Abstract
BACKGROUND
A novel coronavirus has caused an international outbreak. Currently, there are no specific therapeutic agents for coronavirus infections. Convalescent plasma (CP) therapy is a potentially effective treatment option.
METHODS
Patients who had recovered from COVID‐19 and had been discharged from the hospital for more than 2 weeks were recruited. COVID‐19 convalescent plasma (CCP)‐specific donor screening and selection were performed based on the following criteria: 1) aged 18‐55 years; 2) eligible for blood donation; 3) diagnosed with COVID‐19; 4) had two consecutive negative COVID‐19 nasopharyngeal swab tests based on PCR (at least 24 hr apart) prior to hospital discharge; 5) had been discharged from the hospital for more than 2 weeks; and 6) had no COVID‐19 symptoms prior to convalescent plasma donation. In addition, preference was given to CCP donors who had a fever lasting more than 3 days or a body temperature exceeding 38.5°C (101.3°F), and who donated 4 weeks after the onset of symptoms. CCP collection was performed using routine plasma collection procedures via plasmapheresis. In addition to routine donor testing, the CCP donorsʼ plasma was also tested for SARS‐CoV‐2 nucleic acid and S‐RBD‐specific IgG antibody.
RESULTS
Of the 81 potential CCP donors, 64 (79%) plasma products were collected. There were 18 female donors and 46 male donors. There were 34 first‐time blood donors and 30 repeat donors. The average time between CCP collection and initial symptom onset was 49.1 days, and the average time between CCP collection and hospital discharge was 38.7 days. The average volume of CCP collected was 327.7 mL.
All Alanine transaminase (ALT) testing results met blood donation requirements. HIV Ag/Ab, anti‐HCV, anti‐syphilis, and HBsAg were all negative; NAT for HIV, HBV, and HCV were also negative. In addition, all of the CCP donorsʼ plasma units were negative for SARS‐CoV‐2 RNA. Of the total 64 CCP donors tested, only one had an S‐RBD‐specific IgG titer of 1:160, all others had a titer of ≥1:320.
CONCLUSION
Based on a feasibility study of a pilot CCP program in Wuhan, China, we demonstrated the success and feasibility of CCP collection. In addition, all of the CCP units collected had a titer of ≥1:160 for S‐RBD‐specific IgG antibody, which met the CCP quality control requirements based on the Chinese national guidelines for CCP.
In December 2019, a new type of human coronavirus, Severe Acute Respiratory Syndrome Type 2 Coronavirus (SARS‐CoV‐2), was discovered. The infection caused by the virus, named COVID‐19 (coronavirus disease 2019), has been spreading rapidly worldwide. The World Health Organization has declared the outbreak of COVID‐19 to be a pandemic. According to the WHO data, as of April 1, 2020, COVID‐19 had resulted in a total of 823,626 confirmed cases and had killed 40,598 people globally. 1 Most patients with COVID‐19 infection experience a series of clinical manifestations such as fever, cough, myalgia or fatigue, dyspnea, and even acute respiratory distress syndrome (ARDS) and secondary infections. Many critically ill patients have been admitted to intensive care units. 2 , 3 Existing reports have shown that the mortality rate ranges from 1% to 4%. 4 The severity and epidemic potential of COVID‐19 has paralyzed the worldʼs health care system, claimed many lives, and threatened economic stability. Unfortunately, to date, apart from symptomatic treatment and supportive care, no specific antiviral treatment or vaccine has been proven effective. 5
Convalescent plasma (CP) containing SARS‐CoV‐2‐specific antibodies from recovered patients is now being entertained as a potential treatment option. 6 CP has been used to treat several other viral infections, including severe acute respiratory syndrome coronavirus (SARS‐CoV), 7 Ebola virus, 8 Middle East espiratory syndrome coronavirus (MERS‐CoV),9 and avian influenza A(H5N1) virus. 10 A recent report by Shen et al. showed that the transfusion of COVID‐19 convalescent plasma (CCP) had resulted in significant clinical improvement in five critically ill patients with COVID‐19 and ARDS. 11 CCP has been included as a treatment option in the Chinese COVID‐19 treatment guidelines and a viral titer of 1:160 has been recommended as a product quality control indicator. 12 Recently, the US Food and Drug Administration created pathways for using CCP either under an emergency IND or expanded access. 13
For the past few months, CCP has been collected and used in China empirically. In this study, we highlight key elements of a pilot program for collecting CCP in Wuhan, China.
METHODS
Operation protocol for CCP collection
Figure 1 illustrates the workflow of the CCP collection program in Wuhan, China. Potential donors were recruited through mass media and social media such as WeChat. Please see Figure S2 for sample recruitment material in Chinese, with English translation in supplemental material. The primary recruitment targets were patients who had recovered from COVID‐19 and had been discharged from the hospital for more than 2 weeks. Hospital discharge criteria include: 1) body temperature returns to normal for more than 7 days; 2) respiratory symptoms improve significantly; 3) lung imaging shows significant absorption of inflammation; and 4) two consecutive negative COVID‐19 nasopharyngeal swab tests based on PCR (at least 24 hr apart). Consenting participating donors were interviewed via telephone by qualified and trained staff to review a COVID‐19‐specific health history questionnaire and to qualify them based on the following criteria: 1) aged 18‐55 years; 2) eligible for blood donation; 3) diagnosed with COVID‐19; 4) had two consecutive negative COVID‐19 nasopharyngeal swab tests based on PCR (at least 24 hr apart) prior to hospital discharge; 5) had been discharged from the hospital for more than 2 weeks; and 6) had no COVID‐19 symptoms prior to CCP donation. In addition, preference was given to CCP donors who had a fever lasting more than 3 days or a body temperature exceeding 38.5°C (101.3°F), and 4 weeks after symptom onset. 14 If they were deemed qualified at the pre‐screening, they were asked to bring their medical records to an in‐person appointment at the Wuhan Blood Center. A healthcare worker from the blood center examined the donors, reviewed their medical records, and made sure that that the donors had two negative COVID‐19 nasopharyngeal swab tests based on PCR (at least 24 hr apart) and no COVID‐19 symptoms. If they were determined to be eligible for CCP donation, they then underwent pre‐donation testing that included alanine transaminase (ALT), HBsAg, and hemoglobin. If they met all blood donation requirements, they would proceed with a CCP donation via plasmapheresis that was performed according to routine procedures and requirements for plasma donation. After plasma donation, they were monitored for adverse events and awarded with a CCP‐specific blood donation certificate.
Fig. 1.
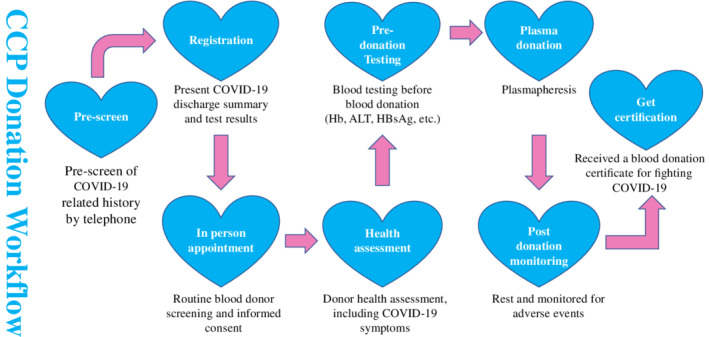
CCP workflow. [Color figure can be viewed at wileyonlinelibrary.com]
Study recruitment, screening, and sample collection
Donor recruitment, consent, screening, and blood specimen collection were conducted at the Wuhan Blood Center. Protocols for donor selection, plasma collection, specimen collection and testing, and clinical trials were approved by the Ethics Review Committee of the Institute of Blood Transfusion, Chinese Academy of Medical Sciences. All CCP donors gave written, informed consent. The CCP products collected from March 3, 2020 to March 18, 2020 were examined for the CCP program and product quality control.
Plasma collection
CCP collection was performed according to routine procedures and requirements for plasma donation. Plasma collection was performed via plasmapheresis (DigiPIa80, Sichuan Nangeer Biotechnology Co., Ltd; XCF 3000, Sichuan Nangeer Biotechnology Co., Ltd). The volume of plasma to be collected was determined based on the donorʼs weight. Female donors and male donors weighing less than 70 kg were given 0.9% sodium chloride (NS) during collection. The volume of the replacement fluid was half of the plasma collected. All plasma products were frozen within 6 hours.
Laboratory testing
Sample collection and preparation
All donor samples were collected in a sodium citrate tube for the ELISA assay and an Ethylene Diamine Tetraacetic Acid (EDTA) tube with gel separator for the NAT assay, and were centrifuged and separated within 4 hours.
Routine blood product testing
All blood products were tested for HCV, HBV, HIV by ELISA and NAT, and for syphilis by ELISA.
Special testing for CCP
Testing for SARS‐CoV‐2 nucleic acid. All collected donor plasma samples were screened for SARS‐CoV‐2 RNA by individual donor testing, using the PerkinElmer New Coronavirus Nucleic Acid Detection Kit (PerkinElmer Healthcare Diagnostics Co., Ltd.). The test kits used were approved and licensed by the FDA, and the assays were performed according to the manufacturersʼ instructions.
Titer of S‐RBD‐specific IgG antibody. For product quality control, S‐RBD‐specific IgG antibody was measured. The S‐RBD‐specific IgG antibody ELISA kit was made in‐house. In brief, 96 well plates (Thermo Scientific) were coated with 100 ng of recombinant Receptor binding domain (RBD) polypeptides per well (Sino Biological). The plates were decanted of the coating solution after overnight coating and were blocked with B3T at 37°C (98.6°F) for 1 hour. Plasma samples were diluted 160‐, 320‐, 640‐, and 1280‐fold with 0.5% triton X‐100 phosphate buffered saline (TPBS) and 5% fetal calf serum (FBS) (Gibco). After washing, the plates had mouse to human secondary antibody added and were observed for horseradish peroxidase (HRP) reaction. The changes in the absorbance at 450 nm and 630 nm were measured using an automatic microplate reader (Sunrise Tecan GmbH), and the OD values were calculated. The results were reported as the S/CO value, which was calculated as the ratio of OD value to the cutoff value. If the ELISA assay was positive, the highest dilutions were reported as the titers, which ranged between 1:160 and 1:1280 (ELISA endpoint dilution titers).
Statistical analysis
The descriptive data were reported. The minimum, maximum, median, average, and SD were calculated by Excel.
RESULTS
CCP donor and product summary
From 81 potential donors for CCP, 64 (79% of donors) plasma products were collected; 17 of the potential donors could not donate plasma because they did not meet the blood donation requirements (2 had hypertension and 15 had abnormal ALT). There were 18 female donors and 46 male donors. Of the 64 donors, 34 were first‐time blood donors and 30 were repeat donors. The average time between CCP collection and symptom onset was 49.1 days, and the average time between CCP collection and hospital discharge was 38.7 days. The average volume of CCP collected was 327.7 mL.
Infectious disease testing
All ALT testing met the blood donation criteria. The anti‐HIV1/2, anti‐HCV, syphilis testing, and HBsAg were all negative; the NAT for HIV, HBV, and HCV were also negative.
In addition, all CCP donorsʼ plasma tested negative for SARS‐CoV‐2 RNA.
Titer of S‐RBD‐specific IgG antibody
Among the total 64 analyzed CCP donors, only one had an S‐RBD‐specific IgG titer of 1:160, all others had a titer ≥1:320.
DISCUSSION
The COVID‐19 pandemic has become a major public health challenge, not only for China but also for countries around the world. The development of an effective and safe CCP program may provide an effective treatment option to help control the pandemic situation.
Here we report a summary of a pilot CCP collection program (Table 1). The program was able to attract recovered patients and successfully collected CCP products. The high rate of program exclusion is not surprising, because this was not a typical blood donation, and some patients may not have been fully recovered from COVID‐19 or were not qualified as blood donors at baseline despite a high level of enthusiasm. It is also very encouraging to note that more than half of the CCP donors are committed repeat blood donors who were willing to come back after being very ill.
TABLE 1.
Summary of CCP products (N = 64)
| Min | Max | Median | Average | SD | |
|---|---|---|---|---|---|
| Volume of CCP collected (mL) | 200 | 400 | 300 | 327.7 | 80.0 |
| Timing of CCP collection: from symptom onset (days) | 37 | 68 | 48 | 49.1 | 7.2 |
| Timing of CCP collection: from hospital discharge | 16 | 57 | 40 | 38.7 | 8.2 |
| Routine TTD testing | All ALT results met donation criteria; ELISA for HIV Ag/Ab, anti‐HCV, anti‐syphilis and HBsAg were all negative; NAT for HIV, HBV, and HCV were also negative | ||||
| SARS‐CoV‐2 NAT | SARS‐CoV‐2 were all negative | ||||
| S‐RBD‐specific IgG titer | All were ≥1:160 | ||||
From blood safety perspective for CCP, it is also notable that we did not observe any positivity for routine TTD testing, or NAT for SARS‐CoV‐2 nucleic acid. This is rather assuring. From product quality control perspective, it is notable that all CCP donors in this pilot program had an S‐RBD‐specific IgG titer of ≥1:160, which met the Chinese national CCP standard.
While the data are very limited, we did demonstrate the success and feasibility of a pilot CCP program. In addition, clinical validation based on well‐designed clinical trials such as RCT will be needed for the safety and therapeutic efficacy of CCP. Clinical correlation with the donor selection criteria and product quality control indicators will help further refine the CCP program requirements.
CONCLUSION
Based on a feasibility study of a pilot CCP program in Wuhan, China, we demonstrated the success and feasibility of CCP collection. All donors who donated CCP during the study period had negative routine TTD, including negative NAT for HIV, HBV, and HCV, and negative NAT for SARS‐CoV‐2. In addition, all donors had a titer ≥1:160 for S‐RBD‐specific IgG antibody, which met the CCP quality control requirements based on the Chinese national guidelines for CCP.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Supporting information
Supplemental material
Figure S2: Recruitment material in Chinese, with English translation in supplemental material.
ACKNOWLEDGMENTS
We would like to thank the following individuals for their guidance, expertise, and assistance with the study design: Peter W. Marks, MD, PhD, Center for Biologics Evaluation and Research, FDA; Anne Eder, MD, PhD, Center for Biologics Evaluation and Research, FDA; Nicole Verdun, MD, Center for Biologics Evaluation and Research, FDA; Tim Uyeki, MD, MPH, MPP, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC), USA; Scott Koepsell MD, PhD, University of Nebraska Medical Center; Annie Winkler, MD, Instrumentation Laboratory; Xuan Qin, PhD, D(ABMM), Seattle Childrenʼs Hospital and University of Washington; Toby L. Simon, MD, CSL Behring; Richard J Benjamin, MD, PhD, CERUS Corporation; Jerry A Holmberg, Grifols S A; Vicente Blanquer, Grifols S A; Daniel Fleta, Grifols S A; Amarant Martinez, Grifols S A; and Liu Yang, Grifols S A.
Financial support: This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant Nos. 2020‐I2M‐CoV19‐006, 2016‐I2M‐3‐024, and 2017‐I2M‐1‐009). Non‐profit Central Research Institute Fund of Chinese Academy of Medical Sciences (Grant No. 2018PT32016).
Contributor Information
Lan Wang, Email: wlan66@126.com.
Yanyun Wu, Email: yxw1366@med.miami.edu.
Zhong Liu, Email: liuz@ibt.pumc.edu.cn.
REFERENCES
- 1. World‐Health‐Organization . Coronavirus disease (COVID‐19) outbreak. [cited 2020 Apr 1]. Available from: https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200401‐sitrep‐72‐covid‐19.pdf?sfvrsn=3dd8971b_2.
- 2. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708‐20. (Published article online). 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Velavan TP, Meyer CG. The COVID‐19 epidemic. Trop Med Int Health 2020;25:278‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cascella M, Rajnik M, Cuomo A, et al. Features, evaluation and treatment Coronavirus (COVID‐19) [Updated 2020 Mar 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554776/?report=reader. [PubMed]
- 6. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID‐19. The Lancet Infectious Diseases 2020;20(4):398‐400. 10.1016/s1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. John MJ, Maria SC, Kenneth BJ, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta‐analysis. J Infect Dis 2015;211:80‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garraud O. Use of convalescent plasma in Ebola virus infection. Transfus Apher Sci 2017;56:31‐4. [DOI] [PubMed] [Google Scholar]
- 9. Arabi YM, Hajeer AH, Luke T, et al. Feasibility of using convalescent plasma immunotherapy for MERS‐CoV infection, Saudi Arabia. Emerg Infect Dis 2016;22:1554‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hui DS, Lee N, Chan PK, et al. The role of adjuvant immunomodulatory agents for treatment of severe influenza. Antiviral Res 2018;150:202‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA 2020;323:1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Health Commission of the Peopleʼs Republic of China . Covid‐19 treatment plan (trial version 6) [cited 2020 Apr 1]. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.
- 13. FDA . Investigational COVID‐19 convalescent plasma—emergency INDs. Available from: https://www.fda.gov/vaccines‐blood‐biologics/investigational‐new‐drug‐ind‐or‐device‐exemption‐ide‐process‐cber/investigational‐covid‐19‐convalescent‐plasma‐emergency‐inds.
- 14. Li L, Tong XL, Chen HW, et al. Characteristics and Serological Patterns of COVID‐19 Convalescent Plasma Donors. Transfusion. 2020. 10.1111/trf.15918 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material
Figure S2: Recruitment material in Chinese, with English translation in supplemental material.


