Abstract
Prolonged viral shedding may pose a threat to the control of coronavirus disease‐2019 (COVID‐19), and data on the duration of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) shedding are still limited, with the associated factors being unknown. All adult patients with laboratory‐confirmed COVID‐19 were included in this retrospective cross‐sectional study in two designated hospitals during 21 January 2020 to 16 March 2020 in Anhui, China. In all patients, data on the duration of SARS‐CoV‐2 RNA shedding were analyzed by reviewing all RNA detection results during hospitalization. In addition, demographic, clinical, treatment, laboratory, and outcome data were also collected from electronic medical records. Factors associated with prolonged viral shedding were analyzed with the Cox proportional hazards model. Among 181 patients, the mean age was 44.3 ± 13.2 years, and 55.2% were male. The median duration of viral shedding from illness onset was 18.0 days (interquartile range [IQR], 15.0‐24.0). Prolonged viral shedding was associated with longer hospital stays (P < .001) and higher medical costs (P < .001). The severity of COVID‐19 had nothing to do with prolonged shedding. Moreover, the median time from onset to antiviral treatment initiation was 5.0 days (IQR, 3.0‐7.0). Delayed antiviral treatment (hazard ratio [HR], 0.976; 95% confidence interval [CI], 0.962‐0.990]) and lopinavir/ritonavir + interferon‐α (IFN‐α) combination therapy as the initial antiviral treatment (HR 1.649; 95% CI, 1.162‐2.339) were independent factors associated with prolonged SARS‐CoV‐2 RNA shedding. SARS‐CoV‐2 showed prolonged viral shedding, causing increased hospital stays and medical costs. Early initiation of lopinavir/ritonavir + IFN‐α combination therapy may help shorten the duration of SARS‐CoV‐2 shedding.
Keywords: associated factors, COVID‐19, outcomes, treatment, viral shedding
Research Highlights
The median duration of viral shedding from illness onset in 181 COVID‐19 patients was 18.0 (15.0‐24.0) days.
Prolonged viral shedding was associated with longer hospital stays and higher medical costs.
The severity of COVID‐19 was not associated with prolonged SARS‐CoV‐2 shedding.
Early initiation of lopinavir/ritonavir + IFN‐α combination therapy may help shorten the duration of SARS‐CoV‐2 shedding.
1. INTRODUCTION
Coronavirus disease‐2019 (COVID‐19) has become a global health emergency, since the first outbreak reported in December 2019 in Wuhan, China. 1 COVID‐19 is caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which is described as highly similar to SARS‐CoV, with 82% nucleotide sequence homology. 2 As of 19 May 2020, 4 735 622 cases of COVID‐19 were recognized in 216 countries/regions worldwide, with nearly 316 289 fatalities. 3
The clinical and epidemiological characteristics of patients with COVID‐19 have been well documented, and from current data, the population seems to be generally susceptible to this virus. 4 , 5 , 6 The fatality rate was ~5.5% in China. Separately, older age, high sequential organ failure assessment scores, and elevated d‐dimer levels were described as risk factors for mortality in patients with COVID‐19. 7 However, although the clinical spectrum appears to be wide, 80% of cases are mild. 8
Patients in the viral replication stage, especially asymptomatic patients, may increase the risk of viral transmission to a greater extent. Prolonged viral shedding has been reported to be associated with fatal or severe patient outcomes, and is critical for controlling diseases caused by viral infection in several studies. 9 , 10 A recent study has shown that SARS‐CoV‐2 in the respiratory tract, especially sputum, is associated with a prolonged viral shedding and high viral load, compared with stool specimens. 11 Zhou et al 7 reported that the median duration of viral shedding in surviving patients with COVID‐19 was 20.0 days. These studies undoubtedly confirm that prolonged SARS‐CoV‐2 shedding poses a huge challenge to the control of COVID‐19, especially for countries or regions that fail to adopt mandatory isolation measures. However, until now, data on the duration of SARS‐CoV‐2 shedding are still limited, with associated factors being unknown.
Here, we conducted a retrospective, multicenter study of 181 hospitalized patients with laboratory‐confirmed SARS‐CoV‐2 infection who were identified during 21 January 2020 to 16 March 2020. We aimed to assess the risk factors for and outcomes of prolonged SARS‐CoV‐2 shedding, focusing on the impact of initial antiviral treatment on viral shedding.
2. MATERIALS AND METHODS
2.1. Study design and data collection
This retrospective study included all adult hospitalized patients (≥18 years old) diagnosed with COVID‐19 during 21 January 2020 to 16 March 2020 from the First Affiliated Hospital of Anhui Medical University and Second People's Hospital of Fuyang (Anhui, China). Patients aged less than 18 years old or with missing virologic data were excluded from this study.
Epidemiological, demographic, clinical, laboratory, treatment, and outcome data were extracted from electronic medical records. All data were checked by two physicians (YZ and YL) and a third researcher (QZ) adjudicated any difference in interpretation between the two primary reviewers. This study was approved by the Ethics Committees of the First Affiliated Hospital of Anhui Medical University and Second People's Hospital of Fuyang.
2.2. Virologic investigations
SARS‐CoV‐2 infection was confirmed in all patients by testing respiratory specimens with real‐time reverse transcription‐polymerase chain reaction (RT‐PCR). SARS‐CoV‐2 RNA was detected by local Centers for Disease Control and Prevention, local health institutions, the First Affiliated Hospital of Anhui Medical University or the Fuyang Second People's Hospital. Respiratory specimens (throat‐swab or sputum specimens) were usually collected every day or every other day for SARS‐CoV‐2 PCR re‐examination after clinical remission of symptoms. Cycle threshold (Ct) values greater than 38 were considered as negative results, and those less than or equal to 35 were considered positive. Respiratory specimens with Ct values greater than 35 and less than or equal to 38 underwent repeat testing, and the results were judged as positive if they were consistent with the former. Positive results of SARS‐CoV‐2 RNA were reported only when RT‐PCR results for both ORF1ab and N were positive. The duration of SARS‐CoV‐2 RNA shedding was defined as the time from illness onset until the test was negative on two occasions, without a positive test afterwards. Notably, for asymptomatic patients, the start date of SARS‐CoV‐2 RNA shedding was defined as the time of the first positive RNA detection.
2.3. Definitions
Based on current data, we conducted a comparative analysis that included two cohorts of patients: those with prolonged shedding (≥21 days) and those with short‐term shedding (<21 days). In addition, the COVID‐19 illness severity was defined according to the Chinese management guideline for COVID‐19, in which patients were divided into four types, namely, mild, general, severe, and critical. 12 In this study, patients with severe or critical status were defined as severe, while those with mild or general status were defined as nonsevere. Separately, initial antiviral therapy was defined as the antiviral agents that were used at the initial stage after onset, so the adjusted therapy according to the development of the disease afterwards was excluded. In addition, antiviral agents and antibiotics used for less than 72 consecutive hours were excluded. The immunoglobin agents used in this study was human immunoglobulin (pH 4) for intravenous injection. The criteria for starting steroids and immunoglobulins in the patients were according to the Chinese management guideline for COVID‐19 (version 7.0). 12 Exposure history was defined as exposure to people with confirmed SARS‐CoV‐2 infection or to the regions in or around the city of Wuhan.
2.4. Statistical analysis
Continuous variables that were in line with normal distribution were presented as the means () ± SD and compared by t test, while those that were not in line with normal distribution were presented as medians (interquartile range [IQR]) and compared by the Mann‐Whitney U test. Categorical variables were described as n (%) and compared by χ 2 or Fisher's exact test. To identify risk factors for prolonged duration of SARS‐CoV‐2 RNA shedding, we conducted a Cox proportional hazards model. Outcome was defined as the time from onset to SARS‐CoV‐2 RNA negativity (ie, duration of SARS‐CoV‐2 RNA shedding). The covariates included in this analysis were age, sex, current smokers, comorbidity, severity of disease, laboratory findings, treatment, and time from onset to antiviral treatment initiation. What needs further explanation is that variables that did not meet the proportional hazards hypothesis were further analyzed using the time‐dependent Cox regression model. Separately, we used Kaplan‐Meier survival analysis and the log‐rank test to make initial comparisons between two groups (ie, severe vs nonsevere patients, patients who started antiviral treatment < 5 days from onset vs those who started antiviral treatment ≥ 5 days from onset, patients with lopinavir/ritonavir monotherapy as the initial antiviral therapy vs those with lopinavir/ritonavir + interferon [IFN]‐α, and patients with lopinavir/ritonavir + IFN‐α as the initial antiviral treatment vs those with lopinavir/ritonavir + IFN‐α + arbidol). Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS), version 16.0 (SPSS Inc, Chicago, IL), and a two‐tailed P < .05 was considered significant for all analyses.
3. RESULTS
3.1. Patients and demographics
From 21 January 2020 to 16 March 2020, a total of 203 hospitalized patients with COVID‐19 were screened from the First Affiliated Hospital of Anhui Medical University and the Second People's Hospital of Fuyang (Anhui, China), of which 15 patients were aged less than 18 years old and seven patients had missing virologic data. A total of 181 adult hospitalized patients with virologic results were included in the final analysis, and all of them had resolution of SARS‐CoV‐2 shedding and had been discharged. The demographics of the patients are presented in Table 1, and the mean age of the 181 patients was 44.3 ± 13.3 years, ranging from 18 years to 82 years, and 55.2% of them were male.
Table 1.
Comparative analysis of demographical, clinical, and laboratory findings between prolonged and short‐term groups of SARS‐CoV‐2 shedding duration
| Variables | Overall | Prolonged group (n = 65) | Short‐term group (n = 116) | P |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 44.3 ± 13.2 | 46.5 ± 14.3 | 43.1 ± 12.5 | .093 |
| Male sex | 100/181 (55.2) | 42/65 (64.6) | 58/116 (50.0) | .058 |
| Exposure history | 152/181 (84.0) | 54/65 (83.1) | 98/116 (84.5) | .805 |
| Current smokers | 19/181 (10.5) | 10/65 (15.4) | 9/116 (7.8) | .108 |
| Comorbidity | ||||
| Baseline disease | 38/181 (21.0) | 17/65 (26.2) | 21/116 (18.1) | .202 |
| Hypertension | 24/181 (13.3) | 8/65 (12.3) | 16/116 (13.8) | .777 |
| Diabetes | 12/181 (6.6) | 5/65 (7.7) | 7/116 (6.0) | .667 |
| Bacterial pneumonia | 48/181 (26.5) | 20/65 (30.8) | 28/116 (24.1) | .332 |
| Symptoms | ||||
| Fever | 150/181 (82.9) | 57/65 (87.7) | 93/116 (80.2) | .238 |
| Cough | 95/181 (52.5) | 34/65 (52.3) | 61/116 (52.6) | .971 |
| Sputum | 29/181 (16.0) | 12/65 (18.5) | 17/116 (14.7) | .503 |
| Fatigue | 21/181 (11.6) | 6/65 (9.2) | 15/116 (12.9) | .456 |
| Severe status of COVID‐19 | 34/181 (18.8) | 15/65 (23.1) | 19/116 (16.4) | .268 |
| Laboratory finding on admission | ||||
| White blood cell count, ×109/L | 5.0 (3.9‐6.4) | 4.8 (3.8‐5.5) | 5.2 (4.1‐6.8) | .099 |
| White blood cell count, <4 × 109/L | 49/181 (27.1) | 22/65 (33.8) | 27/116 (23.3) | .125 |
| Lymphocyte count, ×109/L | 1.2 (0.8‐1.5) | 1.1 (0.7‐1.5) | 1.2 (0.9‐1.5) | .243 |
| Lymphocyte count, <0.8 × 109/L | 41/181 (22.7) | 19/65 (29.2) | 22/116 (19.0) | .133 |
| Hemoglobin, g/L | 137.8 ± 13.5 | 138.5 ± 18.4 | 137.3 ± 15.5 | .646 |
| Platelet count, ×109/L | 187.0 ± 65.8 | 175.9 ± 63.6 | 193.2 ± 66.4 | .089 |
| Albumin, g/L (180)a | 41.1 ± 4.2 | 40.6 ± 4.2 | 41.4 ± 4.2 | .232 |
| ALT, U/L (180)a | 23.0 (14.3‐36.8) | 23.0 (14.5‐39.0) | 23.0 (14.0‐34.0) | .281 |
| Creatinine, μmol/L (180)a | 66.3 ± 15.3 | 67.7 ± 14.3 | 65.5 ± 15.9 | .373 |
| Lactate dehydrogenase, U/L (179)a | 232.0 (195.0‐295.0) | 234.0 (199.5‐311.5) | 226.0 (193.8‐291.5) | .493 |
| Creatine kinase, U/L (165)a | 65.0 (43.5‐93.0) | 61.0 (44.0‐96.0) | 66.0 (43.0‐91.5) | .769 |
| C‐reactive protein, pg/mL, (177)a | 9.9 (2.8‐30.0) | 9.0 (2.6‐33.1) | 9.0 (4.0‐27.5) | .865 |
| D‐dimer, μg/mL (173)a | 0.3 (0.2‐0.6) | 0.3 (0.2‐0.7) | 0.3 (0.2‐0.5) | .289 |
| IL‐6, pg/mL (179)a | 12.5 (5.0‐29.7) | 17.5 (5.9‐43.2) | 9.0 (4.0‐27.5) | .033 |
| Procalcitonin, ≥0.1 ng/mL | 21/181 (11.6) | 8/65 (12.3) | 13/116 (11.2) | .824 |
| CD3+ CD4+ T cell count/μL (162)a | 467.5 ± 216.2 | 468.2 ± 244.1 | 467.0 ± 199.3 | .974 |
| CD3+CD8+ T cell count/μL (162)a | 294.5 (194.5‐410.8) | 271.5 (167.5‐409.0) | 306.0 (223.5‐414.5) | .364 |
| CD4+/CD8+ ratio (162)a | 1.6 ± 0.7 | 1.7 ± 0.7 | 1.6 ± 0.7 | .250 |
Note: [ ± SD/n(%)/M(IQR)].
Abbreviations: ALT, alanine aminotransferase; COVID‐19, coronavirus disease‐2019; IL‐6, interleukin‐6; IQR, interquartile range; M, median; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
The number of patients who conducted this laboratory examination on admission.
3.2. Duration of SARS‐CoV‐2 RNA shedding
The median duration of SARS‐CoV‐2 shedding was 18.0 days (IQR, 15.0‐24.0), ranging from 5 days to 39 days. Only two (1.1%) patients had undetectable SARS‐CoV‐2 RNA within 6 days after symptom onset, 12 (6.6%) tested negative 7 to 10 days after onset, 102 (56.4%) tested negative 11 to 20 days after onset, 35 (19.3%) tested negative 21 to 27 days after onset, and 30 (16.6%) tested positive more than 27 days after onset. A total of 116 (64.1%) patients tested negative within 20 days of illness onset, and they were included in the short‐term viral shedding group. Notably, the median duration of SARS‐CoV‐2 shedding from the first positive RNA detection in seven asymptomatic patients was 9.0 days, ranging from 7.0 to 16.0 days. In addition, all of them had been in close contact with patients with confirmed COVID‐19, and three of them had exposure to family clusters. However, it is worth mentioning that five of them experienced brief nonspecific symptoms such as mild cough after admission.
3.3. Clinical characteristics and laboratory findings
A comparative study of clinical characteristics and laboratory findings between the two groups is also shown in Table 1. A total of 152 (84.0%) patients had an exposure history, and 19 (10.5%) patients were current smokers. The differences in demographic and epidemiologic characteristics between the two groups were not statistically significant. Thirty‐eight (21.0%) patients had a baseline disease, with hypertension (24/181, 13.3%) and diabetes (12/181, 6.6%) being more common, and 48 (26.5%) patients were diagnosed with bacterial pneumonia. In terms of symptoms, fever (150/181, 82.9%) and cough (95/181, 52.5%) were the most common in this study. A total of 34 (18.8%) patients had severe COVID‐19 status. The differences in comorbidities, symptoms, and severity were also not statistically significant. Leukopenia occurred in 49 (27.1%) patients, while lymphocytopenia occurred in 41 (22.7%) patients. The median levels of lactate dehydrogenase, creatine kinase, C‐reactive protein, and D‐dimer were 232.0 U/L (IQR, 195.0‐295.0), 65.0 U/L (IQR, 43.5‐93.0), 9.9 pg/mL (IQR, 2.8‐30.0), and 0.3 μg/mL (IQR 0.2‐0.6), respectively. Separately, we also describe the results of the T lymphocyte subset in Table 1, in which the CD4/CD8 ratio of patients with COVID‐19 was 1.6 ± 0.7. The differences in laboratory findings between the two groups were not statistically significant, except for interleukin‐6 (IL‐6) (P = .033). The median IL‐6 level of 17.5 pg/mL (IQR, 5.9‐43.2) in the prolonged group was higher than the median level of 9.0 pg/mL (IQR, 4.0‐27.5) in the short‐term group.
3.4. Treatment and outcomes
The main characteristics of the treatment and outcomes of patients and the differences between the two groups are described in Table 2. All patients received antiviral treatment in this study, and lopinavir/ritonavir monotherapy (59.1%), lopinavir/ritonavir + IFN‐α (23.8%) and lopinavir/ritonavir + IFN‐α + arbidol (10.5%) were most commonly used as the initial antiviral treatments. Moreover, lopinavir/ritonavir + IFN‐α as the initial antiviral treatment (P = .007) and earlier antiviral treatment initiation from onset (P = .006) seemed to be associated with short‐term shedding duration. Forty‐six (25.4%) patients received systemic corticosteroids, while 22 (12.2%) patients received intravenous immunoglobin during their hospital stay. Notably, 99 (54.7%) patients received antibiotics, and patients in the prolonged group were more likely to have been exposed to antibiotics (P = .045). Notably, traditional Chinese medicine (P = .009) and chloroquine (P = .001) seem to be more likely to be used in patients with prolonged shedding durations, especially when the initial antiviral drug has been used for more than 10 consecutive days. The median time from illness onset to discharge was 23.0 days (IQR, 19.0‐28.5). In terms of patient outcomes, the median length of hospital stay was 17.0 days (IQR, 14.0‐21.0) and the median medical cost was 3027.0 dollars (IQR, 2286.0‐4730.5). A prolonged shedding duration is associated with an increased length of hospital stay (P < .001) and medical costs (P < .001).
Table 2.
Comparative analysis of treatment and outcomes between prolonged and short‐term groups of SARS‐CoV‐2 RNA shedding duration
| Variables | Overall | Prolonged group (n = 65) | Short‐term group (n = 116) | P |
|---|---|---|---|---|
| Initial antiviral therapy | ||||
| Lopinavir/ritonavir monotherapy | 107/181 (59.1) | 42/65 (64.6) | 65/116 (56.0) | .260 |
| Lopinavir/ritonavir + IFN‐α | 43/181 (23.8) | 8/65 (12.3) | 35/116 (30.2) | .007 |
| Lopinavir/ritonavir + IFN‐α + arbidol | 19/181 (10.5) | 10/65 (15.4) | 9/116 (7.8) | .108 |
| Others | 11/181 (6.1) | 5/65 (7.7) | 6/116 (5.2) | .512 |
| Time from illness onset to antiviral treatment initiation, d | 5.0 (3.0‐7.0) | 6.0 (3.0‐10.0) | 4.0 (3.0‐7.0) | .006 |
| Treatment in hospital stay | ||||
| Corticosteroid | 46/181 (25.4) | 20/65 (30.8) | 26/116 (22.4) | .215 |
| Antibiotics | 99/181 (54.7) | 42/65 (64.6) | 57/116 (49.1) | .045 |
| Traditional Chinese medicine | 139/181 (76.8) | 57/65 (87.7) | 82/116 (70.7) | .009 |
| Intravenous immunoglobin | 22/181 (12.2) | 12/65 (18.5) | 10/116 (8.7) | .052 |
| Lopinavir/ritonavir | 172/181 (95.0) | 61/65 (93.8) | 111/116 (95.7) | .584 |
| IFN‐α | 106/181 (58.6) | 44/65 (67.7) | 62/116 (53.4) | .062 |
| Arbidol | 53/181 (29.3) | 20/65 (30.8) | 33/116 (28.4) | .742 |
| Chloroquine | 29/181 (16.0) | 18/65 (27.7) | 11/116 (9.5) | .001 |
| Ribavirin | 9/181 (5.0) | 6/65 (9.2) | 3/116 (2.6) | .049 |
| Outcomes | ||||
| Time from illness onset to discharge | 23.0 (19.0‐28.5) | 31.0 (27.5‐35.0) | 20.0 (18.0‐23.0) | <.001 |
| Hospital length of stay, d | 17.0 (14.0‐21.0) | 26.0 (19.5‐30.0) | 15.0 (12.0‐17.0) | <.001 |
| Hospitalization costs, dollars | 3027.0 (2286.0‐4730.5) | 4262.0 (3280.0‐6200.5) | 2645.0 (2082.3‐3308.8) | <.001 |
Note: [n(%)/M(IQR)].
Abbreviations: IFN, interferon; IQR, interquartile range; M, median; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
3.5. Factors associated with prolonged viral shedding
In this study, 34 (18.8%) patients were included in the severe group, and the results of the log‐rank test suggests that the severity of COVID‐19 seems to have nothing to do with a prolonged duration of SARS‐CoV‐2 shedding (P = .575; Figure 1A). Moreover, the median time from illness onset to initial antiviral treatment was 5.0 days (IQR, 3.0‐7.0), and RNA clearance was significantly faster in patients who started antiviral treatment less tha 5 days after illness onset than in patients who started antiviral treatment more than or equal to 5 days after illness onset (P = .001; Figure 1B). However, lopinavir/ritonavir + IFN‐α as the initial antiviral treatment may help shorten the shedding duration compared with lopinavir/ritonavir monotherapy (P = .012; Figure 1C) and lopinavir/ritonavir + IFN‐α + arbidol (P = .002; Figure 1D). In a multivariable Cox proportional hazards model, the time from illness onset to antiviral treatment (hazard ratio [HR], 0.976; 95% confidence interval [CI], 0.962‐0.990) and lopinavir/ritonavir + IFN‐α as the initial antiviral therapy (HR 1.649; 95% CI, 1.162‐2.339) were ultimately identified as independent factors associated with the duration of SARS‐CoV‐2 RNA shedding (Table 3).
Figure 1.
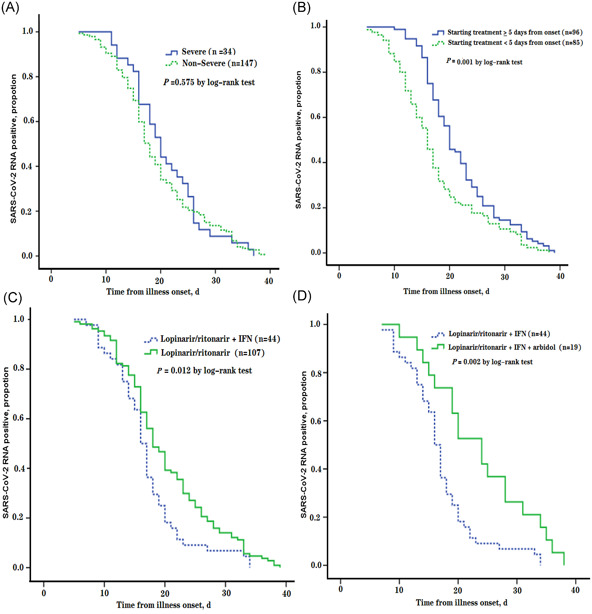
A, Cumulative proportion of patients who had severe status of COVID‐19 vs nonsevere ones, by day after illness onset. B, Cumulative proportion of patients who started antiviral treatment less than 5 days vs more than or equal to 5 days, by day after illness onset. C, Cumulative proportion of patients who received lopinavir/ritonavir +interferon (IFN) combination therapy as the initial antiviral treatment vs lopinavir/ritonavir monotherapy, by day after illness onset. D, Cumulative proportion of patients who received lopinavir/ritonavir + IFN combination therapy as the initial antiviral treatment vs lopinavir/ritonavir + IFN + arbidol combination therapy, by day after illness onset. COVID‐19, coronavirus disease‐2019
Table 3.
Multivariable analysis of factors associated with duration of SARS‐CoV‐2 RNA shedding in 181 patients during 21 Jan 2020 to 16 Mar 2020 from Anhui, China
| Variable | Unadjusted HR (95% CI) | P | Adjusted HR (95% CI) | P |
|---|---|---|---|---|
| Age | 0.995 (0.984‐1.006) | .347 | 0.999 (0.988‐1.011) | .896 |
| Male sex | 0.831 (0.618‐1.118) | .222 | 0.759 (0.559‐1.031) | .078 |
| Current smokers | 0.930 (0.577‐1.499) | .767 | … | … |
| Hypertension | 0.984 (0.639‐1.516) | .943 | … | … |
| Diabetes | 0.854 (0.475‐1.535) | .598 | … | … |
| Bacterial pneumonia | 0.929 (0.667‐1.293) | .663 | … | … |
| Severe status of COVID‐19 | 0.903 (0.621‐1.314) | .595 | … | … |
| White blood cell count, ×109/L | 1.026 (0.961‐1.096) | .440 | … | … |
| IL‐6, pg/mL | 1.000 (0.998‐1.001) | .578 | … | … |
| Corticosteroids | 0.829 (0.593‐1.159) | .272 | … | … |
| Antibiotics | 0.802 (0.597‐1.076) | .141 | 0.761 (0.561‐1.032) | .079 |
| Lopinavir/ritonavir monotherapy | 0.868 (0.645‐1.170) | .353 | … | … |
| Lopinavir/ritonavir + IFN‐α combination therapy | 1.645 (1.163‐2.327) | .005 | 1.649 (1.162‐2.339) | .005 |
| Lopinavir/ritonavir + IFN‐α + arbidol combination therapy | 0.636 (0.394‐1.027) | .064 | 0.648 (0.380‐1.105) | .111 |
| Time from illness onset to antiviral treatment in da | 0.982 (0.968‐0.995) | .008 | 0.976 (0.962‐0.990) | .001 |
Note: Variables with HR < 1 increase the duration of SARS‐CoV‐2 shedding. HRs in multivariable analysis were adjusted for age and sex.
Abbreviations: CI, confidence interval; COVID‐19, coronavirus disease‐2019; HR, hazard ratio; IFN, interferon; IL‐6, interleukin‐6; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
By use of the time‐dependent Cox regression model.
4. DISCUSSION
Since December 2019, COVID‐19 has rapidly spread in China and many countries around the world. As with SARS‐CoV and Middle East respiratory syndrome‐coronavirus (MERS‐CoV), SARS‐CoV‐2 is a member of the coronavirus family, being a β‐coronavirus. The continued shedding of this virus in infected patients (especially asymptomatic patients) will undoubtedly increase the risk of transmission and viral clearance is one of the criteria for isolation release and discharge in China. However, in light of current data, there is limited knowledge about the duration of SARS‐CoV‐2 shedding, and the associated factors remain unknown. This retrospective study aimed to identify factors associated with a prolonged duration of SARS‐CoV‐2 shedding, focusing on the impact of initial antiviral treatment. Delayed antiviral treatment and lopinavir/ritonavir + IFN‐α combination therapy as the initial antiviral treatment were ultimately identified by the Cox proportional hazards model as independent associated factors in this study.
Virologic detection can help us understand viral shedding, especially molecular biology detection. Multiple previous studies reported data on viral shedding by qualitative or quantitative RNA detection using RT‐PCR. 9 , 10 , 11 Thus, the duration of SARS‐CoV‐2 RNA shedding was described in this study by reviewing the results of RNA detection. The median duration of SARS‐CoV‐2 RNA shedding in the respiratory tract was 18.0 days, which is consistent with the result of a median of 20.0 days in an analysis of COVID‐19 by Zhou et al. 7 We must admit the bias of the results in this retrospective study, especially for the results of patients whose RNA detection is positive again after two negative tests. Regardless, these results may provide theoretical value for the length of patient isolation and antiviral treatment. Similarly, MERS‐CoV secretion from lower respiratory specimens can be sustained for up to 18 to 27 days after illness onset. 13 Thus, these results indicated that SARS‐CoV‐2 had a similar prolonged duration of viral shedding to MERS‐CoV. However, the viral load profile of SARS‐CoV‐2 which peaked 4 to 7 days after illness onset is similar to that of influenza, 11 , 14 but contrasts with that of SARS‐CoV at ~10 days and that of MERS‐CoV at the second week after symptom onset. 15 , 16 Thus, sufficient attention should be paid to the risk of community transmission in the early stages of illness.
This study also confirmed that a prolonged duration of SARS‐CoV‐2 RNA shedding was associated with increased hospital stays and medical costs. In addition, SARS‐CoV‐2 RNA shedding was also detected in seven asymptomatic patients, all of whom showed a shorter duration of viral shedding. Since the start date for shedding duration in asymptomatic patients is difficult to determine, their shedding duration results may be underestimated. In fact, patients with asymptomatic infection of SARS‐CoV‐2 have also been confirmed in multiple studies. 17 , 18 Theoretically, asymptomatic carriers might arise when host antiviral defenses are either strong or decoupled. 19 Viral shedding in the respiratory tract in asymptomatic patients will be a huge challenge for controlling the transmission of COVID‐19, so the pattern of SARS‐CoV‐2 shedding in asymptomatic patients will be the focus of further study. In summary, for countries that could adopt compulsory isolation measures such as China, increased hospital stays and medical costs may place a greater burden on limited medical resources, while for countries that could not, the continued shedding of SARS‐CoV‐2 may increase the risk of community transmissions. Therefore, further research is needed to identify factors associated with prolonged shedding duration.
The severity of COVID‐19 seems to have no correlation with shedding duration, which was not in line with our expectations. On the one hand, in this study, no fatal cases and more than two negative RNA detections from all patients may have helped minimize the bias of results of the shedding duration results. On the other hand, the limitation of no fatal cases, two categories of severity (severe and nonsevere) and the sample size may help explain the bias of the interpretation of severity. Limited data imply that the duration of SARS‐CoV‐2 shedding in nonsevere patients was not significantly different from that in severe patients. 7 These results may provide a certain theoretical basis for clinicians. However, a meta‐analysis showed that the mean duration of A(H1N1)pdm09 shedding generally increased with the severity of the clinical presentation. 20 Differences in the methods and patients of studies may help explain these conflicting results. In this study, early laboratory findings do not seem to help identify patients with prolonged SARS‐CoV‐2 shedding. A retrospective study on COVID‐19 implied that the RNA clearance ratio was correlated with a decline in creatine kinase and lactate dehydrogenase levels. 21 These results may imply that the creatine kinase and lactate dehydrogenase levels of patients at admission do not help to identify patients as high risk of prolonged SARS‐CoV‐2 shedding duration, although the decline of these levels may help RNA clearance.
Delayed initiation of antiviral treatment was an independent risk factor associated with prolonged SARS‐CoV‐2 shedding. Similarly, in studies of the influenza, early oseltamivir treatment was reported to be significantly associated with survival benefit and shorter virus shedding duration, compared with later treatment. 10 , 22 , 23 The median time from onset to antiviral treatment was 5.0 days among the 181 patients in our study. However, more than half of the patients received antiviral therapy more than 5 days after the onset, and their viral shedding duration was obviously longer. Most patients initiated antiviral treatment at the time of laboratory confirmation, and earlier administration of optimal treatment might help reduce viral shedding, the hospital stay length, and medical costs.
Due to the lack of undeveloped vaccines, unknown effective antiviral agents, and high risk of transmission, optimal treatment strategies for COVID‐19 with available antiviral agents urgently need to be investigated. In this study, lopinavir/ritonavir, IFN‐α, and arbidol were the most commonly used initial treatment options for patients with COVID‐19, while chloroquine and traditional Chinese medicine were more likely used for patients in the prolonged shedding group. Statistical analysis can only emphasize association and cannot explain causality. By reviewing the patient data, we believe that this phenomenon may be the result of the empirical option that clinicians made, so chloroquine and traditional Chinese medicine are not suitable as factors for prolonged shedding duration. Thus, to minimize bias as much as possible, in this study, we only included antiviral agents, which were used as the initial antiviral treatments, as factors for prolonged shedding duration. Compared with lopinavir/ritonavir monotherapy and lopinavir/ritonavir + IFN‐α + arbidol, lopinavir/ritonavir + IFN‐α combination therapy was significantly helpful to shorten the duration of SARS‐CoV‐2 RNA shedding and was eventually identified as an independent protective factor for prolonged viral shedding. Lopinavir/ritonavir as the treatment for patients with COVID‐19 has been widely reported. 7 , 21 A study conducted in Korea pointed out that the viral load of SARS‐CoV‐2 was significantly reduced after lopinavir/ritonavir administration, 24 but there are also studies indicating that lopinavir/ritonavir treatment does not help improve virus clearance and mortality. 25 Thus, this result may imply that lopinavir/ritonavir and interferon combination therapy could help shorten the shedding duration, while lopinavir/ritonavir monotherapy may have no association with virus clearance. In summary, this conclusion may provide a rationale for clinicians to make optimal antiviral treatment decisions. It is worth noting that corticosteroid therapy was not associated with a prolonged SARS‐CoV‐2 shedding duration in this study, although it was reported to be associated with a prolonged duration of viral shedding in two studies on critically ill patients with MERS‐CoV and transplant recipients with human coronavirus. 26 , 27 Differences in the underlying conditions of patients may help explain this phenomenon.
There are some limitations in this study. First and foremost is the design of a retrospective study, so the estimated duration of SARS‐CoV‐2 RNA shedding is limited by the frequency of respiratory specimens collected and the lack of quantitative data on viral loads. Second, not all laboratory tests, such as creatine kinase and T lymphocyte subset, were performed in all patients, so their roles in the duration of SARS‐CoV‐2 RNA shedding might be underestimated. Third, the interpretation of this study is also limited by the sample size. However, by including all adult patients from the two larger designated hospitals in this province, we think patients in this study are representative of cases diagnosed with COVID‐19 in Anhui, China.
In conclusion, to the best of our knowledge, this is the first study on the risk factors for prolonged SARS‐CoV‐2 shedding. A significant finding of this study is that delayed antiviral treatment and lopinavir/ritonavir + IFN‐α combination therapy as the initial antiviral treatment are independent factors associated with a prolonged duration of SARS‐CoV‐2 RNA shedding. A prolonged duration of SARS‐CoV‐2 shedding is associated with increased hospital stays and medical costs. Notably, RNA detection of SARS‐CoV‐2 in seven asymptomatic patients was also described, and the viral shedding duration of these patients was lower than that of symptomatic patients. Therefore, the pattern of SARS‐CoV‐2 shedding in asymptomatic patients should be the focus of future studies.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
YZ designed this investigation. YZ and YL collected the original data. QZ adjudicated any difference in interpretation of data. YZ, QZ, and KZ contributed to the statistical analysis of data. YZ and ZW contributed to the writing of the paper. ZW and YX contributed to the modification of the paper.
ACKNOWLEDGMENT
There is no foundation for this study.
Zuo Y, Liu Y, Zhong Q, Zhang K, Xu Y, Wang Z. Lopinavir/ritonavir and interferon combination therapy may help shorten the duration of viral shedding in patients with COVID‐19: A retrospective study in two designated hospitals in Anhui, China. J Med Virol. 2020;92:2666–2674. 10.1002/jmv.26127
Yan Zuo and Yunlei Liu share co‐first authorship.
REFERENCES
- 1. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92(4):401‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JF, Kok KH, Zhu Z, et al. Genomic characterization of the 2019 novel human‐pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Coronavirus disease 2019 (COVID‐19) situation report. May 20, 2020.
- 4. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao Y, Liu X, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS‐CoV‐2: a systematic review and meta‐analysis [published online ahead of print April 03, 2020]. J Med Virol. 10.1002/jmv.25822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou F, Yu T, Du R, et al. Clinical courses and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus disease 2019 (COVID‐19) and cardiovascular disease. Circulation. 2020;141(20):1648‐1655. [DOI] [PubMed] [Google Scholar]
- 9. Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Guo Q, Yan Z, et al. Factors associated with prolonged viral shedding in patients with avian influenza A(H7N9) virus infection. J Infect Dis. 2018;217(11):1708‐1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐19 [published online ahead of print April 01, 2020]. Nature. 10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- 12. National Health Commission of the People's Public of China . Chinese management guideline for COVID‐19 (version 7.0). March 4, 2020.
- 13. Min CK, Cheon S, Ha NY, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. 2016;6:25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng PK, Wong DA, Tong LK, et al. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363(9422):1699‐1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oh MD, Park WB, Choe PG, et al. Viral load kinetics of MERS coronavirus infection. N Engl J Med. 2016;375(13):1303‐1305. [DOI] [PubMed] [Google Scholar]
- 17. Lu S, Lin J, Zhang Z, et al. Alert for non‐respiratory symptoms of coronavirus disease 2019 (COVID‐19) patients in epidemic period: a case report of familiar cluster with three asymptomatic COVID‐19 patients [published online ahead of print March 19, 2020]. J Med Virol. 10.1002/jmv.25776 [DOI] [PubMed] [Google Scholar]
- 18. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID‐19 screened among close contacts in Nanjing, China. Sci China: Life Sci. 2020;63(5):706‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fung SY, Yuen KS, Ye ZW, Chan CP, Jin DY. A tug of war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect. 2020;9(1):558‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fielding JE, Kelly HA, Mercer GN, Glass K. Systematic review of influenza A(H1N1)pdm09 virus shedding: duration is affected by severity, but not age. Influenza Other Respir Viruses. 2014;8(2):142‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yuan J, Zou R, Zeng L, et al. The correlation between viral clearance and biochemical outcomes of 94 COVID‐19 infected discharged patients. Inflamm Res. 2020;69(6):599‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chan PK, Lee N, Zaman M, et al. Determinants of antiviral effectiveness in influenza virus A subtype H5N1. J Infect Dis. 2012;206(9):1359‐1366. [DOI] [PubMed] [Google Scholar]
- 23. Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A (H7N9) virus. Clin Infect Dis. 2018;66(7):1054‐1060. [DOI] [PubMed] [Google Scholar]
- 24. Cao B, Wang Y, Wen D, et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe COVID‐19. N Engl J Med. 2020;382(19):1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lim J, Jeon S, Shin HY, et al. Case of the index patient who caused tertiary transmission of COVID‐19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID‐19 infected pnuemonia monitored by quantitative RT‐PCR. J Korean Med Sci. 2020;35(6):e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arabi YM, Mandourah Y, Al‐Hameed F, et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757‐767. [DOI] [PubMed] [Google Scholar]
- 27. Ogimi C, Greninger AL, Waghmare AA, et al. Prolonged shedding of human coronavirus in hematopoietic cell transplant recipients: risk factors and viral genome evolution. J Infect Dis. 2017;216(2):203‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]


