Abstract
Covid‐19 pandemic is deeply affecting transplant activity worldwide. It is unclear whether solid organ transplant recipients are at increased risk of developing severe complications and how they should be managed, also concerning immunosuppression. This is a report about the course and management of SARS‐CoV‐2 infection in liver transplant recipients from a single center in Northwestern Italy in the period March‐April 2020. Three patients who were treated at our institution are reported in detail, whereas summary data are provided for those managed at peripheral Hospitals. Presentation varied from asymptomatic to rapidly progressive respiratory failure due to bilateral interstitial pneumonia. Accordingly, treatment and changes to immunosuppression were adapted to the severity of the disease. Overall mortality was 20%, whereas Covid‐related mortality was 10%. Two cases of prolonged (>2 months) viral carriage were observed in two asymptomatic patients who contracted the infection in the early course after transplant. Besides depicting Covid‐19 course and possible treatment scenarios in liver transplant patients, these cases are discussed in relation to the changes in our practice prompted by Covid‐19 epidemic, with potential implications for other transplant programs.
Keywords: COVID‐19, early infection, immunosuppression, viral carriage
1. INTRODUCTION
After the initial outbreak in Hubei province in China in December 2019, COVID‐19, a zoonosis caused by SARS‐CoV‐2 virus, 1 has rapidly spread worldwide and was declared a Public Health Emergency of International Concern by World Health Organization on January 30, 2020.
Shortly after, the first confirmed case of a COVID‐positive patient was reported in Italy on January 31, 2020. Although containment measures were timely implemented by Italian Government, the outbreak spreads rapidly across Northern Italy and hits our Health system with unforeseen intensity. As of May 9th, 28 368 cases (7039 per million people) have been reported in Piedmont (our Region in Northwestern Italy), with 140 patients still admitted to intensive care units (ICU). 2 Similarly to what has happened elsewhere, entire hospitals have been converted from their usual activity to COVID hospitals. In the meanwhile, surgical activity has been restricted to emergency and high‐risk oncological cases.
Transplantation activity has been primarily affected by a decrease of available donors, likely due to the diversion of ICU resources toward the care of COVID‐19 patients. 3 However, despite concerns regarding the risk of donor‐transmitted infection, peri‐operative infection, and staff safety, liver transplantation (LT) has been considered an emergent and life‐saving procedure and it has not been deliberately interrupted or reduced.
There is still limited knowledge about the course of COVID‐19 in liver transplant recipients, especially in the case of early post‐LT infection. 4 , 5 Recent series from USA 6 and Spain 7 have suggested that recipients of solid organ transplant affected by COVID‐19 may present higher mortality as compared to the general population. However, these series reported pooled data for recipients of different organs and lacked an in‐depth presentation of cases.
This is a preliminary single‐center report about the course of COVID‐19 in liver transplant recipients. Three patients who were admitted at our institution are reported in detail, whereas a more general description is provided for seven more patients who were managed elsewhere or as outpatients, in which we were involved more indirectly. These cases are discussed also in the light of the changes to our practice they prompted and of the implications for other transplant programs.
2. CASE REPORTS
Patient 1 was a 69‐year‐old gentleman with no major comorbidities, who received LT for hepatocellular carcinoma (HCC) arising on HBV and alcohol‐related cirrhosis with model for end‐stage liver disease score of 18 on March 5th, 2020. After an initially regular post‐LT course, he was incidentally diagnosed as SARS‐CoV‐2‐positive with a nasopharyngeal swab (NPS) on his 5th post‐LT day (POD) after his bed neighbor, who was recovering from a liver resection for HCC, had tested positive the previous day. This last patient, who was the first patient being diagnosed COVID‐19 in our unit and died of the disease 13 days later, also infected 8 staff members before being isolated, prompting a steep change in our practice (see Discussion). Negative SARS‐CoV‐2 RNA test in the donor ruled out donor‐recipient transmission. At the moment of diagnosis, patient 1 presented mild symptoms (rare coughs, temperature 37.5°C, no oxygen requirement). A computed tomography (CT) obtained on POD 6 did not show evidence of pneumonia (Figure 1). He was transferred to a COVID unit on POD 9 where he was administered hydroxychloroquine (HCQ) 200 mg bid for 16 days (Figure 2). Mycophenolate mofetil (MMF) dosing was reduced from 750 mg td to 500 mg td, and target tacrolimus (Tac) trough level was set at 5‐7 ng/mL. Blood tests (Figure 3) were significant for mild lymphopenia, with levels comparable with those pre‐LT. He had an otherwise asymptomatic and uneventful course and was discharged home in quarantine on POD 27. More than two months after infection, he is alive and symptom‐free, but still waiting for confirmation of viral clearance, as he tested positive on the last NPS performed on day 42 after the first proof of infection.
FIGURE 1.
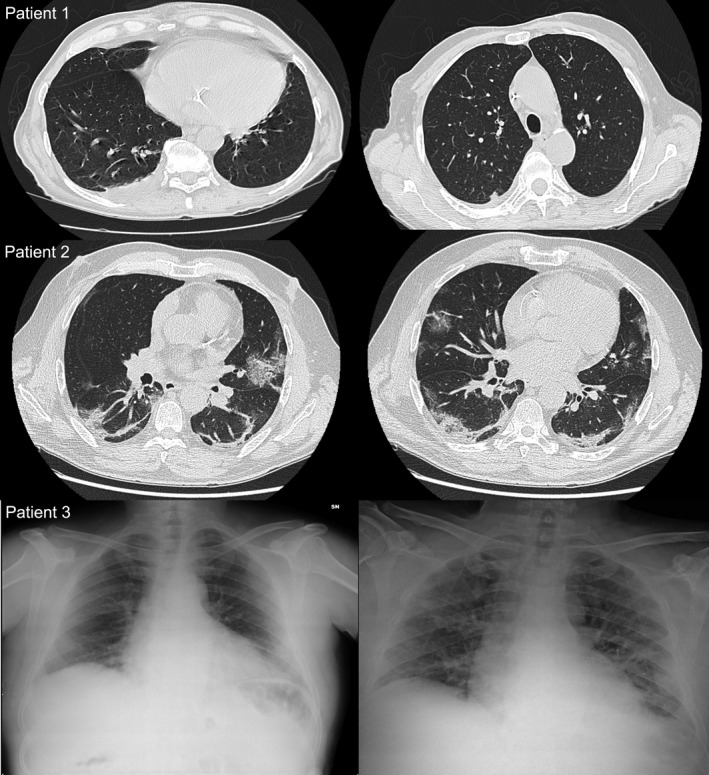
Radiology findings. Only minimal alterations, not typical for COVID‐19, were observed in patient 1, whereas patients 2 and 3 had findings compatible with bilateral interstitial pneumonia. Patient 3, left panel: initial X‐ray showing no sign of pneumonia; right panel: X‐ray upon re‐admission, compatible with bilateral interstitial pneumonia
FIGURE 2.
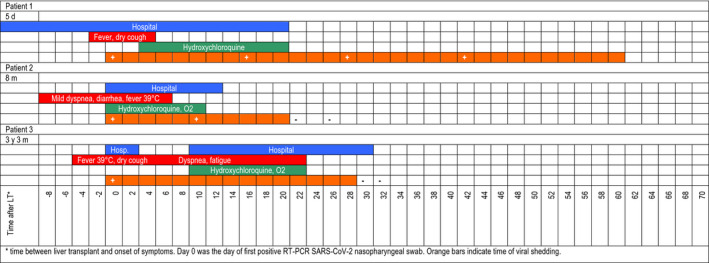
Synoptic view of the course of the disease, including duration of hospital admission, symptoms, treatment, and time of viral shedding
FIGURE 3.
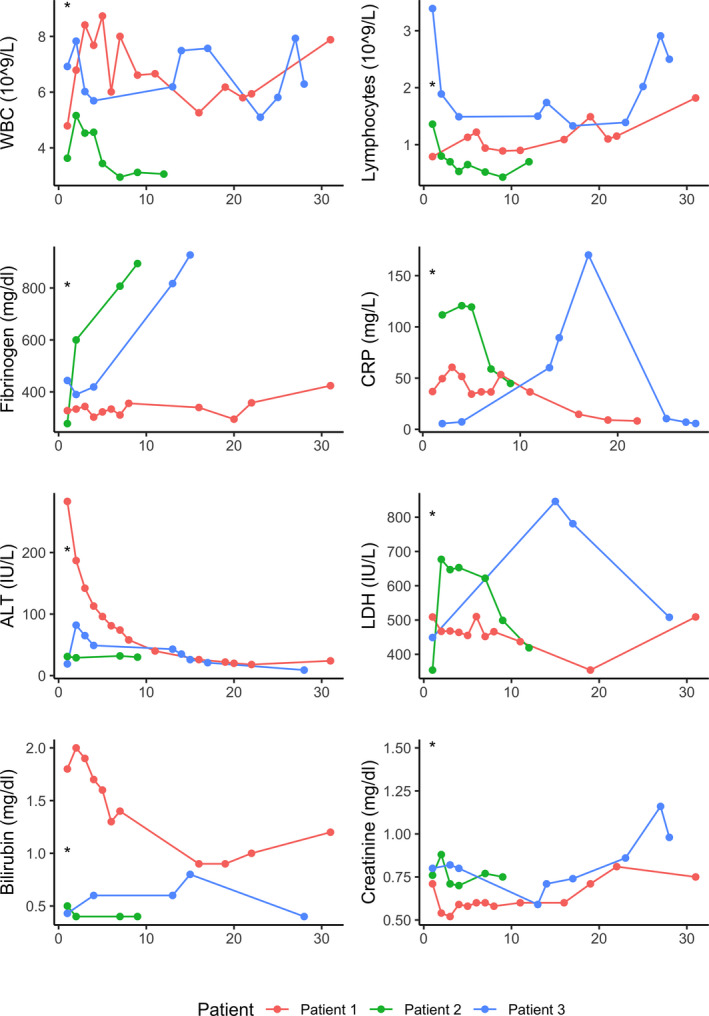
Line plots depicting time trend of laboratory values. First values represent last available values before COVID‐19 diagnosis. Asterisks represent the moment of COVID‐19 infection
Patient 2 was a 59‐year‐old gentleman who was admitted for Covid‐19‐related bilateral interstitial pneumonia (Figure 1) 8 months after LT for HCC in the setting of HBV‐related and alcoholic cirrhosis. His medical history was significant for obesity with a body mass index of 31. Before admission, he had normal hepatic function and his immunosuppression (IS) consisted in meltdose Tac 1.5 mg od and Everolimus 1 mg + 0.75 mg. He presented on March 19th with a 9‐day history of fever (max 39°C), diarrhea, and mild dyspnea. On admission, vital parameters were as follows: blood pressure (BP) 140/90 mmHg, heart rate (HR) 100 bpm, respiratory rate (RR) 26 bpm, and oxygen saturation (SpO2) 96% in room air. He was admitted and started on HCQ 200 mg bid and O2 therapy by facial mask, but never required continuous positive airway pressure (CPAP) or mechanical ventilation to achieve oxygen saturation within normal limits. IS regimen was not modified. His laboratory values were significant for lymphopenia (minimum 0.43 × 10 8 /L) and transient elevation of fibrinogen, C‐reactive protein (CRP), and lactate dehydrogenase (LDH) (maximum levels 894 mg/dL, 120 mg/L and 677 IU/L, respectively). He did not develop severe complications, was progressively weaned from O2, and discharged home in quarantine on April 1st, 2020. Virological clearance was confirmed by two consecutive negative NPS on April 10th and 15th.
Patient 3, a 56‐year‐old gentleman with a history of smoking and LT for HCV‐related cirrhosis in December 2016, presented on March 8th, 2020, due to a weeklong history of fever, sore throat, dry cough, and odynophagia. Before admission, his liver function tests were normal and IS consisted in prolonged‐release Tac 1 mg od and Eve 1.5 mg td. Vital signs on admission were T 38.2°C, BP 120/80 mm Hg, HR 60 bpm, RR 12, and SpO2 99% in room air. Although his chest X‐ray was normal (Figure 1) and blood tests were only mildly altered (WBC 7.83 109/L, Lymphocytes 1.89 109/L, CRP 5.6 mg/L, ALT 82 IU/L), Covid‐19 diagnosis was confirmed by RT‐PCR on nasopharyngeal swab. Given the mild presentation, patient was discharged home in quarantine after a 48‐hour observation period, with no specific treatment except suspension of Tac. Nine days after, however, he was transferred back to our Institution from another hospital, where he presented for persistence of symptoms and appearance of mild dyspnea and fatigue. On admission, he had BP 115/70 mm Hg, T 37.3°C, and SpO2 96%. Chest X‐ray demonstrated findings compatible with bilateral interstitial pneumonia (Figure 1). He was admitted and started on HCQ 200 mg bid. His Eve dosing had to be adjusted due to elevated trough levels (maximum 20.4 ng/mL), possibly due to pharmacological interaction with HCQ (Figure 4). During admission, he required O2 therapy by facial mask, from which he was progressively weaned off. Results of blood tests are depicted in Figure 3. He recovered well and tested negative for SARS‐Cov‐2 RT‐PCR on three consecutive days (April 6th, 7th, and 8th) and was discharged home on April 9th.
FIGURE 4.
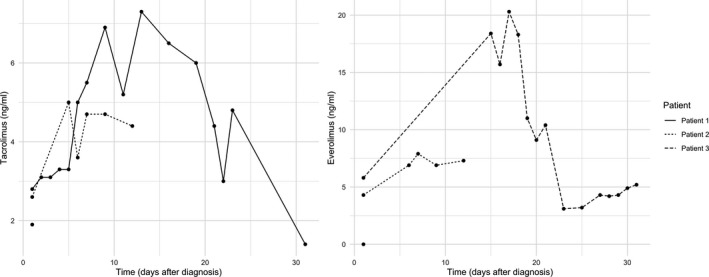
Line plots depicting trough levels of tacrolimus and everolimus. First values represent last available values before COVID‐19 diagnosis
Summary data for the whole series are provided in Table 1, but some details are worth mentioning. First, all patients had good liver function at the moment of diagnosis.
TABLE 1.
Synoptic table of patient course and treatment
| Sex | Age | Time post‐LT | Location of management | Symptoms | Interstitial pneumonia | Treatment | Baseline IS | Changes to IS | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 a | M | 69 | 5 d | Ward | Fever, dry cough | No | HCQ | Tac + MMF +Pred | ↓MMF | Alive |
| Patient 2 a | M | 59 | 8 mo | Ward | Mild dyspnea, diarrhea | Yes | HCQ, O2 | Tac + Eve | None | Alive |
| Patient 3 a | M | 56 | 3 y3 mo | Ward | Sore throat, dry cough, fever | Yes | HCQ, O2 | Tac + Eve | Stop Tac | Alive |
| Patient 4 | M | 58 | 2 mo | Home | None | No | None | Tac + MMF +Pred | None | Alive |
| Patient 5 | F | 64 | 4a4 mo | Ward | Fever, lack of appetite, diarrhea | Yes | Steroids, O2 | Tac + Pred | ↑Pred | Alive |
| Patient 6 | M | 64 | 8a | Ward | Fever | Yes | HCQ, O2, CPAP | Tac + MMF | Stop MMF, ↓Tac | Alive |
| Patient 7 | M | 64 | 9 y3 mo | Ward | Fever | Yes |
LPV/RTV, HCQ, O2 |
Tac + MMF | Stop Tac | Alive |
| Patient 8 | M | 62 | 11a | Ward | Fever | Yes | DRV/RTV, HCQ, steroids, O2 | Tac + MMF | Stop Tac | Dead b |
| Patient 9 | M | 75 | 11a8 mo | Ward | Diarrhea, myalgia, fever, cough | Yes | Steroids, O2, CPAP | Tac + MPA | Stop MPAStop Tac | Dead |
| Patient 10 | F | 85 | 22a7 mo | Ward c | None | No | None | Tac | None | Alive |
Abbreviations: CPAP, continuous positive airway pressure; DRV/RTV, darunavir/ritonavir; Eve, everolimus; HCQ, hydroxycholoroquine; IS, immunosuppression; LPV/RTV, lopinavir/ritonavir; LT, liver transplantation; MMF, mycophenolate mofetil; MPA, mycophenolic acid; Pred, prednisone; Tac, tacrolimus.
Cases of the first three patients are reported in detail in the manuscript.
Deceased for unrelated cause after negative COVID‐19 test.
Patient admitted for rectus abdomins abscess, accidentally diagnosed with SARS‐CoV‐2 infection during admission.
Patient 4 was also a bed neighbor of our index patient, and he had been discharged from hospital before COVID‐19 was made in this last. He tested positive for SARS‐CoV‐2 RNA on a NPS performed 2 months after his LT, without having been in contact with other known COVID‐19 patients. Thus, it is likely that he contracted the infection in the early post‐LT course, similarly to patient 1. As he was presenting no symptoms, no specific treatment has been administered and no changes have been made to his immunosuppression.
Patient 8 contracted the infection during a hospital admission for head trauma and, despite developing bilateral pneumonia, recovered from the disease and died of unrelated causes during the same hospital admission.
Patient 10 was admitted for a rectus abdominis muscle abscess and accidentally found to be positive for SARS‐CoV‐2 RNA on a surveillance NPS. As she presented no respiratory symptoms or evidence of pneumonia, no specific treatment was administered and IS was left unchanged.
In general, IS was reduced according to disease presentation and completely withdrawn in the only patient who died due to COVID‐19. Six patients were administered HCQ, 3 high‐dose steroids, and 2 antivirals (lopinavir/ritonavir and darunavir/ritonavir). Overall, mortality was 20% and COVID‐related mortality was 10%.
3. DISCUSSION
Covid‐19 is a new disease and several questions concerning its course and management are still open, all the more in the specific population of solid organ transplant recipients. According to recent observation about the course of COVID‐19 in Northern Italy 9 and data gathered from past severe acute respiratory distress syndrome (SARS) and middle‐east respiratory syndrome (MERS) epidemics, 8 immunocompromised patients do not seem to be at increased risk of developing severe complications. However, data from recent series have suggested that solid organs transplant recipients contracting COVID‐19 may suffer from increased mortality as compared to the general population. 6 , 7
Data about the course of COVID‐19 in LT recipients are still limited, especially for patients in the early stage after LT. Qin et al 5 reported a case of a patient who presented with fever on POD 2 and tested positive for SARS‐CoV‐2 on POD 12, after a CT scan demonstrated bilateral ground glass opacifications in its lungs. Tacrolimus and steroid doses were diminished, and patient was administered oseltamivir and intra‐venous immunoglobulins. Oxygen requirement was limited to high‐flow nasal cannulae, and he was discharged home on POD 58. Lagana et al 4 have reported a case of likely COVID‐related acute hepatitis in a 5‐month‐old recipient of living donor LT for biliary atresia. Although long‐term follow‐up was not available, the patient was recovering after treatment with HCQ and reduction of IS. Notably, transient worsening of liver enzymes was observed after treatment with steroid pulses, which were administered for concomitant features of acute cellular rejection. In the series by Pereira et al, 6 3 patients presented COVID‐19 < 1 month after transplant, with two of them presenting mild/moderate disease, but it is was not specified whether they were recipients of a LT or of another solid organ.
We report here 10 cases of SARS‐CoV‐2 infection in LT recipients at different times after transplant. In our series, COVID‐related mortality was 10%. Considering that 8/10 patients were <70 years of age, this figure is higher to what we would expect in an age‐matched group in Italy, 10 in keeping with other series. 6 , 7 However, this could be an overestimate due to undetected asymptomatic cases. As of today, several National and International studies are under way to assess outcome and risk factors for severe complications in transplant patients affected by COVID‐19, which will likely provide more precise estimates.
Two patients in our series (patients 1 and 4) contracted the infection in the early days after LT, but both of them presented with an indolent form of the disease. In particular, no treatment or changes to IS were considered in patient 4, as in his case COVID‐19 went undetected during the likely 2 months of infection. This suggests that a higher IS load does not necessarily represent a risk factor for severe complications. However, increased IS could be associated to delayed viral clearance, as both patients exhibited prolonged viral shedding (>2 months).
Concerning IS, there is currently no clear evidence supporting systematic reduction or withdrawal of IS in transplant patients affected by COVID‐19. 9 So far, our approach has been modulating IS load according to the severity of disease and concomitant treatments, considering that rejection treatment could potentially be more detrimental than stable IS. Furthermore, as patients with a severe course of the disease present higher levels of inflammatory cytokines, IS could theoretically be beneficial by controlling pulmonary hyperinflammation and reducing cytokine storm due to interleukins and chemokine overproduction. Thus, it remains unclear whether increased mortality in transplant patients is associated with IS or rather with reduced organ function and/or associated comorbidities.
Our experience as a transplant unit also sheds some light into an otherwise gloomy scenario. Our Institution, a public university hospital hub for complex diseases in our Region, has managed sparing part of its resources for urgent operations and transplants. Our experience with patient 1 prompted a deep reorganization of our activity. Donors and recipients, as well as surgical patients in general, are systematically tested for SARS‐CoV‐2 using RT‐PCR on NPS or bronchoalveolar lavage samples. After transplant, patients are admitted into a COVID‐free ICU and afterward into a dedicated ward. Family visits have been suspended, and relatives are given news about patients’ course on the phone. Other protected facilities, including a radiology department and an outpatient clinic, have also been implemented. Follow‐up is done by telemedicine for patients not requiring physically attending clinic. While our transplant program is still active, we have not observed other hospital‐acquired COVID‐19 cases. In our opinion, notwithstanding that all efforts are mandatory to avoid infection of transplant patients, 11 risks associated with the current pandemic should be weighed against that of candidate death or drop‐out from the list. Obviously, any reasoning has to be contextualized to local logistics and resources. 12 , 13
In conclusion, age‐matched mortality seems higher in LT recipients affected by COVID‐19 as compared to the general population. Future studies are necessary to determine the role of organ function, associated comorbidities, and IS. Finally, our experience suggests that maintenance of transplant activity in the midst of COVID‐19 pandemic is possible, provided protected pathways are ensured.
AUTHOR CONTRIBUTIONS
DP involved in study design, data collection and analysis, and manuscript drafting; FL and FC involved in patient management and manuscript revision; EM performed data collection and manuscript revision; SM, SC, and FT revised the manuscript; FGD and RR revised the manuscript and supervised.
Patrono D, Lupo F, Canta F, et al. Outcome of COVID‐19 in liver transplant recipients: A preliminary report from Northwestern Italy. Transpl Infect Dis. 2020;22:e13353. 10.1111/tid.13353
REFERENCES
- 1. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dipartimento della Protezione Civile ‐ COVID‐19 Italia. http://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1. Accessed May 9th, 2020.
- 3. Angelico R, Trapani S, Manzia TM, Lombardini L, Tisone G, Cardillo M. The COVID‐19 outbreak in Italy: Initial implications for organ transplantation programs. Am J Transplant. 2020. 10.1111/ajt.15904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lagana SM, De Michele S, Lee MJ, et al. COVID‐19 associated hepatitis complicating recent living donor liver transplantation. Arch Pathol Lab Med. 2020. 10.5858/arpa.2020-0186-SA [DOI] [PubMed] [Google Scholar]
- 5. Qin J, Wang H, Qin X, et al. Perioperative presentation of COVID‐19 disease in a liver transplant recipient. Hepatology. 2020. 10.1002/hep.31257 [DOI] [PubMed] [Google Scholar]
- 6. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in Solid Organ Transplant Recipients: Initial Report from the US Epicenter. Am J Transplant. 2020. 10.1111/ajt.15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernandez‐Ruiz M, Andres A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am J Transplant. 2020. 10.1111/ajt.15983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hui DS, Azhar EI, Kim YJ, Memish ZA, Oh MD, Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18(8):e217‐e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl. 2020;26(6):832‐834. [DOI] [PubMed] [Google Scholar]
- 10. Mortality Risk of COVID‐19. https://ourworldindata.org/mortality‐risk‐covid#case‐fatality‐rate‐of‐covid‐19‐by‐age. Accessed May 9th, 2020.
- 11. Michaels MG, La Hoz RM, Danziger‐Isakov L, et al. Coronavirus disease 2019: Implications of emerging infections for transplantation. Am J Transplant. 2020. 10.1111/ajt.15832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gori A, Dondossola D, Antonelli B, et al. Coronavirus disease 2019 and transplantation: a view from the inside. Am J Transplant. 2020. 10.1111/ajt.15853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maggi U, De Carlis L, Yiu D, et al. The impact of the COVID‐19 outbreak on liver transplantation programmes in Northern Italy. Am J Transplant. 2020. 10.1111/ajt.15948 [DOI] [PMC free article] [PubMed] [Google Scholar]


