Abstract
Hypercoagulability has been recognized as a common complication of COVID‐19. Exact mechanisms for this extreme coagulation activation have not yet been elucidated. However, one of the consistent laboratory finding is the increase in fibrinogen, in some cases, marked elevation. High circulating levels of fibrinogen have been linked to thrombosis for years and for this reason, hyperfibrinogenemia is considered one of the mechanisms for COVID‐19 coagulopathy. In this forum article, instead of the prothrombotic role, a protective function for fibrinogen is discussed. Fibrinogen, like the other well‐known acute phase reactants, is increased in COVID‐19 possibly to protect the host.
Keywords: COVID‐19, D‐dimers, fibrinogen, platelets, thrombosis
1. INTRODUCTION
COVID‐19 continues to cause significant mortality around the world. One of the key pathogenic features observed in COVID‐19 is the intense inflammation induced by the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) with the development of a cytokine storm in the most severe cases. From the coagulation perspective, colleagues from different parts of the world have noted several‐fold increase in fibrinogen levels in many patients who require critical care support.1., 2., 3. Why might the fibrinogen levels increase so much?
Fibrinogen is a glycoprotein produced in the body's major synthetic machinery, the liver, which is also an overlooked anti‐infective organ. This is clearly evidenced in sepsis being one of the most common causes of death in patients with liver cirrhosis.4 The liver, in its anti‐infective role, releases several acute phase reactants including fibrinogen, ferritin, C‐reactive protein (CRP), and a plethora of cytokines. Several reports have confirmed marked elevation of these acute phase proteins in patients requiring hospitalization for COVID‐19, suggesting the liver function is in overdrive.2., 3., 5. These different molecules including ferritin, CRP, and fibrinogen play key roles in the body's defense against invading pathogens.
2. THE PROTECTIVE ACUTE PHASE REACTANTS
Ferritin and the regulatory molecule hepcidin are crucial in the iron‐infection axis.6 Every micro‐organism needs iron for their survival. In infectious states, iron is sequestered from the circulation inside ferritin; a process regulated by hepcidin, thus limiting the supply to the pathogens.6 Clinically, this important role of ferritin was observed in negative trials in which routine iron supplementation for preschool children in a malaria‐endemic population resulted in increased risk of severe illness and death.7., 8. Another acute phase reactant, CRP, also plays a protective role in pro‐inflammatory states with high likelihood of adult respiratory distress syndrome (ARDS). In patients with trauma, CRP was shown to defend the human body from histone‐induced endothelial cell damage.9 Mouse experiments by this group showed CRP forms a complex with histones and protects the host from endothelial leakage/damage which would have resulted in lung edema, and pulmonary thrombosis (both commonly noted in COVID‐19).9 A third member of this beneficial group of proteins, haptoglobin, works as an antioxidant, binds circulating extracellular hemoglobin in inflammatory states, and can stimulate the monocyte/macrophage system.10
3. THE PROTECTIVE ACUTE PHASE FIBRINOGEN
Fibrinogen's role among these protective warriors is likely to be two‐fold—regulating the antimicrobial function of the immune cells and clot formation, thus limiting spread of the pathogen. Flick et al showed fibrinogen to be a physiologically relevant ligand for the leukocyte integrin Mac‐1 and thus important in regulating the inflammatory response independent of the clotting function.11 In relation to viral infections, Mac‐1 is a surface receptor for extracellular double stranded RNA (SARS Co‐V 2 is an RNA virus).12 It is possible that high levels of fibrinogen saturate Mac‐1 and therefore reduce the harmful effects from the virus.13., 14. As an acute phase protein, increased concentrations of soluble fibrinogen antagonize leukocyte recruitment, and can contribute to inflammation resolution.15 The several host defense roles of fibrinogen are summarized in a review in which two main mechanisms are detailed, assisting host protective immune function and forming fibrin matrices that serve as a protective barrier.16
4. THE PROTECTIVE FIBRINOGEN AND THROMBOSIS
Thrombus formation limiting the spread of the invading pathogen has long been known to be a host defense mechanism. Different components of the coagulation pathway are highly relevant in the anti‐infective process. These include the central figure, thrombin; anticoagulant proteins like protein C and thrombomodulin; the contact pathway constituents like factor XII; and of, course, fibrinogen.17 The Flick lab attempted to distinguish between fibrinogen‐ and fibrin‐dependent antimicrobial function in vivo by creating FibAEK mice, which lacked the capacity for fibrin polymer formation.18 These animal models retained the functional capacity for platelet interactions but were unable to form polymers and clear intraperitoneal Staphylococcus aureus inoculum suggesting thrombus formation is also important in the antimicrobial process.18 The localized thrombus formation in the lungs is most likely an attempt by the coagulation system to limit the spread of the SARS Co‐V 2. Recent autopsy reports of patients with COVID‐19 demonstrated localized micro‐thrombi in the lung specimens.19
5. HYPERFIBRINOGENEMIA AND THROMBOTIC RISK
Elevated plasma fibrinogen in inflammatory states like trauma and pregnancy has been linked to an increased thrombotic risk, but without evidence for definite causation. In these clinical scenarios, increased coagulation activation including higher levels of fibrinogen is necessary for various physiological reasons, but primarily to limit ongoing (trauma) or impending (pregnancy) hemorrhage. However, despite the high levels of fibrinogen, all pregnant females do not develop thrombosis suggesting the hyperfibrinogenemia is unlikely to be prothrombotic. Similarly, not all trauma or postoperative cases are associated with thrombosis. Indeed, fibrinogen has never been shown to play a direct, causative role in causing thrombosis in these conditions.20 The Wolberg lab conducted animal experiments to demonstrate a link between high levels of fibrinogen and thrombosis and observed that hyperfibrinogenemia did not cause spontaneous thrombosis in vivo.20 They also quoted past observations in which injecting human fibrinogen into mice did not cause spontaneous fibrin deposition. The conclusion from the study was that multiple hits were required to cause thrombosis in addition to elevated fibrinogen levels.20 Certainly, in trauma patients, there can be several hits that can contribute to thrombosis, while in pregnant females, additional complications like pre‐eclampsia or hyperemesis‐induced dehydration could act as additional hits to trigger thrombosis.
6. PLATELET AND CIRCULATING FIBRINOGEN
If fibrinogen behaves as a protective acute phase reactant and as a prothrombotic molecule, could it be coming from two different sites to serve the two different roles? The “two‐layer thrombus” model demonstrated by Stalker et al may provide clues to this duality.21 Their experiments demonstrated a hierarchical organization for thrombus with an inner core of tight thrombus from activated platelets and an outer loose, plasma‐permeable shell, less dependent on activated platelets.21 Interestingly, in the inner core, the activated platelets are packed together by the integrin αIIbβ3, which is the platelet receptor for fibrinogen. The fibrinogen for the inner core of thrombus comes from the platelet α–granules, which also store several coagulation proteins (factor V, XI, and XIII) involved in secondary hemostasis.22 At the same time, the circulating fibrinogen is possibly executing its efforts as an acute phase protein. Although this dual role of fibrinogen has not been examined in detail, that of platelet and circulating von Willebrand factor (VWF), another acute phase reactant, has been studied. Platelet α‐granules contain 20% of the total VWF protein and are the high molecular weight multimeric forms.22., 23. Using pig bone marrow transplantation model, it was shown that platelet VWF markedly ameliorates bleeding in severe von Willebrand disease and in its presence, much lower levels of plasma VWF were required for hemostasis.24 This suggests that the platelet VWF is the predominant form involved in thrombus formation; while the circulating VWF similar to fibrinogen may be more of a participant in the host defense function in inflammatory conditions.
7. PUTTING EVERYTHING TOGETHER AND RELEVANCE TO COVID‐19
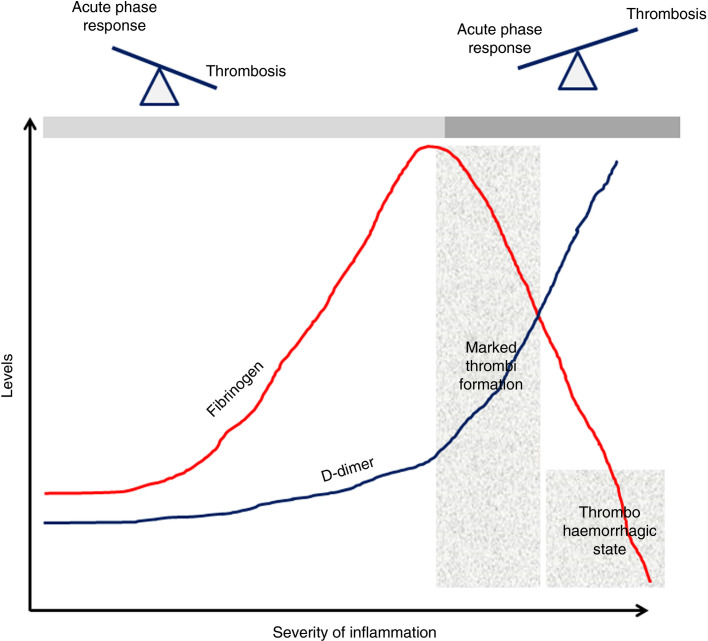
How can all of the above information be put together? In a patient with COVID‐19, the fibrinogen (and VWF) level increases as part of the host defense. In the initial stages, the main role is to regulate the body's intense inflammatory response and the exceedingly high fibrinogen level may not be a disadvantage. At this stage, fibrinogen's acute phase function is predominant to its role in thrombi formation, which is occurring at a low‐grade level (reflected by the mild increase in D‐dimers). This scenario is similar to many other clinical situations in which an acute phase response is seen, such as pregnancy, trauma, and postoperative states, in which the rise in fibrinogen is physiological and an increase in D‐dimers is noted without clinical evidence of thrombi. However, if the underlying inflammatory condition or the host's inflammatory response progresses unabated, the hemostatic system answers by causing widespread thrombi to limit dissemination of the harmful microbes or damage‐associated proteins. Marked thrombi formation results in significant increase in D‐dimers but also leads to exhaustion of platelet granules. Because fibrinogen is no longer released from the platelets, its level starts to decrease in tandem with increasing D‐dimers. This has clearly been shown in one of the most quoted papers in COVID‐19 hypercoagulability literature from Tang et al.1 In this report, a clear distinction between survivors and non‐survivors was identified by the significantly elevated D‐dimer and lower fibrinogen but not high fibrinogen levels.1 The D‐dimer/fibrinogen ratio has been explored in the past as a thrombotic marker. Kucher et al showed higher D‐dimer values and lower fibrinogen levels in patients with pulmonary embolism and even suggested the use of D‐dimer/fibrinogen ratio of >103 as being highly specific for pulmonary clots.25 The authors also commented on the possibility of decreasing fibrinogen levels correlating with increasing pulmonary occlusion rate.25
8. CLINICAL RELEVANCE OF THE HYPOTHESIS
What is the clinical relevance of the protective fibrinogen hypothesis? Monitoring fibrinogen levels along with D‐dimers in critically ill patients with (and without) COVID‐19 is clearly important. If the D‐dimers do not increase in tandem with the fibrinogen, it may be hypothesized that the protective role of fibrinogen is dominating (acute phase response >>> thrombosis). However, if the D‐dimers begin increasing and the fibrinogen levels begin decreasing, this is the time when marked coagulation activation and thrombi formation is occurring (Figure 1 ). Unfortunately, clinicians only notice the fibrinogen levels when they are markedly reduced and the patient may have begun bleeding, which may sometimes be an unsalvageable scenario. An interesting dilemma here is whether fibrinogen replacement should be given to maintain the higher levels rather than considering it when the levels have started decreasing, when such transfusions can be harmful. Unfortunately, most of the clinical studies on fibrinogen have focused on bleeding outcomes with limited considerations looking at benefits outside hemostasis.
Figure 1.
Fibrinogen and the balance between acute phase reaction and thrombus formation
9. SUMMARY
So far, the protective role of fibrinogen as an acute phase reactant remains a hypothesis. Some basic science research suggests this role although clinical studies overlooked any function outside thrombosis causation, that too without definitive proof. It is unusual for an evolutionary mechanism like an acute phase reaction to prove harmful to the host, especially when it occurs in scenarios such as sepsis and trauma, which have affected humans from the early ages. We must begin examining the non‐hemostatic functions of fibrinogen in future research studies. In a similar way, it may be useful to study other coagulation proteins such as VWF for a possible protective role in the acute phase.
CONFLICTS OF INTEREST
The author declares no conflicts of interest.
Footnotes
Manuscript handled by: David Lillicrap
Final decision: David Lillicrap, 31 May 2020
REFERENCES
- 1.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y., et al. China medical treatment expert group for Covid‐19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonetto D.A., Piccolo Serafim L., Gallo de Moraes A., Gajic O., Kamath P.S. Management of sepsis in patients with cirrhosis: current evidence and practical approach. Hepatology. 2019;70(1):418–428. doi: 10.1002/hep.30412. [DOI] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drakesmith H., Prentice A.M. Hepcidin and the iron‐infection axis. Science. 2012;338(6108):768–772. doi: 10.1126/science.1224577. [DOI] [PubMed] [Google Scholar]
- 7.Sazawal S., Black R.E., Ramsan M., et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community‐based, randomised, placebo‐controlled trial. Lancet. 2006;367(9505):133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 8.Tielsch J.M., Khatry S.K., Stoltzfus R.J., et al. Effect of routine prophylactic supplementation with iron and folic acid on preschool child mortality in southern Nepal: community‐based, cluster‐randomised, placebo‐controlled trial. Lancet. 2006;367(9505):144–152. doi: 10.1016/S0140-6736(06)67963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrams S.T., Zhang N., Dart C., et al. Human CRP defends against the toxicity of circulating histones. J Immunol. 2013;191(5):2495–2502. doi: 10.4049/jimmunol.1203181. [DOI] [PubMed] [Google Scholar]
- 10.Quaye I.K. Haptoglobin, inflammation and disease. Trans R Soc Trop Med Hyg. 2008;102(8):735–742. doi: 10.1016/j.trstmh.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Flick M.J., Du X., Witte D.P., et al. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac‐1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113(11):1596–1606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou H., Liao J., Aloor J., et al. CD11b/CD18 (Mac‐1) is a novel surface receptor for extracellular double‐stranded RNA to mediate cellular inflammatory responses. J Immunol. 2013;190:115–125. doi: 10.4049/jimmunol.1202136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoniak S. The coagulation system in host defense. Res Pract Thromb Haemost. 2018;2(3):549–557. doi: 10.1002/rth2.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altieri D.C., Agbanyo F.R., Plescia J., Ginsberg M.H., Edgington T.S., Plow E.F. A unique recognition site mediates the interaction of fibrinogen with the leukocyte integrin Mac‐1 (CD11b/CD18) J Biol Chem. 1990;265(21):12119–12122. [PubMed] [Google Scholar]
- 15.Pillay J., Kamp V.M., Pennings M., et al. Acute‐phase concentrations of soluble fibrinogen inhibit neutrophil adhesion under flow conditions in vitro through interactions with ICAM‐1 and MAC‐1 (CD11b/CD18) J Thromb Haemost. 2013;11(6):1172–1182. doi: 10.1111/jth.12250. [DOI] [PubMed] [Google Scholar]
- 16.Ko Y.P., Flick M.J. Fibrinogen is at the interface of host defense and pathogen virulence in staphylococcus aureus infection. Semin Thromb Hemost. 2016;42(4):408–421. doi: 10.1055/s-0036-1579635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esmon C.T., Xu J., Lupu F. Innate immunity and coagulation. J Thromb Haemost. 2011;9(Suppl 1):182–188. doi: 10.1111/j.1538-7836.2011.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad J.M., Gorkun O.V., Raghu H., et al. Mice expressing a mutant form of fibrinogen that cannot support fibrin formation exhibit compromised antimicrobial host defense. Blood. 2015;126(17):2047–2058. doi: 10.1182/blood-2015-04-639849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolhnikoff M., Duarte‐Neto A.N., de Almeida Monteiro R.A., et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID‐19. J Thromb Haemost. 2020;18(6):1517–1519. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machlus K.R., Cardenas J.C., Church F.C., Wolberg A.S. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood. 2011;117(18):4953–4963. doi: 10.1182/blood-2010-11-316885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stalker T.J., Traxler E.A., Wu J., et al. Hierarchical organization in the hemostatic response and its relationship to the platelet‐signaling network. Blood. 2013;121(10):1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blair P., Flaumenhaft R. Platelet alpha‐granules: basic biology and clinical correlates. Blood Rev. 2009;23(4):177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gralnick H.R., Williams S.B., McKeown L.P., Krizek D.M., Shafer B.C., Rick M.E. Platelet von Willebrand factor: comparison with plasma von Willebrand factor. Thromb Res. 1985;38(6):623–633. doi: 10.1016/0049-3848(85)90205-1. [DOI] [PubMed] [Google Scholar]
- 24.Bowie E.J., Solberg L.A., Jr, Fass D.N., et al. Transplantation of normal bone marrow into a pig with severe von Willebrand's disease. J Clin Invest. 1986;78(1):26–30. doi: 10.1172/JCI112560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kucher N., Kohler H.P., Dornhöfer T., Wallmann D., Lämmle B. Accuracy of D‐dimer/fibrinogen ratio to predict pulmonary embolism: a prospective diagnostic study. J Thromb Haemost. 2003;1(4):708–713. doi: 10.1046/j.1538-7836.2003.00145.x. [DOI] [PubMed] [Google Scholar]