Since December 2019, the world has been battling a pandemic caused by a novel coronavirus species, the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), responsible for the coronavirus disease 2019 or COVID‐19 as defined by the World Health Organization in February 2020. This outbreak was first reported in Wuhan, China, and since then it has spread across the globe. As of 12 May 2020 more than 4.2 million confirmed cases worldwide with greater than 291 000 fatalities have been reported. 1 Importantly, the total number of individuals who tested positive is most likely an underestimate of the gravity and infectivity of the disease.
Patients with COVID‐19 and concomitant type 2 diabetes mellitus (T2DM) present a less favourable prognosis compared to those without T2DM, although their risk of initial infection may not be necessarily increased. 2 , 3 Such association does not come to surprise considering the fact that T2DM is also associated with worse prognosis in other viral infections. 3 In a multicenter cohort of >7300 patients diagnosed with COVID‐19 in China, the presence of T2DM was associated with 49% relative risk increase (RRI) for 28‐day inhospital mortality, even after adjustment for the severity of COVID‐19 (Figure 1A). 4 T2DM was also associated with a 44%, 200% and 95% RRI for acute respiratory distress syndrome (ARDS), acute kidney injury and septic shock, respectively. 4
FIGURE 1.
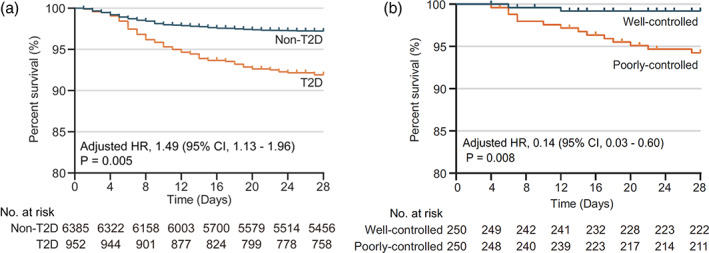
The effects of T2DM and glycemic control on survival in patients with COVID‐19 and admitted to the hospital. A, Kaplan‐Meier curves in figure shows that patients with T2DM have a significantly worse 28‐day survival probability compared to those without T2DM at time of admission. B, Among patients with T2DM, those with worse glycemic variability (ie, ‘poorly controlled’) (fasting glycemia >70 mg/dL and highest 2‐hour post‐prandial glycemia >180 mg/dL) presented a worse survival compared to those with greater glycemic control (ie, ‘well‐controlled’) (fasting glycemia >70 mg/dL and highest 2‐hour post‐prandial glycemia <180 mg/dL). COVID‐19, coronavirus disease 2019; HR, hazard ratio; T2D, type 2 diabetes; T2DM, type 2 diabetes mellitus. Source: Modified with permission from Zhu et al 4
1. PATHOPHYSIOLOGY DRIVING T2DM TO WORSE PROGNOSIS IN COVID‐19
The mechanisms explaining the detrimental effects of T2DM on COVID‐19‐related survival remain to be elucidated. Evidence of increased systemic inflammation [ie, interleukin (IL)‐6, C‐reactive protein (CRP)] has been associated with worse outcomes in COVID‐19 patients. 2 , 3
Similarly, patients with T2DM, especially in the setting of uncontrolled hyperglycemia, also present with a systemic chronic inflammatory state. This state may be attributed to a chronic activation of the macromolecular complex NLRP3 inflammasome. The latter is responsible for the production of the proinflammatory cytokines IL‐1 and IL‐18 which can, in turn, induce the expression of IL‐6 and ultimately of CRP. Hence, it is plausible to speculate that the increased systemic inflammation in COVID‐19 may also be mediated by increased NLRP3 inflammasome‐related products. 5 This is highlighted by the current multiple therapeutic attempts to block cytokines resulting from the NLRP3 inflammasome, 5 and considering the exaggerated inflammatory response in patients with T2DM, such patients might present even greater benefits from those therapies.
A recent report of a series of COVID‐19 cases found an endothelial cell involvement by the virus within the vascular beds of different organs, suggesting a direct viral infection of the endothelial cells associated with endothelial inflammation. 6 Patients with T2DM are characterized by endothelial dysfunction and vascular inflammation, which are major cardiovascular risk factors, even in absence of COVID‐19. 7 It is plausible to hypothesize that underlying endothelia dysfunction and inflammation in T2DM might augment the detrimental effects of COVID‐19 on the endothelium and the overall cardiovascular system.
The SARS‐CoV2 virus cell entry is mediated through the binding of the viral surface spike protein to the human angiotensin‐converting enzyme 2 (ACE2) receptor after the spike protein is activated by the transmembrane protease serine 2. The ACE2 protein is ubiquitously expressed, it is found in the lung, heart, intestinal epithelium and the pancreatic β‐cells. In chronic hyperglycemia, expression of ACE2 is further increased, possibly facilitating the virus to bind to it, ultimately resulting in a direct multi‐organ (including the β‐cells) toxic effect mediated by SARS‐CoV2. This would suggest that not only can T2DM worsen COVID‐19, but also the latter can cause a direct injury to the β‐cells. To support this theory, increased incidence of severe diabetic ketoacidosis (DKA) during hospital admission for COVID‐19 as well as the dramatic increase in insulin requirements in those with established disease have been noted. 8 Such effects could be caused by the direct toxic effects of the virus resulting from the increased availability of ACE2 due to chronic hyperglycemia. Clinicians should as well monitor for the new onset of diabetes in patients infected with SARS‐CoV2. 8
2. GLUCOSE‐LOWERING THERAPIES AND GLYCEMIC TARGETS: OUTPATIENT SETTING
There is currently very little guidance regarding the treatment of T2DM during COVID‐19. A recent document by Bornstein et al suggested that in the outpatient setting, the major goal of treatment of T2DM should be to minimize the risk of infections that might lead to hospitalizations. 8 This can be achieved through an optimal glycemic control with a targeted HbA1c <7% and plasma glucose concentrations between 72 and 144 mg/dL. 8 Of note, whether glycemic control can prevent COVID‐19, to date, remains unknown. It is crucial to prevent hypoglycemia in patients with diabetes in general, but specifically in older patients and in those prone to hypoglycemia. Hypoglycemia would increase the risk for hospitalization during a pandemic with limited available hospital resources, and exposing patients to a higher risk for infection by SARS‐CoV2. Sulfonylureas and insulin are commonly prescribed agents; however, they are associated with an increased risk for hypoglycemia, thus frequent blood glucose monitoring is recommended. Of note, among basal insulin therapies, degludec and glargine U300 have been associated with a lower risk for severe hypoglycemia, including nocturnal hypoglycemia, compared to glargine U100. 9 Also considering switching to other glucose‐lowering agents, such as sodium‐glucose cotransporter (SGLT)2 inhibitors, glucagon‐like peptide 1 receptor agonists (GLP1RA) or dipeptidyl peptidase (DPP)4 inhibitors, which are associated with extremely low risk for hypoglycemia, especially when used in monotherapy, seems reasonable. 9
On the other hand, in patients of T2DM and COVID‐19 the use of SGLT2 inhibitors and GLP1RA can be continued, yet with caution. These drugs have known cardiorenal protective properties that can be beneficial knowing COVID‐19‐associated risk for cardiac and renal damage. 4 , 10 Among SGLT2 inhibitors, canagliflozin, dapagliflozin and empagliflozin have shown cardio renal protective effects and should be the preferred choice in patients with heart failure considering the clear benefits of these agents in this population. 9 SGLT2 inhibitors, however, have been associated with increased risk of DKA, 9 therefore patients receiving such treatments should be well instructed on recognizing early signs and symptoms of DKA. For GLP1RA, liraglutide, semaglutide, dulaglutide and albiglutide should be the preferred agents due to their greater cardio renal benefits reported in cardiovascular outcomes trials. 9
Furthermore, SGLT2 inhibitors increase free fatty acids leading to increased production of ketone bodies. 11 The latter have profound anti‐inflammatory properties, and recently proven to inhibit the NLRP3 inflammasome, 11 whose products are dramatically increased in the setting of COVID‐19. 5 Similar to SGLT2 inhibitors, GLP1RA also exert anti‐inflammatory effects, which have been partially attributed to a reduction of the expression of the NLRP3 inflammasome. Maintaining adequate hydration and frequent blood glucose monitoring are crucial to ensure safety of these agents.
3. GLUCOSE‐LOWERING THERAPIES AND GLYCEMIC TARGETS: INPATIENT SETTING
In hospitalized patients with COVID‐19, patients with T2DM require more intensive treatments compared to those without T2DM, including a greater need for antibiotic, systemic corticosteroids, increased oxygen requirement and ventilator support. 4 Among those with T2DM, however, it is unknown whether specific glycemic targets should be recommended. In a recent multicenter retrospective cohort study that included >800 patients with T2DM, an improved glycemic variability (fasting glycemia between 70 and 110 mg/dL and 2‐hour post‐prandial glycemia <180 mg/dL) was associated with reduced levels of IL‐6 and CRP during hospital stay. 4 Importantly, greater glycemic control was associated with a 86% relative risk reduction (RRR) for all‐cause mortality (Figure 1B), as well as 53%, 88% and 76% RRR of developing acute respiratory distress syndrome, acute kidney injury and acute myocardial injury, respectively, compared to those with greater glycemic variability. 4 Based on these data, which are, however, limited by the retrospective nature of the data, achieving a glycemic control with glucose levels between 70 and 180 mg/dL throughout the duration of the hospital stay, seems a reasonable approach in patients admitted for COVID‐19. Clearly, prospective randomized trials investigating different glycemic targets, and possibly using more sophisticated measures of glycemic variability such as continuous glucose monitoring are encouraged.
Furthermore, whether specific antihyperglycemic agents should be used to achieve such target remains unknown. It is reasonable to use intravenous insulin therapy to achieve glycemic control while closely monitoring glycemia to minimize the risk for hypoglycemia and avoid significant glycemic variability. Metformin is one of the most prescribed agents in T2DM. Knowing that COVID‐19 patients are at increased risk for dehydration secondary to the acute illness, we would suggest against using metformin due to the increased risk of lactic acidosis, especially in those with respiratory distress and hypoxemia, which are, in turn, significant triggers for lactic acidosis. With the known increased risk for DKA of SGLT2 inhibitors and the concomitant risk of DKA possibly caused by the virus itself, it may be prudent to interrupt such treatment in hospitalized patients else; closely monitor plasma ketone bodies, electrolytes and pH. 8 GLP1RA may also lead to dehydration, reduced food intake and weight loss. For such reasons, clinicians should closely monitor patients for meeting energy as well as fluid requirements to reduce the risk for dehydration. It may seem appropriate to avoid GLP1RA in patients with limited food and fluid intake to avoid further reduction of oral intake using these agents, especially in the acute setting. Finally, DPP4 inhibitors (ie, sitagliptin, linagliptin and alogliptin) appear safe, and therefore they can be continued. Due to its association with increased risk for heart failure, 9 however, saxagliptin should not represent the first choice of DPP4 inhibitor. Of note, DPP4 is ubiquitously expressed and serves as a receptor for the human coronavirus‐Erasmus Medical Center (hCoV‐EMC), the virus that causes Middle East Respiratory Syndrome (MERS). 12 In culture, DPP4 inhibition reduces the infectivity of the virus. Whether this is also true for SARS‐CoV‐2 remains unknown, but it would ultimately propose the use of DPP4 inhibitors as potential treatment for COVID‐19, independent of their glucose‐lowering effects.
4. CONCLUSION
T2DM is a risk factor for worse prognosis in patients with COVID‐19. In patients with T2DM, with or without COVID‐19, the goal should remain to achieve an optimal glycemic control and minimize the risk for hypoglycemia and related risk for hospitalization.
Continuing agents such as SGLT2 inhibitors or GLP1RA in T2DM patients with COVID‐19 is reasonable due to their established cardiorenal protective effects, in addition to potential anti‐inflammatory effects, yet maintaining hydration and frequent blood glucose monitoring is advised. Of note, the cardiorenal benefits of these agents have only been demonstrated in long‐term treatment, while their effects in the setting of acute infections remain unknown. DPP4 inhibitors also represent an appropriate choice, especially for patients who are prone to hypoglycemia and do not tolerate SGLT2 inhibitors and/or GLP1RA. In those with COVID‐19 and admitted to the hospital, intravenous insulin remains the drug of choice, while discontinuation of oral agents should be considered. Of note, DPP4 inhibitors remain safe and potentially have a protective role, therefore its discontinuation may not be necessary.
Clearly, data resulting from randomized controlled trials are urgently needed to ultimately guide the clinicians to implementing the most appropriate glycemic target as well as the most effective pharmacologic treatment in T2DM patients at risk for or have COVID‐19.
CONFLICT OF INTEREST
Amin Yehya is on the speaker bureau for Zoll, Akcea therapeutics and CareDx. The other authors declare that they have no conflicts of interest.
ACKNOWLEDGEMENT
Salvatore Carbone is supported by a Career Development Award 19CDA34660318 from the American Heart Association.
REFERENCES
- 1.Johns Hopkins Coronavirus Resource Center. COVID‐19 Map. @JohnsHopkins. https://coronavirus.jhu.edu/map.html. Published 2020. Accessed May 12, 2020.
- 2. Guo W, Li M, Dong Y, et al . Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab Res Rev. 2020;e3319. 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maddaloni E, Buzzetti R. Covid‐19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020;e33213321. 10.1002/dmrr.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu L, She ZG, Cheng X, et al . Association of blood glucose control and outcomes in patients with COVID‐19 and pre‐existing type 2 diabetes. Cell Metab. 2020;31(6):1068‐1077.e3. 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ingraham NE, Lotfi‐Emran S, Thielen BK, et al . Immunomodulation in COVID‐19. Lancet Respir Med. 2020;8(6):544‐546. 10.1016/s2213-2600(20)30226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care. 2011;34(Suppl 2):S285‐S290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID‐19. Lancet Diabetes Endocrinol. 2020;8:546‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carbone S, Dixon DL, Buckley LF, Abbate A. Glucose‐lowering therapies for cardiovascular risk reduction in type 2 diabetes mellitus: state‐of‐the‐art review. Mayo Clin Proc. 2018;93(11):1629‐1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li D, Chen Y, Jia Y, et al . SARS‐CoV‐2‐induced immune dysregulation and myocardial injury risk in China: insights from the ERS‐COVID‐19 study. Circ Res. 2020. 10.1161/circresaha.120.317070. [DOI] [PubMed] [Google Scholar]
- 11. Kim SR, Lee SG, Kim SH, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11(1):2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strollo R, Pozzilli P. DPP4inhibition: Preventing SARS‐CoV‐2 infection and/or progression of COVID‐19?. Diabetes Metab Res Rev. 2020. 10.1002/dmrr.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]


