ETHICS STATEMENT
This study has been conducted according to principles of Declaration of Helsinki and Good Clinical research Practice.
To the Editor,
We read with great interest, the recently published article by Chakraborty et al 1 in response to IL‐6 antagonist use, for the management of COVID‐19 ‐related cytokine storm. We agree that to date, no therapy has demonstrated definite efficacy for patients with COVID‐19, hence in this context antiviral for example remdesivir (RDV), 2 as well as, anti‐inflammatory approaches (tocilizumab [TCZ]), 3 have been recently put forward. However, data upon optimal choice of one over the other, or potential need for regimen combination, remains an open question. We, hereby, report our experience with two identical cases of SARS‐CoV‐2 (+) patients, developing respiratory failure, both receiving TCZ following severe inflammatory response, with or without RDV, and we argue that, even though, TCZ administration may dump underlying inflammatory response mediated by IL‐6, cytokine storm cannot be totally diminished by this blockade, since other distal pathways remain active.
Two SARS‐CoV‐2 (+) patients 59 (Patient 1) and 49‐year‐old (Patient 2), respectively, both with medical history of dyslipidemia, otherwise unremarkable, presented in our department with low grade fever and dyspnoea, on 5th day of symptoms. Both patients had received hydroxychloroquine (800 mg LD followed by 400 mg qd) and azithromycin (500 mg LD, followed by 250 mg qd) for 4 days before admission, with no response. Upon presentation, they were both in good condition, showing signs of incipient lower respiratory tract infection and mild hypoxia of 94% and 93% oxygen saturation on room air, respectively. On 3rd day of admission, their clinical condition deteriorated, both presenting high grade fever up to 39.5°C and respiratory failure (pO2/fiO2 < 200), on air entrainment‐masks. In line with increased inflammatory markers, we administrated TCZ in both patients. Patient 2 was also approved to receive RDV, in the context of compassionate use. Defervescence and drop in CRP occurred within the next 24 hours of TCZ infusion, in both patients. However, during the same time, both required noninvasive positive pressure and invasive mechanical ventilatory support respectively, exhibiting laboratory markers and imaging as shown in Figure 1. Interestingly, at this time, patient 1 showed signs of ongoing hemophagocytic syndrome (HLH) (HScore:193, absent NK cell activity), contrary to patient 2 (HScore: 63, mildly increased NK cell activity). On day 5 following administration, both patients presented significant respiratory and radiologic improvement, and were eventually transferred from ICU to medical ward, with low flow oxygen supply, where they continued an uncomplicated course until discharge.
Figure 1.
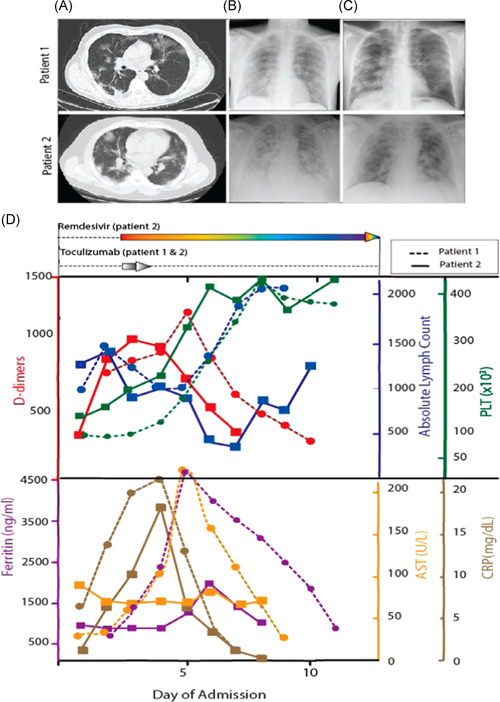
Chest computed tomography showing bilateral infiltrates (A), in line with plain chest X‐ray findings (B). Significant radiologic improvement was noted 5 days later (C).Temporal course of laboratory markers including (D). CRP, ferritin, absolute lymphocyte count, platelet count, SGOT and D‐dimers is shown, in line with administration of RDV and TCZ. Dashed lines: patient 1, continuous lines: patient 2. CRP, C‐reactive protein; RDV, remdesivir; AST, aspartate aminotransferase; TCZ, tocilizumab
A unique pattern of immune dysregulation, associated with sustained cytokine production and hyperinflammation has been recently identified in severe COVID‐19 cases. 4 The primary source and kinetics of the cytokine storm, however, remain elusive. On one hand, there is defective antigen presentation driven by IL‐6, and on the other, an HLH like syndrome mediated by IL‐1β. 4 Exact timing of these processes need to be addressed, while simultaneous progression of both syndromes, similar to bacterial sepsis is possible. It seems that blockade of IL‐6, by TCZ in COVID‐19 patients partially rescues immune dysregulation. 4 , 5 Even though, both of our patients received TCZ and presented prompt decrease in inflammatory markers similar to others, 3 , 5 patient one was consequently driven to HLH. It is possible, that even though, hyperinflammatory response is mainly driven by IL‐6, its blockade can leave alternative pathways intact, pushing response to the other end, that of HLH. Previous authors have noted the need to screen those patients for secondary HLH, as this reflected in HScore more than 169, where respective immunomodulatory therapy could prove beneficial. 6
Whether, systemic cytokine storm or actually direct virus‐induced tissue damage, or development of both, contributes to poor outcomes remains to be seen. In the latter case, we should expect a combination of approaches, rather than, a magic bullet to target SARS‐CoV‐2. Nonetheless, the earlier in the cascade we target, the best chances we have to get optimal results. We argue that, RDV can blunt inflammatory response, by hampering viral replication; thus, preventing inflammatory mediator release to any direction, at its very beginning.
Even though, well‐matched, our cases are far from a randomized clinical trial of that sort. Recently initiated REMDACTA trial could elucidate RDV and TCZ potential combination use. In line with previous authors, 1 we stress the complexity and variety of pathways underlying inflammatory response, that can drive diverse outcomes. We suggest administration of RDV, early before secondary uncontrolled inflammatory response takes place. If the latter does occur, immunomodulation, for example, via TCZ use is an option, but does not solely seem to drive outcomes. Managing hyperinflammation with immunomodulation is important, but source control, with proper and timely antiviral therapy remains critical.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
DV, DZ, CD, AT, KT, and FF were involved in patients' management. AS carried out immunologic profile analysis. CK performed patients' imaging and provided respective material, MM advised patients' management. KA co‐ordinated patients' management and wrote the manuscript. CG advised patients' management and critically corrected the manuscript. All authors contributed to the study's perception, design, have seen and approved the manuscript.
ACKNOWLEDGMENT
Both patients reported in this study singed an informed consent form to have their data anonymously analyzed, utilized, and published.
DATA AVAILABILITY STATEMENT
Data can be made available upon request, according to GDPR.
REFERENCES
- 1. Chakraborty C, Sharma AR, Bhattacharya M, Sharma G, Lee SS, Agoramoorthy G. COVID‐19: consider IL6 receptor antagonist for the therapy of cytokine storm syndrome in SARS‐CoV‐2 infected patients [published online ahead of print May 28, 2020]. J Med Virol. 10.1002/jmv.26078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;395(10236):1569‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu X, Han M, Li T. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970‐10975. http://chinaxiv.org/abs/2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giamarellos‐Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID‐19 patients with severe respiratory failure [published online ahead of print April 17, 2020]. Cell Host Microbe. 10.1016/j.chom.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: a single center experience [published online ahead of print April 6, 2020]. J Med Virol. 10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be made available upon request, according to GDPR.


