Abstract
The COVID‐19 pandemic has produced critical shortages of ventilators worldwide. There is an unmet need for rapidly deployable, emergency‐use ventilators with sufficient functionality to manage COVID‐19 patients with severe acute respiratory distress syndrome. Here, we show the development and validation of a simple, portable and low‐cost ventilator that may be rapidly manufactured with minimal susceptibility to supply chain disruptions. This single‐mode continuous, mandatory, closed‐loop, pressure‐controlled, time‐terminated emergency ventilator offers robust safety and functionality absent in existing solutions to the ventilator shortage. Validated using certified test lungs over a wide range of compliances, pressures, volumes and resistances to meet U.S. Food and Drug Administration standards of safety and efficacy, an Emergency Use Authorization is in review for this system. This emergency ventilator could eliminate controversial ventilator rationing or splitting to serve multiple patients. All design and validation information is provided to facilitate ventilator production even in resource‐limited settings.
Keywords: acute respiratory distress syndrome, COVID‐19 pandemic, critical care, mass casualty incidents, mechanical ventilation, medical device design, respiratory insufficiency
1. INTRODUCTION
A key challenge in the battle against the disease caused by the novel coronavirus SARS‐CoV‐2, COVID‐19, is a potential worldwide shortage of mechanical ventilators. The required number of ventilators is projected to significantly exceed capacity, based on the number of patients expected to contract the disease in the United States and the percentage of these likely to require assisted ventilation (Fauci, Lane, & Redfield, 2020; Ranney, Griffeth, & Jha, 2020; Wang et al., 2020; Weissman et al., 2020). Adding to this burden is the fact that COVID‐19 patients who develop acute respiratory distress syndrome (ARDS) often require prolonged mechanical ventilation (Bhatraju et al., 2020; Cascella, Rajnik, Cuomo, Dulebohn, & Di Napoli, 2020; Phua et al., 2020; Yang et al., 2020). Physicians around the world have been forced to make difficult triage decisions on which patients to treat and which to let go of due to inadequate number of ventilators (Rosenbaum, 2020; Xie et al., 2020). Adding to the challenges of increasing number of devices is the complexity and expense of traditional ICU ventilators further aggravated by the breakdown of regular supply chains as a consequence of the pandemic (Huang et al., 2017; Netland, 2020; Woodyatt, 2020).
A pandemic caused by a potentially lethal and easily transmissible (Sanche et al., 2020) viral pathogen like SARS‐CoV‐2 requires rapid, focused effort in either obtaining or manufacturing sufficient medical equipment to save lives despite the disruption of normal supply chains, difficult working conditions and regulatory restrictions reasonably imposed in normal times that nonetheless jeopardize progress during a state of emergency. In response to the anticipated COVID‐19 crisis, we formed the University of California San Diego Acute Ventilation Rapid Response Taskforce (AVERT) to develop a ventilator with functionality sufficient to safely treat COVID‐19 patients with ARDS, while simultaneously shortening ventilator production time and cost to make ventilators available when and where they are needed.
The ventilator design focuses on safe operation and reliable production while addressing the specific needs of COVID‐19 patients with ARDS: minimizing part count, cost and complexity; reducing or eliminating reliance on scarce parts and resources; ensuring viable implementation in different healthcare systems across the world; and seeking simple assembly, testing and use procedures by healthcare personnel with limited experience in ventilation; and no experience with this type of ventilator system (Krishnamoorthy, Vavilala, & Mock, 2014).
Modern ICU ventilators provide complex control and intricate feedback loops of a wide variety of respiratory parameters and ventilation modalities. Their operation requires highly specialized staff (Morrison, 2020). Regulatory requirements are understandably high, and pandemic crisis‐driven emergency orders of ventilators to medical device manufacturers are difficult to fulfil due to the failure of supply lines and the difficulty in rapidly ramping up production of these technically advanced ventilators. In the meantime, lives are at risk. While several emergency ventilators are commercially available, most do not meet the medical requirements of the complex ARDS‐like pneumonia associated with COVID‐19 which requires pulmonary protective ventilation with careful control of pressure and volume as compliance of the infected lung tissue can rapidly deteriorate, placing the patient at elevated risk of barotrauma and further lung injury. We are left with an unmet need for COVID‐19 pneumonia‐appropriate, rapidly deployable, comparatively simple emergency‐use ventilators.
Based on published literature and clinical experience, we determined the following ventilation features to be essential for safe use in patients in this crisis: pressure control mode of ventilation, respiratory rate (RR), inspiratory time and forward‐compatibility with external modular components such as adjustable positive end‐expiratory pressure (PEEP) valves (Amato et al., 2015; Brower et al., 2000; Fan et al., 2017; Weiss et al., 2016). In addition, basic alarms indicating high and low pressure and volume are necessary to notify the healthcare provider when desired parameters are not being met or if there is a significant problem with the system. Many modern ventilators can sense and synchronize to patient‐initiated breaths to provide the most comfortable form of ventilation in a minimally sedated patient. We did not include a synchronized mode of ventilation in the design of this ventilator, recognizing that patients with COVID‐19 and severe ARDS will require sedation and possibly pharmacologic paralysis to facilitate optimal ventilation (Bourenne et al., 2017; Prevention and Early Treatment of Acute Lung Injury (PETAL) Network, 2019). The advantages of this approach include simplified ventilator settings and simplified troubleshooting with a single‐mode continuous, mandatory, closed‐loop, pressure‐controlled, time‐terminated ventilator (from now on referred to simply as pressure‐controlled). This approach provides predictable delivery of ventilated breaths and streamlined device production. Further design choices were based on the dual goals of safe, effective ventilation and quick production as detailed in the next section.
All ventilators in clinical use are regularly validated and calibrated using lung simulators to comply with U.S. Food and Drug Administration (FDA) standards of safety and efficacy. All devices described in this manuscript were tested in accordance with those practices and FDA regulation protocols utilizing an approved lung simulator (Dual Adult Test Lung; Michigan Instruments) with the associated data visualization software at the University of California San Diego. Our bedrock of safety is the provision to test every one of our devices using this human ventilation simulator, a physical device designed to emulate human respiration with time‐stamped data capture to determine the safety and efficacy of the manufactured ventilators. This testing is conducted under the supervision of a licensed anaesthesiologist exactly the same way commercial ventilators are annually certified during their use in U.S. hospitals (Figure 1).
FIGURE 1.
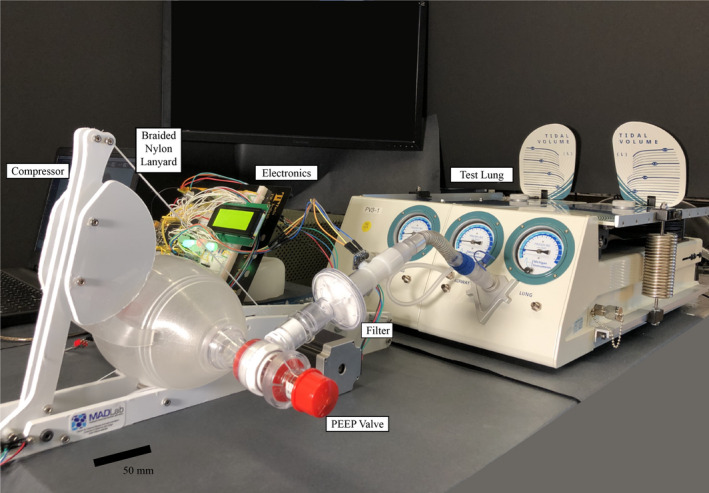
The ventilator was tested on a lung simulator. All parameters were tested to their stated limits (over 200 individual experiments) and according to International Standards Organization standards for pressure‐controlled ventilation. Notice that the dead space is kept to a minimum by reducing the length of tube between the bag and the lung simulator; this configuration was reproducible with a full‐sized simulator manikin and a standard adjustable overbed hospital bedside table. The system shown here is an early prototype with exposed electronics, but is to be supplied with housings as depicted in Figure 2
All models, print files, simulation data, coding and other details necessary to manufacture these ventilators have been included either in this manuscript or in the [Link], [Link], [Link], [Link]. This is in recognition of the urgency of the situation and the coordinated and cooperative effort necessary to save lives once the design has undergone peer review by members of the clinical community and Emergency Use Authorization (EUA) by the FDA (Hinton, 2020) (PEUA200567). Our ventilator design offers the following novel advantages over the current panoply of commercial, emergency‐use FDA‐approved and FDA‐unapproved but widely publicized ventilator designs:
The MADVent ventilator is tailored to treat COVID‐19 patients as, formally (International Standards Organization, 2019b), a single‐mode continuous, mandatory, pressure‐controlled, time‐terminated design. Most low‐cost ventilators function instead as volume‐control ventilators, delivering air into the lungs even to excessive pressure, which can lead to lung injury, especially in ARDS lung‐compromised patients typical in this COVID‐19 pandemic (Amato et al., 2015; Meng et al., 2020).
The MADVent has a novel torque conversion mechanism via a simple pulley and lanyard system to convert the relatively low‐torque, high‐speed rotation of the motor to a high‐torque, reduced speed resuscitation bag compression mechanism. This is superior to the ubiquitous geared rack‐and‐pinion mechanisms of other low‐cost ventilators as it offers greater pressure, at least doubles the maximum ventilation rate, has no backlash, and is far quieter. It is also much more durable, as the nylon geared mechanisms used in other systems are subject to wear and failure much faster than our approach.
Unlike all low‐cost ventilators known to us, we offer a fully alarmed ventilation operation suitable for life support, commensurate with the strict requirements of the FDA for life‐support ventilators, even in a pandemic.
We uniquely determine the volume of air delivered through knowledge of the resuscitation bag characteristics and a model of its compression based on the rotation angle of the motor. This obviates the need for expensive airflow sensors and the complex algorithms necessary to compute the volume from airflow. It also drastically reduces the cost of our ventilator, to about $300 in parts and <$500 including assembly; an airflow sensor approved for use in ventilators is $150 alone. This furthermore offers the possibility of offering other ventilation modes in the future, such as volume‐control or patient‐initiated ventilation.
We have pursued a comprehensive strategy of low cost, worldwide accessible parts in the design. In this pandemic, supply lines are disrupted and the complex designs of many ventilators, open‐source designs included, are simply not produceable due to parts shortages. Our design avoids this problem, from the ability to use 3.3 VDC or 5 VDC pressure sensors to the exclusion of valves and motors that are simply unavailable.
2. EXPERIMENTAL SECTION
2.1. Design strategy for an emergency ventilator in a pandemic
Even amid a pandemic, the process of medical device design requires due consideration and, if possible, mitigation of patient and user risks. In the context of any equipment to be approved for clinical use by the FDA, the ISO standard 14971:2019 (International Standards Organization, 2019a) details the risk management process to be followed. Though any risk management process is inherently flawed, especially for new technology (Fischhoff, 2015), following a process identifies and addresses problems before they can affect a patient. In our case, many such risks were identified, for example the breakage of the lanyard between the motor and the resuscitation bag compression arm. The severity of this failure is critical, while the probability is remote. Any potential risk of this mode of failure was reduced by choosing a lanyard capable of carrying one hundred times the maximum possible loading in the system, selecting a braided construction of abrasion‐resistant polymer fibres, and mandating that the lifetime of this emergency use ventilator is 1 month or less. By doing this, the probability of this failure was reduced to negligible. Other risks, including overheating of the motor or circuit, failure of the pressure sensor, the pinch risk of the ventilator bag compression arm, and 29 other risks we brainstormed about were considered with an assessment of their severity and probability. Evaluating the risks entails consultation of the risk acceptability matrix, a composition of the severity and probability to help guide us on whether we must mitigate or eliminate the risk in some way.
Mechanical ventilation typically requires pressure or volume‐based control of inspiration at a defined rate (Brower et al., 2000; Dellaca', Veneroni, & Farre', 2017; Weiss et al., 2016). Given the relative ubiquity and simplicity of pressure transducers as compared to flow sensors, the pressure‐controlled mode of ventilation was determined to be both safe and best suited to this current project. This has proven fortuitous since, though both volume and pressure limits are included in ARDS recommendations (Brower et al., 2000; Fan et al., 2017), there are data to support the pressure control mode as being particularly safe in ARDS therapy (Amato et al., 2015).
Typically, automatic pressure‐controlled ventilation relies on either an impeller motor that pressurizes air within the ventilator or a reticulated, regulated high‐pressure source from the healthcare environment. Volume‐controlled ventilation relies on the compression of a bag or bellows by a known volume. In order to be truly controlled, each of these methods must measure the pressure or volume—sometimes both—and use this information to appropriately adjust the actuation in a feedback loop. Measuring pressure at the output of the ventilator is far more straightforward, less expensive and less susceptible to calibration and algorithmic errors than measuring volume. Accurate flow sensors for mechanical ventilation are expensive (Corp, 2020), susceptible to supply chain disruptions, and conversion of their output into volumetric flow rate is difficult (Biselli, Nóbrega, & Soriano, 2018) with complex algorithms required to deal with that challenge (Bachiller, McDonough, & Feldman, 2008). Airflow is typically integrated over time to estimate the volume of air passed through a ventilator, and the volume–flow relationship is complicated by sensor accuracy (Heulitt, Holt, & Thurman, 2013); lung compliance (Harris, 2005); humidity, compression and temperature (Lyazidi et al., 2010); and leaks in the system.
Manual ventilation—and automated ventilators from the past—make use of a bag with valves to ventilate a patient's lungs with mechanical compression and release of the bag. Safe ventilation, however, demands care in mechanical compression and release beyond simply compressing a bag. For our ventilator, we adopted a self‐inflating bag‐based mechanical ventilation system, combining its intrinsic simplicity with instrumented sensing of the pressure produced by the system to continuously control the ventilator in a closed feedback loop, eschewing airflow sensors in favour of calibrated determination of how bag volume varies with mechanical compression. This allows the ventilator to reach precise pressure targets within a prescribed inspiratory time while setting safety alarmed thresholds on the volume delivered per breath utilizing an inexpensive and rapidly devised design.
2.2. Using a self‐inflating manual resuscitator bag for safety and ease of adoption
Rather than reinventing the bag and valving system, we have elected to utilize a self‐inflating manual resuscitator bag (SPUR II; Ambu Inc) already in common use worldwide in hospitals and other emergency care settings. These self‐inflating bag systems have been designed to deliver the proper range of tidal volumes with simple manual compression, do not require a pressurized gas source and have the appropriate valves and standard connections to ventilate patients. Other manual resuscitator bags of similar size are compatible with the MADVent system, but may require calibration for safe use of volume alarms and features. We note that adult self‐inflating resuscitation bags have similar geometries and total volumes and are designed to be used interchangeably by hospital personnel. These resuscitator bags are compatible with external PEEP valves that both add no dead space to the system and are essential for the care of patients with COVID‐19 and ARDS. They also have built‐in ports for supplemental oxygen administration and pressure monitoring. Two pressure sensors were used to measure ambient and in‐line pressure (BMP180; Bosch), but these can be replaced by a single differential pressure sensor (SSCMRRN060MDSA5; Honeywell Inc) that can be mounted on a printed circuit board. The differential pressure sensor can be connected to the respiration circuit either in line with the patient tube via a standard connector or at a modified mouthpiece. The mouthpiece placement option may be preferable for patients requiring very low tidal volumes or with especially poor gas exchange, for whom reducing dead space is crucial. In either case, the sensor is able to provide pressure measurement for the entire breath cycle: inhalation, exhalation and the idle time between breaths.
The dead space is the volume within the tubing leading from the patient's lungs to the resuscitator bag. During ventilation, exhaled gases may be cycled back and forth into and out of the patient without removal from the ventilation system, thus decreasing oxygen and increasing carbon dioxide in that volume. In our testing, dead space was effectively minimized by reducing tube length and positioning the MADvent near a full‐sized simulator manikin utilizing a standard adjustable overbed hospital table. This positioning has the advantage of minimizing the need for limited reserves of ventilator tubing in a time of crisis, though for safety would require heavy sedation or paralysis to prevent patient movement. If a more distant positioning of the MADvent is desired, the inspiratory/expiratory splitter valve typically housed at the exit of the Ambu SPUR 2 bag should be moved to a mouthpiece. This will create a traditional ‘Y’ connection at the level of the endotracheal tube, reserving the connection from the ventilator for inspiration and allowing for expiration through a separate limb of the circuit protected by a filter. Our design is forward compatible with a detailed dead space solution meeting the above description suggested by the MIT E‐Vent team (E‐Vent, 2020).
The bag is mounted into a frame under a lever arm that is subsequently used to compress the bag, as shown in Figure 2. The entire ventilator structure, including the bag mounting frame and arm, can be rapidly laser cut from polyoxymethylene (acetal) in 15 min and assembled using readily available hardware. An alternate material choice is polycarbonate, which has superior resistance to commonly used hospital disinfectants such as sodium hypochlorite (bleach). Complete design files are provided for the reader (see [Link], [Link], [Link], [Link]). Two convex compressor extensions are mounted on the lever arm and press into contact with the bag held in place by corresponding concave surfaces via hook‐and‐loop (Velcro) fixtures on the fixed frame of the ventilator, ensuring its stability and maximizing the possible compression volume of the bag. The hook‐and‐loop attachment facilitates quick and simple bag removal in the event the healthcare provider needs to manually ventilate the patient or the bag needs to be exchanged.
FIGURE 2.
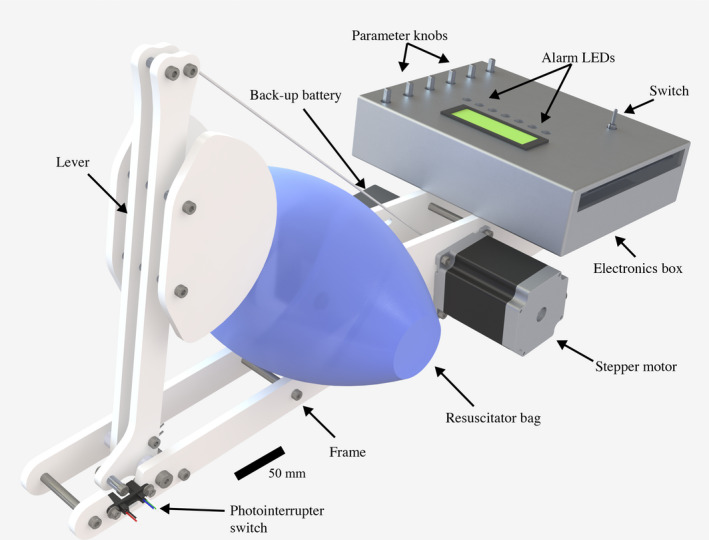
Render of the final version of MADVent, with an electronics enclosure. The enclosure has an interface for the healthcare provider to adjust various ventilation settings such as target pressure, inspiratory time, respiratory rate and alarm thresholds. An liquid crystal display (LCD) screen displays ventilation parameters in real time. LED's and a built in alarm alert the healthcare provider in the event of an emergency
2.3. Lever and pulley mechanism for reliable and quiet actuation
Rather than rely on gear or cam mechanisms to translate the rotational motion of a control motor to a rectilinear motion for bag compression (MIT, 2020a; University of Minnesota, 2020), we use the bag compression arm as a lever to provide substantial mechanical advantage from the motor. Geared and cam mechanisms are subject to wear, have backlash, add cost and complexity and tend to be noisy, a significant issue in the critical care setting. Our approach permits simple direct motor drive via a lanyard attached to the top end of the lever arm and wrapped around a spool attached to the motor's shaft. Lengthening the lever arm or placing the bag closer to the pivot point increases the mechanical advantage.
A stepper motor with 1.89 N‐m of holding torque and a maximum rotation speed of 180 rpm (QSH5718‐76‐28‐189, NEMA 23; Trinamic Motion Control GmbH) was chosen (see [Link], [Link], [Link], [Link] for details) in order to supply the rotation power and control necessary to implement a pressure control feedback loop and likewise produce sufficient rotation speed to enable rapid breath cycling. A microstepping commutation scheme was chosen for quiet operation, precision and the avoidance of resonances. Stepper motors are brushless and therefore can fail only by failure of the bearings or the insulation of the electrical wire within. They feature a mean time between failure of at least 10,000 hr, over a year of continuous operation. Supplies of these motors are unlikely to be affected by the pandemic, as they feature in diverse applications from 3D printing to robotics, consumer devices, automobiles and furniture. The lever arm hinges around a shoulder screw, a type of machine screw characterized by a constant diameter raised portion which is commonly used for simple pivot points, and its lateral movement along this screw is limited by spacers. A torsional spring is mounted at the hinge in order to aid in the return of the lever arm to its zero position at the end of each stroke, as verified for each cycle by a photointerrupter switch (C14D32P‐A3, CUI Devices, Lake Oswego, OR USA). An electronics box is secured to the frame opposite the lever hinge. The system is powered by a universal, medical grade (UL/ISO 60601) 12 VDC wall adapter (90–240 VAC input, SWM30‐12‐NV‐P5; CUI Devices), but a rechargeable lead‐acid back‐up battery (BP1.2‐12‐T1; B B Battery) capable of powering the system for at least 20 min is also installed and automatically begins supplying power when needed, while also indicating with a red LED.
One well‐known limitation of using bipolar stepper motors in any application is the high current they require when operating at low speeds. As the motor pauses for a period of time at each step in order to provide slow rotation, it could theoretically lead to high power consumption and overheating. However, this difficulty was foreseen, and pulse‐width modulation (PWM)‐based current limiting was programmed into the controller to eliminate it. PWM lowers the effective voltage drop across the motor for longer step times, in turn lowering the current draw of the motor. A motor controller was chosen that is capable of significantly higher current than the programmed limit current, preventing the motor controller from overheating. The robust motor controller set up and software limiting, combined with a power supply capable of no more than 3 A of constant draw, comprehensively limits possible thermal issues. As an added measure of safety, the temperature of the motor and circuits are continually monitored using temperature sensors and a visual alarm indicator is displayed in the event of the system overheating. The rotational position of the motor and the arm is tracked during operation to ensure mechanical integrity during operation. The limitations of individual ventilator components were identified and thorough testing performed to ensure no mechanical or electrical problems during operation. A full list of all potential errors and the systems we have in place to mitigate these risks are included in the [Link], [Link], [Link], [Link].
2.4. Estimating the tidal volume delivered by the ventilator from its motor rotation
Though we made the decision to omit flow sensors due to their expense (Corp, 2020) and complexity (Sensirion, 2020), we still required an accurate prediction of the tidal volume in order to safely provide high‐ and low‐volume alarms. This is achieved by monitoring the compression of the bag. The volume delivered by compressing the bag is directly proportional to the decrease in cross‐sectional area, Ai , of the bag as it is compressed by the lever. Thus, if we can relate Ai to the rotation of the motor, then we can predict the tidal volume, V tidal, since we are controlling the rotation of the motor shaft. An exercise in trigonometry provided in the [Link], [Link], [Link], [Link] reveals the relationship between the rotation of the motor shaft, , and the tidal volume produced by the bag, V tidal. This relationship, V tidal(), is validated in Figure 3.
FIGURE 3.
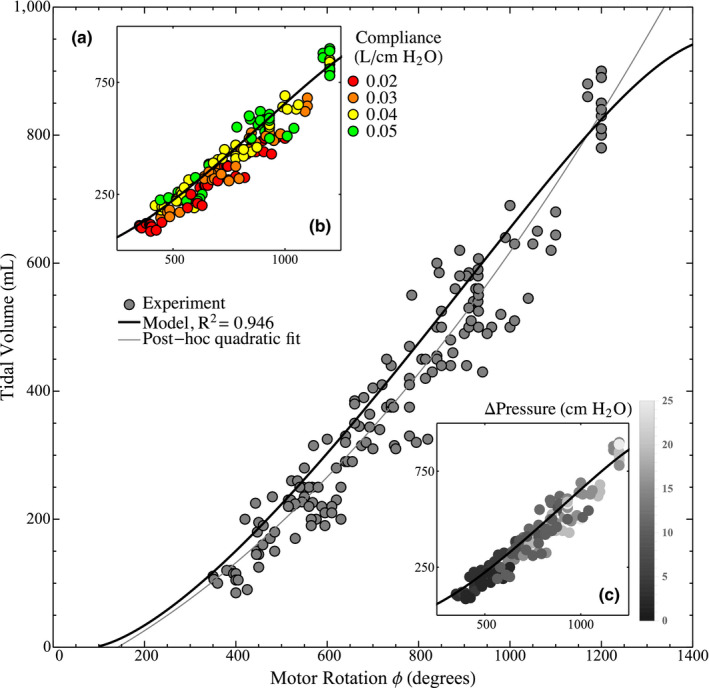
Tidal volume is related to the rotation of the motor via compression of the bag, as indicated (A) by the experimental results compared with a model V tidal = V tidal()constructed from the geometry (see [Link], [Link], [Link], [Link] for the full derivation). Furthermore, a post hoc quadratic curve fit (3.47 × 10–4 + 0.322 − 52.5 with R 2 = .953) is provided showing a slightly improved fit, indicating that a quadratic function can adequately represent the tidal volume as a function of the angle . In B, the volume corresponding to a given motor rotation is seen to increase with compliance—accounting for the spread in the data along with experimental error. In C, the difference between peak pressure and PEEP is seen to increase along the model, as expected due to the ideal gas law
We performed experiments across the full range of ventilation capabilities with four independent parameters, compliance, PEEP, inspiratory time and peak pressure, and two dependent measurements, tidal volume and motor rotation. Figure 3 shows that these potentially confounding variables do not have a large effect on the relationship between volume and motor rotation. A quadratic curve was post hoc least‐squares fit to the data, with a coefficient of determination of R 2 = .953, demonstrating a potential simple representation for the tidal volume to motor angle relationship. The model generally predicts larger volumes as expected since it does not account for the compliance of the lung and thus should match the higher range of data points. The model assumes two rigid bodies are intersecting, but in reality, the lever is rigid while the bag is elastic. As the bag is compressed, its shape changes, which accounts for the relative linearity of the fit curve compared with the model.
The volume–rotation relationship described by our model is embedded in the ventilation code so that the volume alarms are triggered correctly without a flow sensor, accurate to a mean value of 5%. It is important to note that manual resuscitation bags with different structure/geometry than the one used in this calibration (Ambu SPUR II; Ambu Inc.) will not have identical volume–rotation relationships, V tidal(), and volume‐related alarms will therefore be less accurate without another calibration. We expect this effect to be small since adult‐sized, self‐inflating resuscitation bags have similar geometries and total volumes. Recall these bags are all designed for the same purpose and are interchangeably used by hospital personnel.
2.5. Healthcare provider interface design, including life support alarms
The healthcare provider is able to directly set the following six parameters via control knobs on the system: RR, PIP, inspiratory time, high‐pressure alarm threshold, low‐volume alarm threshold and high‐volume alarm threshold. The system is capable of delivering between 10 and 35 breaths per minute (bpm), peak inspiratory pressures between 10 and 35 cm H2O, and inspiratory times between 1 and 3 s. Volume alarms may be set between 200 and 1,000 ml. The set values of each parameter are displayed on a liquid crystal display (LCD) screen. Seven light‐emitting diodes (LEDs) are provided to individually indicate to a clinician the nature of an alarm condition. These include alarms for the high and the low‐volume thresholds, as already mentioned, and alarms for mechanical failure, overheating, pressure sensor disconnection or failure, wall power disconnection and low battery. In urgent situations such as a low or high‐volume ventilation condition, a loud (92 dB) buzzer will also alert clinicians. If conflicting or otherwise incompatible alarm parameters are entered, then the relevant parameters will flash on the screen and an alarm will immediately sound. This condition has been programmed to occur in three cases: when the low‐volume alarm threshold is higher than the high‐volume alarm threshold, when the set peak pressure is higher than the high‐pressure alarm threshold and when the user set inspiratory time is more than 75% of the inspiratory time calculated from the user set RR.
After the parameters have been set, the system waits for activation via a toggle switch before initiating ventilation. During inspiration, the motor rotates an amount proportional to the difference between the intended pressure and the current measured pressure at each time step. The intended pressure at each time step is determined by a monotonically increasing function between p(t = 0) = 0 and p(t = t i) = p p, where p is pressure, t is time, p p is the peak pressure set by the provider, t i is the inspiratory time set by the provider. Once the peak pressure or the inspiratory time has been reached, the motor reverses direction at a set speed until it reaches the zero position, which is defined by the compressor arm photointerrupter switch and confirmed by the motor encoder. The system then enters a waiting period calculated according to the set RR and inspiratory time before beginning the next breath cycle.
If, at any point during the control loop, a single breath cycle generates a volume below the low‐volume alarm threshold, then that alarm is triggered. The system identifies the volume expelled in each breath via an encoder fixed to the motor shaft that reports exactly how much the shaft has rotated. A low volume may indicate significantly decreased compliance in the patient or an endotracheal tube obstruction. Similarly, if a single breath's volume exceeds the high‐volume alarm threshold, then that alarm is triggered, and may indicate a patient becoming disconnected from circuit or another source of a leak in the system. Alarms for pressure are triggered directly from the pressure sensor and similarly can identify issues with lung compliance and circuit integrity.
In addition to alarms for pressure, the system is equipped with temperature sensors that are mounted on the stepper motor and the motor controller, in order to continually monitor temperature and alert the healthcare provider if the measured motor temperature exceeds 65°C; these mechanical components are far removed from the ventilatory circuit. An encoder mounted on the shaft and a photointerrupter switch attached to the lever arm serve to detect mechanical faults that may occur during operation. Details of how these sensors are integrated into the system to produce requisite alarms to alert the healthcare provider, including how they are handled with code for the Arduino and what strategies have been used to avoid false alarms, are provided in the [Link], [Link], [Link], [Link].
2.6. Ventilator validation
All ventilators in clinical use are regularly validated and calibrated using lung simulators to comply with U.S. FDA standards of safety and efficacy. We validated our ventilator using the same procedures, first testing the ability of the alarms to notify the healthcare provider of adverse conditions, then testing the ventilator under normal and extreme operation and finally by testing the ventilator for 24 hr. All devices described in this manuscript were tested in accordance with those practices and FDA regulation protocols utilizing an approved lung simulator (Dual Adult Test Lung; Michigan Instruments) and a ventilator‐specific pressure and volume delivered data acquisition system (MP160; BioPac) at the University of California San Diego.
The alarm system of the MADVent Mark V ventilator was tested by simulating the same alarm conditions that would normally be detected by a commercial ventilator. Excessively high‐ and low‐volume conditions were simulated by changing the PEEP values as shown in Figure 4a,b; each of these conditions triggered the respective alarms on our ventilator. Likewise, high‐pressure events that could be due to a patient coughing or a kink in the ventilation tube, blocking airflow to the patient, produce an alarm in Figure 4c, but only after repeated coughing—as desired. Triggering alarms after a single cough might inappropriately encourage the healthcare provider to find a way to defeat the alarm. The admissible range of operating pressure, PEEP, time and breaths per minute were determined for our system from the lung compliance and the peak and PEEP pressure values as shown in Figure 3.
FIGURE 4.
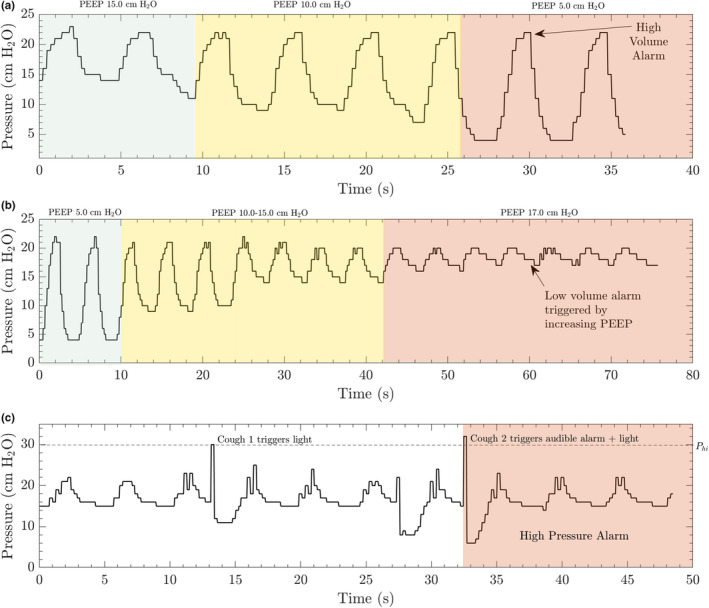
The MADVent Mark V has alarms for high and low volume that may be set between 200 and 1,000 ml. In this example, the system was run at a rate of 13 breaths per minute (ventilation rate), a PEEP value of 15 cm H2O and the compliance on the lung simulator was initially set to 0.03 /cm H2O. A, The high‐volume alarm threshold was set to 500 ml for the first case. PEEP was decreased from 15 to 5 cm H2O in order to increase the tidal volume delivered to the lung simulator. A high‐volume alarm was triggered when the calculated tidal volume exceed the limit set by the healthcare provider. A relevant clinical scenario for this alarm would be a leak in the inspiratory circuit leading to an increase in volume delivered without the target pressure being reached. B, The low‐volume alarm is triggered once the calculated volume drops below the lower limit set by the healthcare provider. This was simulated by increasing the PEEP up to 17 cm H2O. A relevant clinical scenario for this alarm would be the inspiratory line being kinked. C, The high‐pressure scenario was simulated by interrupting the expansion of the lung simulator during inspiration to simulate a patient coughing. The high‐pressure alarm was triggered when the pressure exceeded the set value of 30 cm H2O. Other scenarios are provided in the [Link], [Link], [Link], [Link], including a 24‐hr operation test and 12 adverse ventilation situations per ISO80601‐2‐80:2018 table 201.105 (International Standards Organization, 2018)
Once the alarms were confirmed to operate according to expectations by our anaesthesiologists, with the desired adjustability, sensitivity and absence of failure they are accustomed to from commercially available ventilators, the MADVent Mark V was validated per ISO 80601‐2‐80:2018 (International Standards Organization, 2018). This standard and its references define the expected functionality for a ventilator for the purposes of FDA certification under the current EUA (Hinton, 2020). This includes, notably, a 24‐hr operation test and 12 adverse ventilation situations, the results of which are provided in the [Link], [Link], [Link], [Link] for our ventilator. These tests operate the ventilator to the limits of the potential clinical range of pressure, PEEP, time and breaths per minute, while the lung compliances and resistances in the lung simulator are likewise adjusted to become extreme as per table 201.105 of ISO 80601‐2‐80 (International Standards Organization, 2018). The purpose of these tests is to verify the ventilator still safely functions under extreme operating conditions. The 24‐hr test used a compliance of 0.01 / cm H2O, a pressure of 40 cm H2O, breaths per minute of 30 bpm, a PEEP of 4 cm H2O and a lung resistance of 50 hPa‐ /s. The MADVent showed no deviation from the defined values for these tests, and the ventilator was judged by our anaesthesiologists to be safe for use.
3. RESULTS
The ventilator's operating and alarm capabilities were tested on a lung simulator after its design and fabrication as described in the Methods and [Link], [Link], [Link], [Link]. Under pressure‐control ventilation, the high‐volume, low‐volume and high‐pressure alarms were all successfully triggered when their alarm set points were crossed, as illustrated in Figure 4. For a pressure‐controlled system, a high‐volume alarm could be triggered by too large of a ΔP (Δ pressure = peak inspiratory pressure [PIP] − PEEP), an increase in the patient's compliance or an accidental disconnect/leak in the inspiratory circuit. This was experimentally demonstrated by slowly increasing the ΔP through PEEP reduction in Figure 4a. A low‐volume alarm state could be induced by a blockage in the inspiratory circuit, a decrease in the patient's compliance or too small of a ΔP set by the healthcare provider. This alarm was demonstrated in our system by gradually increasing the PEEP during operation, which gradually lowered the ΔP, and ultimately dropped the tidal volume below the set alarm threshold (Figure 4b). The high‐pressure alarm may be elicited by a patient coughing or ‘fighting’ the ventilator, simulated in our demonstration in Figure 4c, potentially indicating insufficient sedation or as a sign of circuit obstruction (along with the low‐volume alarm).
The overall range of parameters at which the system is capable of operating is listed in Table 1, which align with the specifications recommended for ARDS patients (Amato et al., 2015; Brower et al., 2000; Fan et al., 2017; Weiss et al., 2016). In addition to the testing reported in Figure 3, we also performed tests according to International Standards Organization (ISO) standards (see [Link], [Link], [Link], [Link]), which dictate airway resistance values.
TABLE 1.
Suitable MADVent Mark V operating parameter ranges
| Operating parameter | Tested range |
|---|---|
| Target inspiratory pressure | 10–35 cm H2O |
| Tidal volume (V T) | 200–1,000 ml |
| Respiratory rate (RR) | 6–35 bpm |
| Inspiratory time | 1–3.0 s |
| Low‐pressure alarm threshold | 0–20 cm H2O |
| High‐pressure alarm threshold | 30–60 cm H2O |
| High‐volume alarm threshold | 200–1,000 ml |
| Low‐volume alarm threshold | 200–1,000 ml |
The hardware on the system allows for a volume‐driven approach to ventilation in addition to pressure‐controlled ventilation with continuous feedback. Tests were conducted to characterize the system operating in this mode, but a proper continuous feedback volume‐control system would require an in‐line flow sensor, adding to the cost and complexity of the system and increasing reliance on an intact supply chain. However, we did test the system as a volume‐driven ventilator and the results are included in Figure 5. This mode was solely for evaluation purposes and will not be available to the healthcare provider. The volume‐driven mode includes user‐defined limits for low and high pressure. Baseline conditions were set to 5.0 cm H2O PEEP, a RR of 14 breaths per minute and an initial compliance of 0.03 /(cm H2O). Figure 5a illustrates a drastic change in compliance resulting in the trigger of a high‐pressure alarm. Examples where a high‐pressure alarm would be triggered are a blockage in the endotracheal tube, significant change in patient lung compliance, or bronchospasm. The alarm was programmed to trigger upon two consecutive high‐pressure events, after which the system will release the bag compression arm and commence a new respiration cycle at lower tidal volumes but increased rate in order to meet the minute ventilation set by the healthcare provider. In the event of an accidental disconnection of the endotracheal tube or other significant leak in the system, a low‐pressure alarm will be triggered as illustrated in Figure 5b. Kinking of the endotracheal tube or a sudden change in resistance can lead to a high‐pressure alarm as plotted in Figure 5c.
FIGURE 5.
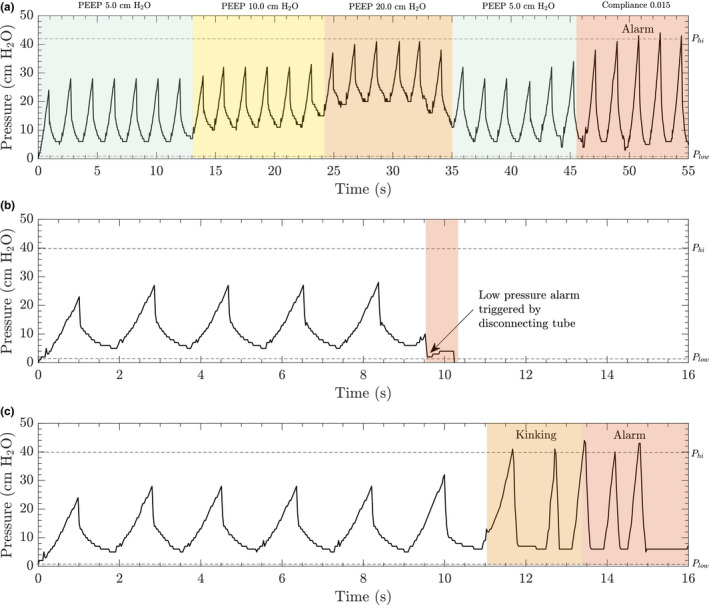
The volume‐driven version of the MADVent comes with alarms for high and low pressure that can be set between 0 and 50 cm H2O defined by the caregiver. The system was initially set at a rate of 34 breaths per minute, a PEEP value of 5 cm H2O was chosen and compliance on the lung simulator set to 0.03 /(cm H2O). A, The low‐ and high‐pressure alarm thresholds were set to 2 and 42 cm H2O, respectively. PEEP values were increased from 5 to 20 cm H2O and lowered back down to 5.0 cm H2O to ensure that the in‐line pressure sensor could detect and display changes in pressure values. A high‐pressure condition was simulated by decreasing patient lung compliance. The system triggered an alarm once the pressure went above 42 cm H2O. B, The low‐pressure alarm is triggered once the in‐line pressure value drops below the lower limit. A low‐pressure situation was simulated by disconnecting the endotracheal tube to trigger an alarm which results in the system immediately stopping. C, In the event that the tubing is kinked or there is a blockage in the endotracheal tube, the pressure begins to rise until the upper threshold is reached. This triggers a high‐pressure alarm and causes the system to resume ventilation at a lower volume, but at an increased rate according to the set minute ventilation
4. DISCUSSION
A number of solutions have been proposed to address the anticipated shortage of traditional ventilators during the COVID‐19 outbreak (Abir et al., 2020; For Critical Care in Medicine Society, 2020), including other low‐cost ventilators (Al Husseini et al., 2010; Darwood, McCanny, Kwasnicki, Martin, & Jones, 2019). Splitting one ventilator among two or more patients, re‐purposing continuous positive airway pressure (CPAP) machines, placing large orders for existing high‐cost commercial ventilators and bringing retired ventilators out of storage are some of the proposed solutions to meet the demand for reliable ventilators. Although there have been several cases (Abir et al., 2020; Rosenthal, Pinkowski, & Goldstein, 2020) of healthcare workers around the world splitting ventilators for shared use among two or more patients, this method remains controversial and requires further testing to better ensure safety of all patients on the shared circuit (For Critical Care in Medicine Society, 2020). Placing large orders for ventilators has put a strain on supply chains, many of which are located in countries that are severely affected by the pandemic. Bringing retired ventilators out of storage and re‐purposing CPAP machines could have unintended consequences due to component failures and a lack of testing for off‐label use.
There are currently multiple groups working in parallel to develop ventilation solutions with the similar goal of providing care to patients with COVID‐19. Notable devices are the Puritan Bennett™ 560 (PB560) developed by Medtronic and released under a temporary license to the public, the E‐Vent in development at the Massachusetts Institute of Technology (MIT, 2020a) and the Coventor developed at the University of Minnesota (University of Minnesota, 2020). The PB560 is a fully functioned portable ventilator system, and with its functions come increased cost and increased complexity, both of which are issues when ventilators need to be produced quickly and in great quantity, especially with over‐burdened supply lines in times of crisis. The MADVent, E‐Vent and Coventor ventilators are all less expensive and simpler to manufacture than the PB560.
The following information on the MIT E‐Vent is representative of the publicly available information at the time of this publication's writing, but may not remain accurate as their development process continues (MIT, 2020a; University of Minnesota, 2020). The MIT E‐Vent is described as a volume‐control system with the option of being triggered by spontaneous inhalation. The question of calibration is mentioned in the MIT E‐Vent's result summary (MIT, 2020b), but follow‐up data releases do not mention this, although their implementation of a spirometer to measure flow does partially address this. The E‐Vent does have the advantage of multiple rounds of testing in a porcine model in addition to a robust team of volunteers working on its development (MIT, 2020b).
Although the Coventor (University of Minnesota, 2020) recently received FDA EUA, details on controls, features, patient safety and clinician controls are not publicly available. It is not clear what degree of patient monitoring is possible with the Coventor, what respiratory parameters can be adjusted, or the presence and function of alarms based on publicly available information. At the time of this publication, it is estimated that the MADVent Mark V will cost around $250. This is likely less than the E‐Vent, whose publicly cited costs are as high as $500 and lack recent robust citation, and certainly less than the publicly disclosed $1,000 cost of the Coventor ($150 advertised initial prototype component‐only cost) (MIT, 2020a; University of Minnesota, 2020). The MIT E‐Vent and the MADVent have similar alarm and failure mode functions, but little is currently known about the Coventor's function or safety features.
Compared to these other low‐resource ventilator examples, the UCSD MADVent Mark V is the only device offering pressure‐controlled ventilation combined with adjustable volume alarms. Absolute pressures have always been a feature of lung‐protective ventilation, and the change in pressure during each respiratory cycle has increasingly been associated with optimal management of ARDS (Amato et al., 2015; Brower et al., 2000; Fan et al., 2017). Despite the relative simplicity of our mechanical system, the electronics of the system allow clinicians wide‐ranging control over ventilation characteristics and alarms. A conclusion on which device is most appropriate or effective in the current crisis cannot be responsibly made until all devices under consideration have publicly available testing, calibration and safety monitoring information. Low‐cost, scalable ventilator technologies such as this may also have applications for use in rural environments, low‐resource environments, natural disaster response and other mass casualty scenarios (Branson, Johannigman, Daugherty, & Rubinson, 2008; Health Systems Research Inc., 2005).
The MADVent Mark V pressure‐controlled ventilator works by controlled compression of a self‐inflating bag‐valve resuscitator until a target inspiratory pressure is reached. The peak pressure is set by the healthcare provider, and the controlled compression is to ensure this pressure is achieved in a gradual manner to maintain patient safety. An in‐line pressure sensor continually monitors pressure and provides feedback to control a lever arm that compresses the self‐inflating bag until the set peak pressure is attained. The system reaches the peak pressure at the inspiratory time per the set RR, both as selected by the healthcare provider and serving to define the remaining expiratory time and idle time between breaths. We prefer this pressure‐controlled version of the MADVent as it is continually regulated by means of a feedback loop between the pressure sensor and the motor, in order to accommodate changes in lung compliance and enable finer control over the delivery of mechanical ventilation. Though we have chosen the pressure‐controlled version for our final configuration, the hardware on the system is also capable of supporting a volume‐driven ventilation system that relies on compressing the bag by a specific amount corresponding to the volume set by the healthcare provider (Figure 5). This version would also monitor in‐line pressure during the breath cycle using the same sensors as the pressure‐controlled version. Here, we make the distinction between pressure‐controlled and volume‐driven approaches by pointing out there is no continuous feedback from any sensed tidal volume delivered to the patient and the compression of the bag, because there is no integrated flow sensor for this purpose. In the future, if it is determined that breath triggering is a necessary feature, the MADVent Mark V already has the hardware in place to provide this feature. This would allow the ventilator to be used in patients with lower levels of sedation and who are capable of initiating breaths but require the support of a ventilator. The system is set up to easily accommodate an in‐line viral filter to ensure that the air expired to the room is free of pathogens. An in‐line humidifier can also be added at the inlet as patients with ARDS typically require humidified inspiratory gas to improve mucociliary function (Chidekel et al., 2012).
Patients with COVID‐19 and ARDS can require mechanical ventilation for over 2 weeks (Anesi, Manaker, Finlay, & Bloom, 2020; Anzueto et al., 2004). All electrical components in the system were chosen to provide reliable continuous operation for such patients over weeks of use. The mechanical components chosen are all capable of withstanding the standard operational load due to the weight of the motor and that of the battery. The components of the ventilator were placed to balance the system across the width and length of the frame, and to provide easy access for maintenance and disinfection. The materials of the ventilator may be sanitized with conventional disinfectants such as 1.5% hydrogen peroxide and 70% ethanol. As part of the design, we attempted to integrate as many standard hospital items as possible. These items, such as the bag‐valve resuscitator and PEEP valve, are staples of the hospital environment and have already undergone rigorous testing for safety, longevity and compatibility with conventional disinfectants.
5. CONCLUSION
The lack of adequate ventilatory support has already caused preventable deaths in the first few months of the COVID‐19 pandemic, and more can be expected unless ventilators can quickly be provided to areas overburdened with COVID‐19 patients, both now and in the inevitable future surges of infection. The MADVent is capable of safely meeting the diverse ventilation requirements of COVID‐19 patients because its parameters are adjustable over the broad ranges required for ARDS patients. The combination of off‐the‐shelf components and laser cut parts in addition to our choice of mechanically driven pressure control makes our design both low cost and rapidly manufacturable. The essential qualities of safety, effectiveness, low cost and rapid manufacturability make it a feasible option for scaled production and use in current and future health crises.
The MADVent Mark V ventilator generates a pressure curve up to a set level in a prescribed rise time. A widely available resuscitator bag is used to drive flow with a simple mechanical system controlled by a widely available stepper motor, controller and system‐on‐a‐chip computer. Standard control of PEEP is provided with a disposable off‐the‐shelf valve. Volume and pressure alarms are provided for safety and additional alarms provided for electronics temperature and device failure detection to ensure that healthcare providers will be informed if this life support system shows signs of failure. Tidal volumes and pressure waveforms were tested and verified on a lung simulator according to FDA specifications, confirming the prototype is effective over the intended operating range.
As we continue to refine the design of the MADVent, we intend to add additional features to bring our low‐cost ventilator even closer to the expansive capabilities of standard ICU mechanical ventilators, though still at a reduced cost, to facilitate broader adoption. Much of the high cost associated with modern ventilators is a consequence of thorough adherence to safety regulations and ensuring the manufacturer is responsive to patient outcomes per FDA requirements. Our ventilator is not a substitute for these well‐designed and produced systems. Instead, our system—like many other recent low‐cost ventilators arising in this emergency—is a ventilator of last resort during a pandemic or mass casualty event. The design focuses upon patient safety, simplicity of manufacturing and modularity. The system, in its current state of development, can easily accommodate new modules that enable more sophisticated features, such as flow monitoring, which can enable additional ventilation modes and provide healthcare operators more information regarding a patient's breathing.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
L.P. and J.F. contributed equally. L.P., J.P. and J.F. conceived the project and studies. A.V., R.W., W.C. and J.S. designed the ventilator operating mechanism and scheme in a garage in the initial stages of this pandemic guided by L.P., J.F. and J.P.. A.V., R.W., W.C. and J.S. fabricated ventilator prototypes. M.S. designed, prototyped and coded the software and electronics to control and operate the ventilator system. M.S., E.S, C.K., D.U., A.V., R.W., W.C. and J.S. revised and improved the ventilator mechanics, controls, and control coding, and its suitability for mass and rapid manufacture. J.P. and L.P. created the ventilator validation protocol with J.F.'s assistance from ISO documentation. Testing and data collection were conducted by A.V., R.W., W.C., P.S., D.E.L., L.P., J.S. and T.V. using lung simulator systems. P.S., D.E.L., L.P., J.P., S.M., W.M. and J.S. provided ample input on ventilator features and functions needed for ARDS patients. A.V., R.W. and W.C. devised analyses of the data and ventilator mechanics with J.F.'s help. A.V., R.W., W.C., J.S., L.P., J.P. and J.F. wrote the initial manuscript with significant revisions by D.E.L., L.P., J.P. and J.F. All authors have read and approved the manuscript.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Video S1
ACKNOWLEDGEMENTS
The authors are grateful to the Office of Naval Research (Grant N00014–20–P–2007), Kratos Defense and Security Solutions (Gift R–86X16–VX16) and the University of California San Diego for provision of funds and facilities in support of this work. This work was performed in part at the Medically Advanced Devices Laboratory at the University of California, San Diego and at the Simulation and Training Center in the Center for Future Medicine at the University of California San Diego's School of Medicine. The authors would also like to thank Alex Grant and the prototyping laboratory at the Qualcomm Institute for valuable feedback during the design process, Shiv Patel, Matt Wilkinson (RCP, RRT, Pima Medical Institute) and the staff at the University of California San Diego School of Medicine for assisting with testing, Cody Noghera of the Jacobs School of Engineering for industrial support, and Victoria Cajipe at the University of California San Diego Office of Innovation and Commercialization for help with licensing and regulatory matters. The authors are finally grateful to the administration and staff of the University of California San Diego for finding creative ways to enable our work to proceed despite this pandemic.
Vasan A, Weekes R, Connacher W, et al; Acute Ventilation Rapid Response Taskforce (AVERT) . MADVent: A low‐cost ventilator for patients with COVID‐19. Med Devices Sens. 2020;3:e10106. 10.1002/mds3.10106
REFERENCES
- Abir, M. , Nelson, C. , Chan, E. W. , Al‐Ibrahim, H. , Cutter, C. , Patel, K. , & Bogart, A. (2020). Critical care surge response strategies for the 2020 COVID‐19 outbreak in the United States. Tech. Rep., Santa Monica, CA: RAND Corporation. https://www.rand.org/pubs/research_reports/RRA164‐1.htm [Google Scholar]
- Al Husseini, A. M. , Lee, H. J. , Negrete, J. , Powelson, S. , Servi, A. T. , Slocum, A. H. , & Saukkonen, J. (2010). Design and prototyping of a low‐cost portable mechanical ventilator. Transactions of the ASME Journal of Medical Devices, 4, 027514. 10.1115/1.3442790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato, M. B. , Meade, M. O. , Slutsky, A. S. , Brochard, L. , Costa, E. L. V. , Schoenfeld, D. A. , … Brower, R. G. (2015). Driving pressure and survival in the acute respiratory distress syndrome. New England Journal of Medicine, 372, 747–755. 10.1056/NEJMsa1410639 [DOI] [PubMed] [Google Scholar]
- Anesi, G. L. (2020). Coronavirus disease 2019 (COVID‐19): Critical care issues. Manaker, S., Finlay, G., & Bloom, A., eds. UpToDate. Waltham, MA: UpToDate Inc. https://www.uptodate.com/contents/coronavirus‐disease‐2019‐covid‐19‐critical‐care‐and‐airway‐management‐issues. Accessed on June 15, 2020. [Google Scholar]
- Anzueto, A. , Frutos–Vivar, F. , Esteban, A. , Alía, I. , Brochard, L. , Stewart, T. , … Pelosi, P. (2004). Incidence, risk factors and outcome of barotrauma in mechanically ventilated patients. Intensive Care Medicine, 30, 612–619. 10.1007/s00134-004-2187-7 [DOI] [PubMed] [Google Scholar]
- Bachiller, P. R. , McDonough, J. M. , & Feldman, J. M. (2008). Do new anesthesia ventilators deliver small tidal volumes accurately during volume‐controlled ventilation? Anesthesia & Analgesia, 106, 1392–1400. 10.1213/ane.0b013e31816a68c6 [DOI] [PubMed] [Google Scholar]
- Bhatraju, P. K. , Ghassemieh, B. J. , Nichols, M. , Kim, R. , Jerome, K. R. , Nalla, A. K. , … Mikacenic, C. (2020). COVID‐19 in critically ill patients in the Seattle region—Case series. New England Journal of Medicine, 382(21), 2012–2022. 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biselli, P. J. C. , Nóbrega, R. S. , & Soriano, F. G. (2018). Nonlinear flow sensor calibration with an accurate syringe. Sensors, 18, 2163– 10.3390/s18072163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourenne, J. , Hraiech, S. , Roch, A. , Gainnier, M. , Papazian, L. , & Forel, J.‐M. (2017). Sedation and neuromuscular blocking agents in acute respiratory distress syndrome. Annals of Translational Medicine, 5, 291–303. 10.21037/atm.2017.07.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branson, R. D. , Johannigman, J. A. , Daugherty, E. L. , & Rubinson, L. (2008). Surge capacity mechanical ventilation. Respiratory Care, 53, 78–88. [PubMed] [Google Scholar]
- Brower, R. G. , Matthay, M. A. , Morris, A. , Schoenfeld, D. , Thompson, B. T. , Wheeler, A. , & Acute Respiratory Distress Syndrome Network . (2000). Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine, 342, 1301–1308. [DOI] [PubMed] [Google Scholar]
- Cascella, M. , Rajnik, M. , Cuomo, A. , Dulebohn, S. C. , & Di Napoli, R. (2020). Features, evaluation and treatment coronavirus (COVID‐19). In StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. [PubMed] [Google Scholar]
- Chidekel, A. , Zhu, Y. , Wang, J. , Mosko, J. J. , Rodriguez, E. , & Shaffer, T. H. (2012). The effects of gas humidification with high‐flow nasal cannula on cultured human airway epithelial cells. Pulmonary Medicine, 2012, 1–8. 10.1155/2012/380686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corp, S. (2020). Flow sensor. Retrieved from https://www.sensirion.com/en/about‐us/newsroom/sensirion‐specialist‐articles/flow‐sensor‐solutions‐in‐modern‐medical‐ventilators/ [Google Scholar]
- Darwood, A. , McCanny, J. , Kwasnicki, R. , Martin, B. , & Jones, P. (2019). The design and evaluation of a novel low‐cost portable ventilator. Anaesthesia, 74, 1406–1415. 10.1111/anae.14726 [DOI] [PubMed] [Google Scholar]
- Dellaca', R. L. , Veneroni, C. , & Farre', R. (2017). Trends in mechanical ventilation: Are we ventilating our patients in the best possible way? Breathe, 13, 84–98. Retrieved from https://breathe.ersjournals.com/content/13/2/84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- E‐Vent, M. (2020). Plumbing. Retrieved from https://e‐vent.mit.edu/mechanical/plumbing/ [Google Scholar]
- Fan, E. , Del Sorbo, L. , Goligher, E. C. , Hodgson, C. L. , Munshi, L. , Walkey, A. J. , … American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine . (2017). An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. American Journal Respiratory Critical Care Medicine, 195, 1253–1263. [DOI] [PubMed] [Google Scholar]
- Fauci, A. S. , Lane, H. C. , & Redfield, R. R. (2020). COVID‐19 — navigating the uncharted. New England Journal of Medicine, 382, 1268–1269. 10.1056/NEJMe2002387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischhoff, B. (2015). The realities of risk‐cost‐benefit analysis. Science, 350, aaa6516. 10.1126/science.aaa6516 [DOI] [PubMed] [Google Scholar]
- For Critical Care in Medicine Society. (2020). Consensus statement on multiple patients per ventilator. Retrieved from https://www.sccm.org/Disaster/Joint‐Statement‐on‐Multiple‐Patients‐Per‐Ventilato [Google Scholar]
- Harris, R. S. (2005). Pressure‐volume curves of the respiratory system. Respiratory Care, 50, 78–99. [PubMed] [Google Scholar]
- Health Systems Research Inc. (2005). Bioterrorism and other public health emergencies: Altered standards of care in mass casualty events. Retrieved from https://archive.ahrq.gov/research/altstand/altstand.pdf [Google Scholar]
- Heulitt, M. J. , Holt, S. J. , & Thurman, T. L. (2013). Accuracy of small tidal volume measurement comparing two ventilator airway sensors. Journal of Pediatric Intensive Care, 2, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton, D. (2020). Emergency use authorization for ventilators. Retrieved from https://www.fda.gov/media/136423/download [Google Scholar]
- Huang, H.‐C. , Araz, O. M. , Morton, D. P. , Johnson, G. P. , Damien, P. , Clements, B. , & Meyers, L. A. (2017). Stockpiling ventilators for influenza pandemics. Emerging Infectious Diseases, 23, 914–921. 10.3201/eid2306.161417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Standards Organization ISO/TC 121/SC 3. (2018). ISO 90601–2‐80‐2018(en) medical electrical equipment — part 2–80: Particular requirements for basic safety and essential performance of ventilatory support equipment for ventilatory insufficiency. Retrieved from https://www.iso.org/standard/68844.html [Google Scholar]
- International Standards Organization ISO/TC 210. (2019a). ISO 14971:2019 medical devices — application of risk management to medical devices. Retrieved from https://www.iso.org/standard/72704.html [Google Scholar]
- International Standards Organization ISO/TC 121/SC 4. (2019b). ISO 19223:2019(en) lung ventilators and related equipment — vocabulary and semantics. Retrieved from https://www.iso.org/standard/51164.html80601 [Google Scholar]
- Krishnamoorthy, V. , Vavilala, M. S. , & Mock, C. N. (2014). The need for ventilators in the developing world: An opportunity to improve care and save lives. Journal of Global Health, 4(010303), 1–4. 10.7189/jogh.04.010303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyazidi, A. , Thille, A. W. , Carteaux, G. , Galia, F. , Brochard, L. , & Richard, J.‐C. (2010). Bench test evaluation of volume delivered by modern ICU ventilators during volume‐controlled ventilation. Intensive Care Medicine, 36, 2074–2080. 10.1007/s00134-010-2044-9 [DOI] [PubMed] [Google Scholar]
- Meng, L. , Qiu, H. , Wan, L. , Ai, Y. , Xue, Z. , Guo, Q. , … Xiong, L. (2020). Intubation and ventilation amid the covid‐19 outbreak: Wuhan's experience. Anesthesiology, 132(6):1317–1332. 10.1097/ALN.0000000000003296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIT . (2020). MIT emergency ventilator (E‐Vent) project. Retrieved from https://e‐vent.mit.edu/ [Google Scholar]
- MIT . (2020). MIT E‐Vent testing. Retrieved https://e‐vent.mit.edu/testing‐results/ [Google Scholar]
- Morrison, S. (2020). Ford and GM are making tens of thousands of ventilators. It may already be too late. Retrieved from https://www.vox.com/recode/2020/4/10/21209709/tesla‐gm‐ford‐ventilators‐coronavirus [Google Scholar]
- Netland, T. (2020). A better answer to the ventilator shortage as the pandemic rages on. Retrieved from https://www.weforum.org/agenda/2020/04/covid‐19‐ventilator‐shortage‐manufacturing‐solution/ [Google Scholar]
- Phua, J. , Weng, L. I. , Ling, L. , Egi, M. , Lim, C.‐M. , Divatia, J. V. , … Du, B. . (2020). Intensive care management of coronavirus disease 2019 (COVID‐19): Challenges and recommendations. The Lancet Respiratory Medicine, 8(5), 506–517. 10.1016/S2213-2600(20)30161-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevention & Early Treatment of Acute Lung Injury (PETAL) Network . (2019). Early neuromuscular blockade in the acute respiratory distress syndrome. New England Journal of Medicine, 380, 1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranney, M. L. , Griffeth, V. , & Jha, A. K. (2020). Critical supply shortages — the need for ventilators and personal protective equipment during the COVID‐19 pandemic. New England Journal of Medicine, 382(18), e41. 10.1056/NEJMp2006141 [DOI] [PubMed] [Google Scholar]
- Rosenbaum, L. (2020). Facing COVID‐19 in Italy—ethics, logistics, and therapeutics on the epidemic's front line. New England Journal of Medicine, 382(20), 1873–1875. 10.1056/NEJMp2005492 [DOI] [PubMed] [Google Scholar]
- Rosenthal, B. M. , Pinkowski, J. , & Goldstein, J. (Eds.) (2020). ‘The Other Option Is Death’: New York starts sharing of ventilators. Retrieved from https://www.nytimes.com/2020/03/26/health/coronavirus‐ventilator‐sharing.html [Google Scholar]
- Sanche, S. , Lin, Y. T. , Xu, C. , Romero‐Severson, E. , Hengartner, N. , & Ke, R. (2020). High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerging Infectious Diseases, 26. 10.3201/eid2607.200282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensirion . (2020). CMOSens technology for gas flow and differential pressure. Retrieved from https://www.sensirion.com/en/about‐us/company/technology/cmosens‐technology‐for‐gas‐flow/ [Google Scholar]
- University of Minnesota . (2020). COVID‐19 ventilator. https://med.umn.edu/covid19Ventilator [Google Scholar]
- Wang, D. , Hu, B. O. , Hu, C. , Zhu, F. , Liu, X. , Zhang, J. , … Peng, Z. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Journal of the American Medical Association, 323, 1061–1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, C. H. , Baker, D. W. , Weiner, S. , Bechel, M. , Ragland, M. , Rademaker, A. , … Persell, S. D. (2016). Low tidal volume ventilation use in acute respiratory distress syndrome. Critical Care Medicine, 44, 1515–1522. 10.1097/CCM.0000000000001710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman, G. E. , Crane‐Droesch, A. , Chivers, C. , Luong, T. B. , Hanish, A. , Levy, M. Z. , … Halpern, S. D. (2020). Locally informed simulation to predict hospital capacity needs during the COVID‐19 pandemic. Annals of Internal Medicine, 1‐9. 10.7326/M20-1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodyatt, A. (2020). The world is scrambling to buy ventilators in the covid‐19 pandemic. One country has only four of them – for 12 million people. Retrieved from https://www.cnn.com/2020/04/18/africa/covid‐19‐ventilator‐shortage‐intl‐scli/index.html [Google Scholar]
- Xie, J. , Tong, Z. , Guan, X. , Du, B. , Qiu, H. , & Slutsky, A. S. (2020). Critical care crisis and some recommendations during the COVID‐19 epidemic in China. Intensive Care Medicine, 46(5), 837–840. 10.1007/s00134-020-05979-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Yu, Y. , Xu, J. , Shu, H. , Xia, J. , Liu, H. , … Shang, Y. (2020). Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: A single‐centered, retrospective, observational study. The Lancet Respiratory Medicine, 8(5), 475–481. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Video S1


