell mediated and humoral immune response to the SARS‐CoV‐2 virus. 6 , 7 The virus enters the cell via the angiotensin‐converting enzyme‐2 (ACE‐2) by Toll‐like receptor‐7 (TLR −7). 8 TLR‐7 activation leads to the production of TNF‐alpha, alpha‐interferon, and the secretion of interleukin (IL)‐6 and IL‐12. This results in the formation of CD8+‐specific cytotoxic T cells and, through the CD4 + helper T‐cell, leads to the formation of antigen‐specific B cells and antibody production. 9 In SOT‐infected patients, the suppressive naïve T cells caused by immunosuppressant drugs failed to recognize the pathogens, which most probably lead to the delayed or failed response of specific cellular and humoral immunity. 10
In conclusion, SOT recipients represent a frail immune status due to the long‐term immunosuppressive therapy, the diagnosis of COVID‐19 is more complex, and they may more susceptible to SARS‐CoV‐2 virus re‐infected. For this reason, clinical manifestations may differ from general population and different diagnostics and treatment approaches may be needed.
CONFLICT OF INTEREST
The authors of this manuscript have no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
Xia Z and Liu X contributed equally and shared first authorship. Hu X collected the study data. All authors contributed to the drafting, review, and final approval of this manuscript.
REFERENCES
- 1. Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID‐19 based on current evidence. J Med Virol. 2020;92(6):548‐551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JF, Yip CC, To KK, et al. Improved Molecular Diagnosis of COVID‐19 by the Novel, Highly Sensitive and Specific COVID‐19‐RdRp/Hel Real‐Time Reverse Transcription‐PCR Assay Validated In Vitro and with Clinical Specimens. J Clin Microbiol. 2020;58(5):e00310‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Winichakoon P, Chaiwarith R, Liwsrisakun C, et al. Negative Nasopharyngeal and oropharyngeal swabs do not rule out COVID‐19. J Clin Microbiol. 2020;58(5):e00297‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Du Z, Zhu F, Guo F, Yang B, Wang T. Detection of antibodies against SARS‐CoV‐2 in patients with COVID‐19. J Med Virol. 2020. 10.1002/jmv.25820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Padoan A, Cosma C, Sciacovelli L, Faggian D, Plebani M. Analytical performances of a chemiluminescence immunoassay for SARS‐CoV‐2 IgM/IgG and antibody kinetics. Clin Chem Lab Med. 2020;58(7):1081–1088. [DOI] [PubMed] [Google Scholar]
- 6. Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baruah V, Bose S. Immunoinformatics‐aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019‐nCoV. J Med Virol. 2020;92(5):495‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fung SY, Yuen KS, Ye ZW, Chan CP, Jin DY. A tug‐of‐war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect. 2020;9(1):558‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu J, Mohan C. Toll‐like receptor signaling pathways–therapeutic opportunities. Mediators Inflamm. 2010;2010:781235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020;323(18):1775–1776. [DOI] [PubMed] [Google Scholar]
ACKNOWLEDGEMENTS
This research was supported by the National Natural Science Foundation of China, Grant No. 81600587 to Zhiping Xia.
Xia and Liu are contributed equaly.


