Dear Editor,
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, is currently spreading worldwide, causing the worst pandemic experienced this century. 1 During the present outbreak, reports have been accumulating that various types of cutaneous manifestations were observed in COVID‐19 patients. 2 , 3 , 4 , 5 , 6 , 7 We read with interest the recent article by Amatore et al. 3 describing a COVID‐19 case who presented with a febrile rash consisting of annular, polycyclic and circinate erythema, presumably specific to COVID‐19. Recently, we experienced a case of polycyclic erythema, which was very similar to theirs, in a patient with respiratory distress whose eosinophilic granulomatosis with polyangiitis (EAGP) was later confirmed by skin biopsy.
A 30‐year‐old Chinese male had a high fever (>38°C) with respiratory distress on a flight to Japan, where he worked, and was referred to us. Laboratory examination revealed elevated white (9.2–10.5 × 104 cells/µL, normal range 3.3–8.6 × 104 cells/µL) and red (615–692 × 104 cells/µL, normal range 435–555 × 104 cells/µL) blood cell counts, eosinophilia (15–28%, normal range <5%), a decreased platelet count (2–7 × 104 cells/µL, normal range 15–42 × 104 cells/µL) and high levels of D‐dimers (10–22 µg/mL, normal range <0.1 µg/mL), fibrin‐fibrinogen degradation products (6.3–35.3 µg/mL, normal range <5.0 µg/mL) and C‐reactive protein (3.05–4.59 µg/dL, normal range <0.14 µg/dL). His high red blood cell count had been clinically observed previously, without treatment. Neither anti‐neutrophil cytoplasmic antibody nor anti‐nuclear antibody was detected. The chest computed tomogram revealed bilateral interstitial shadows (Fig. 1), like those frequently seen in COVID‐19 cases, so a pulmonologist segregated him on suspicion of COVID‐19. During treatment with 6 g/day of intravenous ceftriaxone, erythema suddenly developed. Multiple circinate and annular erythema with slight pruritus were observed on his body, arms (Fig. 2a), palms (Fig. 2b) and legs (Fig. 2c). Ecchymosis after bruising was found in his right leg (Fig. 2c). A skin biopsy specimen taken from the skin lesion on his right thigh revealed mild perivascular infiltrates with lymphocytes and eosinophils. Some vessels had markedly infiltrated eosinophils with erythrocyte extravasation (Fig. 2d and inset). Afterwards, RT‐PCR tests on the patient's sputum and nasopharyngeal swabs were negative for SARS‐CoV‐2 RNA detection. Based on these laboratory and histological findings, we diagnosed the patient with EGPA. Steroid pulse therapy for three successive days promptly relieved all his symptoms, in parallel with the normalization of his chest radiogram and laboratory data. His treatment was then changed to oral administration of 50 mg/day prednisolone for 2 weeks, after which the prednisolone dose was gradually tapered to 25 mg/day. Although the eruption reappeared transiently, it was successfully treated with olopatadine hydrochloride. No relapse occurred.
Figure 1.
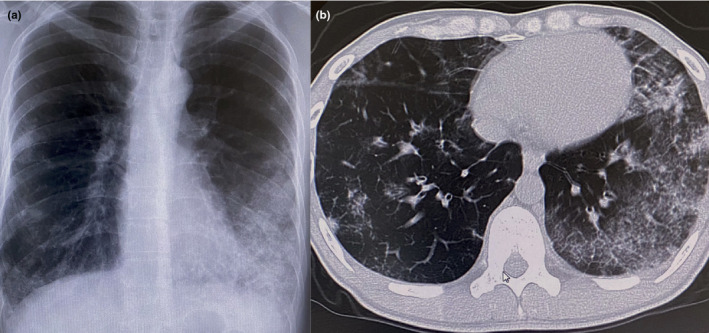
Chest roentgenogram (a) and computed tomogram (b) showing bilateral interstitial shadows.
Figure 2.
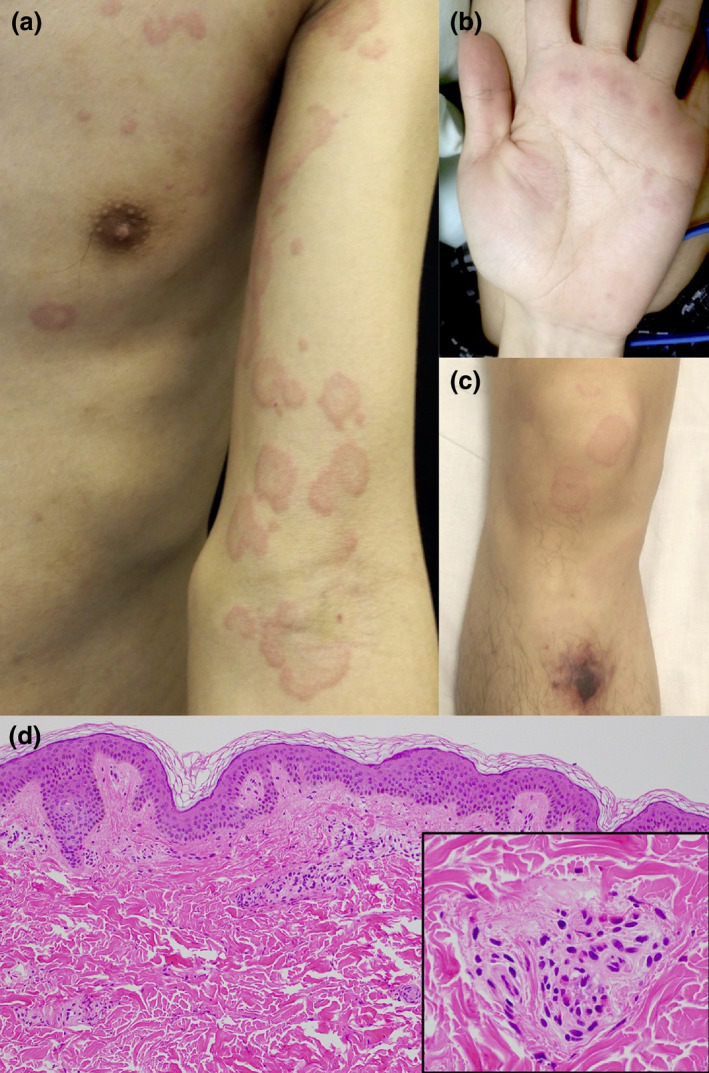
Urticarial and circinate erythema of the patient's arm (a), palm (b) and leg (c). Histologic examination showing perivascular infiltrates with lymphocytes and eosinophils (d and inset).
Various clinical signs, laboratory parameters and imaging modalities are suggestive of COVID‐19. Additionally, recent reports have implicated distinctive skin manifestations, such chilblain‐like erythema, livedoid eruptions, 2 , 8 morbilliform rash, 9 urticarial rash 4 and varicella‐like rash, 6 , in COVID‐19. Here, we found bilateral interstitial pneumonia accompanied with a hypercoagulation state, which is a typical pulmonary manifestation of COVID‐19. In addition, we also observed polycyclic erythema, manifesting very similarly to the COVID‐19 case described by Amatore et al. 3 These findings might have supported a diagnosis of COVID‐19. However, because eosinophilia and eosinophilic infiltrates in skin lesions are an exceptional feature of COVID‐19, 10 EGPA was the more appropriate diagnosis for our case. The patient's complete response to steroid therapy, which usually causes deterioration in COVID‐19 cases, supports our diagnosis. It is important to be aware of the similar clinical manifestations between EGPA and COVID‐19.
Conflict of interest
None.
Funding source
None.
Acknowledgement
This patient in this manuscript has given written informed consent to the publication of his case details.
References
- 1. Ng OT, Marimuthu K, Chia PY et al. SARS‐CoV‐2 infection among travelers returning from Wuhan, China. N Engl J Med 2020; 382: 1476–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alramthan A, Aldaraji W. A case of COVID‐19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol 2020. 10.1111/ced.14243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amatore F, Macagno N, Mailhe M et al. SARS‐CoV‐2 infection presenting as a febrile rash. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henry D, Ackerman M, Sancelme E, Finon A, Esteve E. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Magro C, Mulvey JJ, Berlin D et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res 2020; 220: 1–13. 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marzano AV, Genovese G, Fabbrocini G et al. Varicella‐like exanthem as a specific COVID‐19‐associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol 2020; 83: 280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tammaro A, Adebanjo GAR, Parisella FR, Pezzuto A, Rello J. Cutaneous manifestations in COVID‐19: the experiences of Barcelona and Rome. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandez‐Nieto D, Jimenez‐Cauhe J, Suarez‐Valle A et al. Characterization of acute acro‐ischemic lesions in non‐hospitalized patients: a case series of 132 patients during the COVID‐19 outbreak. J Am Acad Dermatol 2020; 83: e61–e63. 10.1016/j.jaad.2020.04.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Su CJ, Lee CH. Viral exanthem in COVID‐19, a clinical enigma with biological significance. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil responses during COVID‐19 infections and coronavirus vaccination. J Allergy Clin Immunol 2020. 10.1016/j.jaci.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]


