Abstract
Solid organ transplant recipients are considered at high risk for COVID‐19 infection due to chronic immune suppression; little data currently exists on the manifestations and outcomes of COVID‐19 infection in lung transplant recipients. Here we report 8 cases of COVID‐19 identified in patients with a history of lung transplant. We describe the clinical course of disease as well as preexisting characteristics of these patients.
Keywords: COVID‐19, immune suppression, lung transplant
Solid organ transplant recipients are considered at a high risk for severe COVID‐19 disease due to chronic immunosuppression, though their risk compared with that of the general population remains unclear. Management of lung transplant recipients can be particularly challenging given high levels of maintenance immunosuppression, high incidence of rejection, constant exposure of the graft to the external environment, and diagnostic uncertainty in patients with respiratory symptoms. There are currently limited data on manifestations, management, and outcomes in lung transplant recipients who develop this novel infection. 1
We report our early experience with COVID‐19 infections at a high‐volume lung transplant center in Philadelphia, Pennsylvania. We identified 8 lung transplant recipients diagnosed with COVID‐19 between March 26, 2020, and April 30, 2020. Patient‐specific information can be found in Table S1. Recognizing the limitations of the COVID‐19 nasopharyngeal swab test, patients were considered COVID‐19 positive based on PCR results, or consensus opinion when considering chest CT findings and clinical presentation. Five patients were COVID‐19 PCR positive (62.5%). Seven patients were male (87.5%) with a mean age of 60.8 years. Five patients (62.5%) had undergone bilateral lung transplantation, and three (37.5%) were single left lung recipients. The diagnoses resulting in lung transplant were idiopathic pulmonary fibrosis (five patients, 62.5%), COPD (2 patients, 25%), and cystic fibrosis (1 patient, 12.5%). Five of eight patients (62.5%) were transplanted within the past year. Common comorbidities were diabetes mellitus (35.7%), hypertension (37.5%), hyperlipidemia (37.5%), chronic kidney disease (37.5%), and atrial fibrillation (37.5%). All patients were being maintained on the standard three drug immunosuppression regimen including a calcineurin inhibitor, a nucleotide‐blocking agent, and steroids at the time of diagnosis.
The most common presenting symptoms were dyspnea (75%), cough (75%), subjective fever (50%), and gastrointestinal symptoms (37.5%); only two patients (25%) had a measured fever (> 38.0 C degrees) on the day of diagnosis. Two patients developed COVID pneumonia within 7 and 14 days, respectively, of their lung transplant. We believe these were healthcare‐acquired infections, since multiple providers were diagnosed around that time and donors were retrospectively tested negative. The remaining patients all presented to the emergency department and were admitted for further inpatient care. Three patients (37.5%) were admitted to our COVID‐designated intensive care unit at diagnosis.
All patients underwent a chest CT scan. The predominant findings were bilateral ground glass opacities, present in all patients, and consolidation in five patients (62.5%) Figure 1. In all single lung transplant recipients, both the native lung and the allograft were involved. Lymphopenia (mean TLC 0.56 K/mm3) with associated elevation of inflammatory biomarkers such as CRP (mean 9.66 mg/dL), D‐dimer (mean 1881 ng/mL), and ferritin (mean 471 ng/mL) was noted in all eight patients. Three patients (37.5%) were noted to have superimposed bacterial infections. Patients were stratified into stages from mild to very severe disease 2 , two patients (25%) had very severe disease, three patients (37.5%) had severe disease, and three patients (37.5%) had moderate disease. A summary of pertinent clinical and laboratory data is reported in Table 1.
FIGURE 1.
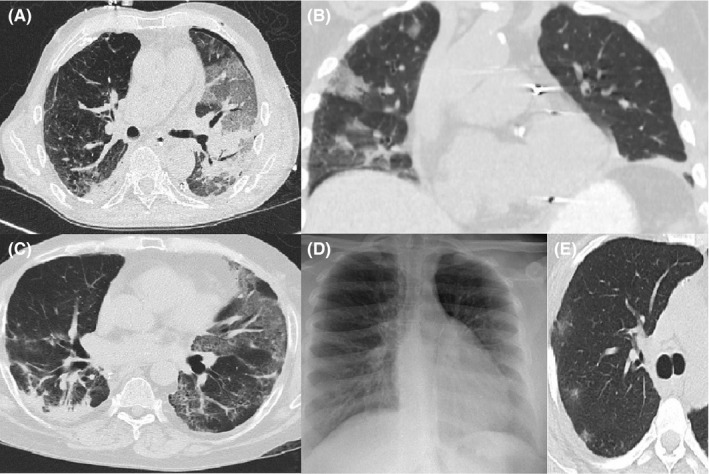
Image A: axial CT image at time of diagnosis of patient 1 (left lung allograft) demonstrating predominant allograft involvement with ground glass, interstitial prominence, and consolidation. Image B: coronal CT image at time of diagnosis of patient 2 (left lung allograft) demonstrating only involvement of native right lung with peripheral ground glass. Image C: axial CT image at time of diagnosis for patient 6 (bilateral lung allograft) demonstrating bilateral involvement with interstitial and GGO pattern in left allograft and posterior consolidation in right allograft. Images D and E: normal chest plain film (D) for patient 4 (bilateral lung allograft) at time of diagnosis but demonstrates subtle peripheral GGO nodules on simultaneous axial CT image (E)
Table 1.
Patient specific data including patient demographics and presenting symptoms, laboratory values, treatments received, and patient outcomes
| Variable | Value (% of total) |
|---|---|
| Demographics | |
| Sex |
Male: 7 (87.5%) Female: 1 (13%) |
| Age years | Median: 59, 69 (Range: 43‐75) |
| Body mass index | Median: 26, 28 (Range: 20.3‐31.6) |
| Prior smoker | 7 (87.5%) |
| Interstitial lung disease/ idiopathic pulmonary fibrosis | 5 (62.5%) |
| Atrial fibrillation | 3 (37.5%) |
| Chronic kidney disease | 3 (37.5%) |
| Diabetes mellitus type 2 | 3 (37.5%) |
| Hyperlipidemia | 3 (37.5%) |
| COPD | 2 (25%) |
| Deep vein thrombosis | 2 (25%) |
| Hypertension | 2 (25%) |
| Congestive heart failure | 1 (12.5%) |
| Coronary artery disease | 1 (12.5%) |
| Cystic fibrosis | 1 (12.5%) |
| Obstructive sleep apnea | 1 (12.5%) |
| Transplant details | |
| Time since transplant |
0‐3 mo: 4 (50%) 3‐12 mo: 1 (12.5%) 12‐24 mo: 2 (25%) >24 mo: 1 (12.5%) |
| Indication for transplant |
IPF: 5 (62.5%) COPD: 2 (25%) CF: 1 (12.5%) |
| Type of transplant |
Double lung: 5 (62.5%) Left lung: 3 (37.5%) |
| Induction agent used |
Basiliximab: 6 (75%) Alemtuzumab: 2 (25%) |
| Baseline immunosuppression |
Calcineurin inhibitor: 8 (100%) Mycophenolate: 5 (62.5%) Azathioprine: 2 (25%) Steroids: 8 (100%) |
| Presenting symptoms/signs | |
| Symptoms |
Cough: 6 (75%) Dyspnea: 6 (75%) Fever: 4 (50%) Fatigue: 3 (37.5%) Nausea/Vomiting/Diarrhea: 3 (37.5%) Chills: 2 (25%) Nasal congestion: 1 (12.5%) Myalgia: 1 (12.5%) |
| Maximum temperature on day of diagnosis—mean (range) | 98.6° Fahrenheit ( 97.8‐100.5 F) |
| S/F ratio at diagnosis—mean (range) | 361 (188‐461) |
| Laboratories | |
| COVID nasopharyngeal PCR positive | 5 (62.5%) |
| Absolute lymphocyte count (1.0 ‐ 4.8 K/mm3)—mean |
Admission: 0.56 Nadir: 0.27 |
| D‐Dimer (0 ‐ 500 ng/mL)—mean |
Admission: 1881 Peak: 2766 |
| C reactive protein (0.0 ‐ 0.4 mg/dL)—mean |
Admission: 9.66 Peak: 9.03 |
| Ferritin (8‐388 ng/mL)—mean |
Admission: 471 Peak: 5881 |
| Lactate dehydrogenase (87‐241 U/L)—mean |
Admission: 287 Peak: 247 |
| Troponin (ng/mL) at time of diagnosis—mean | 0.16 |
| Interleukin‐6 (<5.0 pg/mL)—mean |
Admission: 49.1 Peak: 83.3 |
| Interleukin‐10 (<2.0 pg/mL), on admission—mean | 3.17 |
| Bacterial culture |
K oxytoca: 1 (12.5%) P aeruginosa: 1 (12.5%) Methicillin‐resistant S aureus: 1 (12.5%) |
| Imaging | |
| Chest X‐ray |
Normal: 1 (12.5%) Bilateral alveolar abnormalities: 7 (87.5%) Lobar consolidation: 1 (12.5%) Pleural effusion: 4 (50%) |
| Chest CT—allograft |
GGO: 8 (100%) Consolidation: 5 (62.5%) Interstitial abnormalities: 4 (50%) Pleural effusion: 2 (25%) |
| Chest CT—native lung (no./total no.) |
GGO: 3/3 Consolidation: 1/3 Interstitial abnormalities: 2/3 Pleural effusion: 1/3 |
| Severity staging |
Mild: 0 Moderate: 3 (37.5%) Severe: 3 (37.5%) Very severe: 2 (25%) |
| Treatment | |
| Clinical trials (remdesivir) | 2 (25%) |
| Pulse steroid (> 125 mg methylprednisolone/day) | 6 (75%) |
| Anakinra | 1 (12.5%) |
| Intravenous immunoglobulin | 4 (50) |
| Tocilizumab | 2 (25%) |
| Nucleotide‐blocking agent held | 6 (75%) |
| No change to immunosuppression | 2 (25%) |
| Maximum respiratory support needed | |
| Ambient air | 3 (37.5%) |
| Regular nasal cannula | 1 (12.5%) |
| High‐flow nasal cannula | 2 (25%) |
| Mechanical ventilation | 2 (25%) |
| Outcomes | |
| Hospital admission | 8 (100%) |
| ICU admission | 3 (37.5%) |
| Death | 2 (25%) |
| Other organ dysfunction |
Acute kidney injury: 3 (37.5%) New renal replacement therapy: 0 Circulatory shock: 2 (25%) Liver failure: 1 (12.5%) Acute pancreatitis: 1 (12.5%) |
| New donor‐specific antibodies | 1 (12.5%) |
| Disposition |
Death: 2 (25%) Still hospitalized: 0 (0%) Discharged to rehabilitation facility: 1 (12.5%) Discharged home: 5 (62.5%) |
| Hospital LOS, patients discharged/dead—mean (range) | 8 (2‐16) days |
| Oxygen needs on discharge (no./total no.) |
None: 4/6 Nasal cannula: 2/6 |
Abbreviations: CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; GGO, ground glass opacities; IPF, idiopathic pulmonary fibrosis; LOS, length of stay; S/F, spo2/fio2 ratio.
Without consensus guidelines to define treatment algorithms in this cohort, treatment decisions were made by a multidisciplinary COVID team and our transplant pulmonologists. All patients had nucleotide‐blocking medications held, calcineurin inhibitors were titrated to appropriate therapeutic levels, and steroid dosing was increased. Six patients (75%) received pulse‐dose steroids (defined as at least 3 days of>/=125 mg daily of methylprednisolone). All patients were started on empiric antibiotics selected based on individual risk factors. Two patients (25%) were enrolled in a blinded clinical trial assessing the utility of remdesivir in the treatment of COVID‐19. Cytokine storm was treated with a combination of anakinra (IL‐1 receptor antagonist) and IVIG in one patient (12.5%), and tocilizumab (IL‐6 receptor antagonist) in two patients (25%).
Two patients (25%) required mechanical ventilation, one of whom was prone. Two patients (25%) required high‐flow nasal cannula, one patient with regular nasal cannula (12.5%) and three patients (37.5%) were maintained without supplemental oxygen. With a mean follow‐up of 24 days, the mortality was 25%. Both patients who died were transplanted within 2 weeks prior to diagnosis; each had been successfully extubated post‐transplant and subsequently required mechanical ventilation after COVID‐19 diagnosis. One patient developed progressive sepsis and the other patient necrotizing pancreatitis, leading to death. Of the surviving patients, all six (75%) have been discharged with stable graft function.
Establishing a diagnosis of COVID‐19 pneumonia can be particularly challenging in lung transplant recipients who present with respiratory symptoms. Similar to prior reports, gastrointestinal symptoms appear to be more common in transplant recipients. Fever does not appear to be a reliable sign and was observed in only 25% of our patients. In the appropriate clinical setting, chest CT findings of bilateral ground glass opacities and consolidation are important, especially given the high false‐negative rate of COVID‐19 PCR. In our series, 37.5% of patients had a negative swab PCR, despite a clear clinical diagnosis. No trends in differences in outcomes or need for respiratory support were noted between PCR‐negative and PCR‐positive patients. The involvement of the native lung in single lung transplant patients was helpful to differentiate COVID‐19 from acute rejection.
We observed a short‐term mortality of 25% which is similar to previous reports in solid organ transplant recipients. 3 , 4 Induction therapy in the past year can leave patients unable to mount an adequate T‐cell response, potentially making them vulnerable to infections. 62.5% of our patients developed infection within a year from induction, and both patients who died received basiliximab induction within the prior 2 weeks. However, it is encouraging that most patients (75%) continue to improve or have fully recovered, despite having moderate to severe disease requiring inpatient care. Additionally, recovered patients seem to have a preserved lung function. Long‐term impact of this novel infection on lung transplant recipients remains to be seen.
AUTHOR CONTRIBUTIONS
Catherine N. Myers MD and John Harwood Scott MD reviewed patient charts, compiled data in a central spreadsheet, and drafted the manuscript. Sameep Sehgal MD oversaw and guided the chart review and manuscript preparation. Gerard J. Criner MD, Francis C. Cordova MD, Albert James Mamary MD, Nathaniel Marchetti DO, Kartik V. Shenoy MD, Jonathan A. Galli MD, Patrick D. Mulhall MD, James C. Brown MD, and Norihisa Shigemura MD, PHD, all reviewed the data, discussed the results, and contributed to the final manuscript. The Temple University COVID‐19 Research Group entails the entire clinical staff who cared for the patients described in this manuscript.
Supporting information
Tab S1
Appendix S1
Myers CN, Scott JH, Criner GJ, et al. COVID-19 in lung transplant recipients. Transpl Infect Dis. 2020;22:e13364. 10.1111/tid.13364
Please see Appendix S1 for full personnel listing.
REFERENCES
- 1. Aigner C, Dittmer U, Kamler M, Collaud S, Taube C. COVID‐19 in a lung transplant recipient. J Heart Lung Transplant. 2020;2498(20);31511‐31514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chowdhury JM, Patel M, Zheng M, Abramian O, Criner GJ. Mobilization and preparation of a large urban academic center during the COVID‐19 pandemic. Ann Am Thorac Soc. 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and kidney transplantation. N Engl J Med. 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marcus R, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab S1
Appendix S1


