Abstract
Identifying drugs effective in the new coronavirus disease 2019 (COVID‐19) is crucial, pending a vaccine against SARS‐CoV2. We suggest the hypothesis that cannabidiol (CBD), a non‐psychotropic phytocannabinoid, has the potential to limit the severity and progression of the disease for several reasons:‐ (a) High‐cannabidiol Cannabis sativa extracts are able to down‐regulate the expression of the two key receptors for SARS‐CoV2 in several models of human epithelia, (b) cannabidiol exerts a wide range of immunomodulatory and anti‐inflammatory effects and it can mitigate the uncontrolled cytokine production responsible for acute lung injury, (c) being a PPARγ agonist, it can display a direct antiviral activity and (d) PPARγ agonists are regulators of fibroblast/myofibroblast activation and can inhibit the development of pulmonary fibrosis, thus ameliorating lung function in recovered patients. We hope our hypothesis, corroborated by preclinical evidence, will inspire further targeted studies to test cannabidiol as a support drug against the COVID‐19 pandemic.
Linked Articles
This article is part of a themed issue on The Pharmacology of COVID‐19. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.21/issuetoc
Abbreviations
- CBD
cannabidiol
- COVID‐19
corona virus disease 2019
- CYP450
cytochromes P450
- FDA
Food and Drug Administration
- MCP‐1
monocyte chemoattractant protein
- MIP‐2
macrophage inflammatory protein 2
- SARS‐CoV2
severe acute respiratory syndrome‐coronavirus‐2
- TMPRSS2
transmembrane serine protease 2
- TRP
transient receptor potential
1. HYPOTHESIS
An aberrant release of cytokines and proinflammatory molecules is closely related to lung injury, multiorgan failure and ultimately poor prognosis in the new severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV2) pandemic (Huang et al., 2020).
Such uncontrolled release of cytokines, namely IL‐1β, IL‐6 and CCL2 (chemokine (C‐C motif) ligand 2, alternatively known as monocyte chemoattractant protein (MCP)‐1), paralleled with the decrease in natural killer (NK) cells may result in the so‐called “cytokine storm.” Immune dysregulation, rather than viraemia levels per se, has been related to the massive proinflammatory cytokine secretion by alveolar macrophages and subsequent CD4+ and CD8+ T cell dysfunction observed in SARS‐CoV infection (Channappanavar et al., 2016). Hence, until specific vaccines become available, the use of antiviral agents alone may not be sufficient to stop the cytokine storm and respiratory distress in severely ill patients. In the attempt of reducing their overall mortality, it is therefore essential to identify new therapeutics options that are able to mitigatie the cytokine storm (Huang et al., 2020). Nonetheless, redundancies within the complex cytokine network still represent a major obstacle to treatment with monoclonal antibodies. The ideal drug candidate should be already in use for other indications, have a favourable safety profile, a multitargeted action, should be able to synergistically mitigate the cytokine storm and should act as an immunomodulatory rather than an immunosuppressant drug.
In a recent paper, high‐cannabidiol (CBD) Cannabis sativa extracts have been reported to down‐regulate angiotensin‐converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) enzymes, crucial viral gateways in oral, lung and intestinal epithelia constituting important routes of SARS‐CoV2 invasion (Wang et al., 2020). By down‐regulating ACE2 and TMPRSS2 enzymes, the authors suggested that high‐cannabidiol products, such as mouth washes, as a preventative strategy in COVID‐19 infection to limit SARS‐CoV2 entry into susceptible hosts. While this article puts forward the concept that cannabinoids‐containing products may serve as a preventative treatment for topical use, there is evidence suggesting that cannabidiol immunomodulatory activities may play a role in later stages of the disease.
We herein explore the hypothesis that systemic administration of cannabidiol could have the potential to limit COVID‐19 disease progression and post‐infectious sequelae.
Non‐psychotropic phytocannabinoid, cannabidiol is considered one of the most interesting emerging molecules in the field of pharmacology, since it exerts a wide range of therapeutic effects, ranging from anticonvulsive, sedative, hypnotic, antipsychotic, anti‐cancer, anti‐inflammatory and neuroprotective activities (Iffland & Grotenhermen, 2017). Lacking of the unwanted psychotropic effects of marijuana derivatives, cannabidiol has little binding affinity to cannabinoid receptors (acting as allosteric modulator of cannabinoid CB1 receptors) and a favourable safety profile in humans (Iffland & Grotenhermen, 2017). Cannabidiol acts as a powerful antioxidant acting at various receptor sites, including PPARγ (NR1C3), 5‐HT1A, adenosine A2 A and transient receptor potential (TRP) channel receptors, to directly or indirectly cause a wide range of anti‐inflammatory and immunomodulatory effects. A complete review of cannabidiol receptor targets is beyond the purpose of the present article and the readers are invited refer to an extensive review on this subject (Iffland & Grotenhermen, 2017).
Such pleiotropic pharmacological activity has been tested in various pathological conditions, including respiratory diseases resembling COVID‐19‐induced respiratory distress. Acute lung injury refers to a characteristic form of parenchymal lung disease, featured by bilateral pulmonary infiltrates, alveolar‐capillary vasculitis with neutrophil infiltration and proinflammatory cytokines release, comparable to COVID‐19. By acting at adenosine A2A receptors, cannabidiol caused a marked amelioration of the reduced pulmonary function (Ribeiro et al., 2012; Ribeiro et al., 2015). This was a consequence of a significant decrease in lung resistance and elastance due to the reduction of leukocyte migration into the lung, accompanied by a marked inhibition of both pro‐inflammatory cytokines (TNF‐α and IL‐6) and chemokine (MCP‐1/MIP‐2/CXCL2) release (Ribeiro et al., 2012, Ribeiro et al., 2015).
Although limited to interesting preclinical studies, scattered evidence also points towards a possible use of cannabidiol in viral infections. Indeed, several plant‐derived compounds have evolved to display antiviral activity, including many phenol‐based compounds, such as terpenoids.
Cannabidiol and other cannabinoids exert their activity through the interaction with the nuclear PPARs (O'Sullivan & Kendall, 2010). The PPARs belong to the family of nuclear hormone receptors and their activity is regulated by steroids and lipid metabolites. Three different PPAR isoforms (PPARα, PPARβ, also called δ, and PPARγ) have been identified and they have been shown to regulate the expression of genes related to lipid and glucose homeostasis and inflammatory responses.
PPARγ agonism in resident alveolar macrophages significantly limits pulmonary inflammation and promotes host recovery following respiratory viral infections (Huang et al., 2019). As it has been demonstrated during acute pneumonia, alveolar macrophage largely express PPARγ. PPARγ activation is also responsible for the control of cytokine over‐secretion with consequent amelioration of the tissue damage. It is therefore likely that in addition to directly causing an improvement in lung dynamics, cannabidiol could significantly counteract the onset of the cytokine storm from resident macrophages. Interestingly, prophylactic or therapeutic administration of PPARγ agonists led to a reduction of morbidity and mortality during influenza A virus infection (Bassaganya‐Riera, Song, Roberts, & Hontecillas, 2010). Not coincidentally, the opportunity of thiazolidinediones repurposing for the treatment of COVID‐19 patients has been recently suggested, based on their activity on PPARγ receptors (Carboni, Carta, & Carboni, 2020). Nonetheless, full PPARγ agonists bear several unwanted side effects that could limit their clinical applicability in COVID‐19 infection. The use of thiazolidinediones indeed suffered a back‐box warning from the FDA, given the risk of cardiovascular complications such as acute myocardial infarction, heart failure and stroke. Conversely, being a weak PPARγ agonist, cannabidiol may overcome these limitations and be voided of these side effects (Graham et al., 2010).
Moreover, PPARγ agonists may directly inhibit viral replication by different human viruses such as human immunodeficiency virus, respiratory syncytial virus, hepatitis B virus, and hepatitis C virus. Noteworthy, this experimental evidence was corroborated by a recent study showing a direct antiviral action against hepatitis C virus in vitro (Lowe, Toyang, & McLaughlin, 2017).
Recent reports show that a subset of COVID‐19 survivors can develop post‐infectious sequelae with persistently impaired lung function and pulmonary fibrosis (Ng, Li, Lee, & Ma, 2020). PPARγ receptors represent a potential therapeutic target in fibrotic lung diseases, given their ability of regulating fibroblast/myofibroblast activation and collagen secretion in murine models (Milam et al., 2008). Notably, cannabidiol has been shown to reduce pulmonary inflammation and fibrosis in animal models of asthma (Vuolo et al., 2019). It is therefore conceivable that cannabidiol being a PPARγ receptor agonist, could potentially limit the start of late‐onset pulmonary fibrosis in COVID‐19‐recovered patients.
Although cannabidiol seems to be a relatively safe molecule in humans as shown in different trials that have been conducted (Millar et al., 2019) or are ongoing especially for the treatment of neurological disorders. However, there are currently no data about the efficacy and relative toxicity of cannabidiol in COVID‐19.
Even if cannabidiol was (incorrectly in our opinion) considered as a mere therapeutic supplement, there is still a lack of data regarding the relative toxicity profile when co‐administered with other drugs used in the current anti‐COVID‐19 protocols. According as a precautionary principle, a possible strategy would be testing cannabidiol therapeutic potential in COVID‐19 patients (aged 18 years or older) either at an early stage of the disease to stop the cytokine storm and development of respiratory distress, or alternatively, to evaluate its effectiveness in COVID‐19 recovered patients to prevent pulmonary fibrosis. Cannabidiol effects in vivo largely depend on its dose and the bioavailability of its receptor targets in various pathological conditions. Different plasma concentrations of cannabidiol may be required in order to activate the distinct pathways responsible for its multifaceted activity. Indeed, subtherapeutic dosing (0.3 mg·kg−1·day−1) has been suggested to account for cannabidiol lack of effectiveness in Crohn's disease (Millar et al., 2019). In humans, cannabidiol has been tested across a wide dosage range, varying from <1 up to 50 mg·kg−1·day−1 depending on the trials and on the explored pathological condition, with both in vitro and in vivo studies suggesting an immunosuppressive action at higher concentrations or doses. Both in human immunodeficiency virus (HIV) and in post‐Ebola syndrome, cannabidiol has been proposed as a therapeutic agent to control immune activation at doses of 10–20 mg·kg−1·day−1 and 1.7–10 mg·kg−1·day−1 (100 mg·day−1 titrating up to 600 mg·day−1), respectively (Costiniuk et al., 2019; Reznik, Gardner, & Ashby, 2016). We suggest that cannabidiol should be given orally, starting at 100 mg·day−1 titrating up to 300 mg·day−1 (2.5 mg·kg−1·day−1) as this dosage did not produce any relevant adverse effects even after prolonged administrations (up to 18 weeks) in human clinical trials. However, it is worth mentioning that most of the published clinical trials lack data on the effective plasma concentrations reached by orally administered cannabidiol in vivo. This has also implications for its safety profile, since cannabidiol acts as an in vitro inhibitor of several CYP450 isoforms (Millar et al., 2019). As previously underlined, drug–drug interaction studies between cannabidiol and anti‐COVID‐19 treatments are lacking, therefore monitoring patients for potential drug interactions would be required. Similarly, CYP inhibitors are predicted to increase cannabidiol plasma concentrations, thus patients should be carefully monitored for adverse effects. Nonetheless, we do not anticipate any serious adverse side effects, since the proposed dose of cannabidiol is generally well tolerated in humans and the concentration (IC50) required to inhibit the CYP450 is significantly higher than the plasma concentration of cannabidiol achieved following oral administration. Finally, regarding the possible concerns about immunosuppression during acute infections, we believe it is important to underline the observation that cannabidiol did not cause an increase in mortality in acutely infected animals, rather in pneumococcal meningitis animal survival was increased and TNF‐α concentrations decreased at the doses of 2.5, 5 and 10 mg·kg−1 (Barichello et al., 2012).
The COVID‐19 pandemic is testing the world. The off‐label use of readily available therapeutics able to limit the severity of the disease must be scrupulously scrutinized, pending a vaccine against SARS‐CoV2. In keeping with this, we consider cannabidiol a promise candidate drug to bet on, based on the encouraging preclinical studies and its relative safety profile in humans (Figure 1). Further evidence will be needed to confirm its beneficial activities and turn cannabidiol into a useful addition to the treatment of COVID‐19.
FIGURE 1.
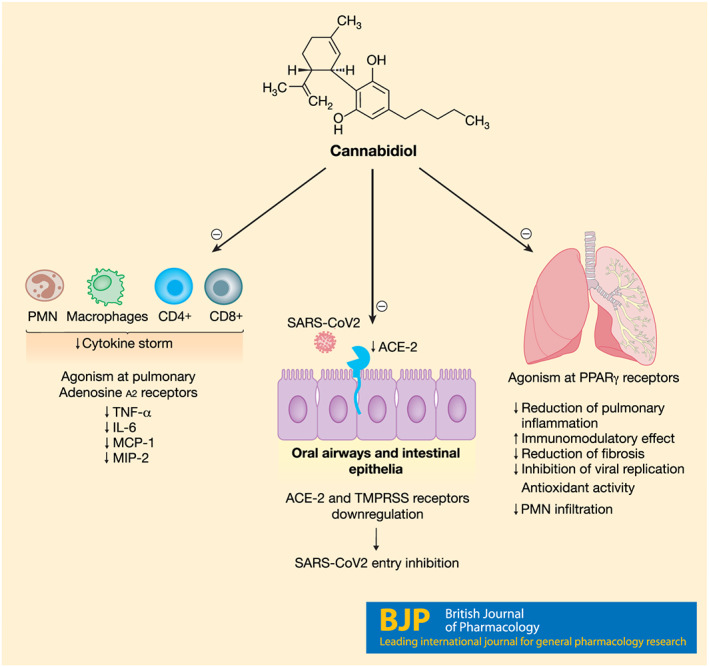
The potential of cannabidiol (CBD) in SARS‐CoV2 infection. Cannadibidiol recognizes several receptor targets and displays a multifaceted immunomodulatory activity that could limit the severity of SARS‐CoV2 infection: it downregulates ACE‐2 receptors, SARS‐CoV2 gateway, it can mitigate cytokines release and reduce pulmonary inflammation and fibrosis through PPARγ receptors
2. CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Esposito G, Pesce M, Seguella L, et al. The potential of cannabidiol in the COVID‐19 pandemic. Br J Pharmacol. 2020;177:4967–4970. 10.1111/bph.15157
REFERENCES
- Barichello, T. , Ceretta, R. A. , Generoso, J. S. , Moreira, A. P. , Simões, L. R. , Comim, C. M. , … Teixeira, A. L. (2012). Cannabidiol reduces host immune response and prevents cognitive impairments in Wistar rats submitted to pneumococcal meningitis. European Journal of Pharmacology, 697, 158–164. 10.1016/j.ejphar.2012.09.053 [DOI] [PubMed] [Google Scholar]
- Bassaganya‐Riera, J. , Song, R. , Roberts, P. C. , & Hontecillas, R. (2010). PPAR‐γ activation as an anti‐inflammatory therapy for respiratory virus infections. Viral Immunology, 23, 343–352. 10.1089/vim.2010.0016 [DOI] [PubMed] [Google Scholar]
- Carboni, E. , Carta, A. R. , & Carboni, E. (2020). Can pioglitazone be potentially useful therapeutically in treating patients with COVID‐19? Medical Hypotheses, 22, 109776 10.1016/j.mehy.2020.109776 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar, R. , Fehr, A. R. , Vijay, R. , Mack, M. , Zhao, J. , Meyerholz, D. K. , & Perlman, S. (2016). Dysregulated type I interferon and inflammatory monocyte‐macrophage responses cause lethal pneumonia in SARS‐CoV‐infected mice. Cell Host & Microbe, 19(2), 181–193. 10.1016/j.chom.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costiniuk, C. T. , Saneei, Z. , Routy, J. , Margolese, S. , Mandarino, E. , Singer, J. , … Klein, M. B. (2019). Oral cannabinoids in people living with HIV on effective antiretroviral therapy: CTN PT028—Study protocol for a pilot randomised trial to assess safety, tolerability and effect on immune activation. BMJ Open, 9(1), e024793 10.1136/bmjopen-2018-024793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, D. J. , Ouellet‐Hellstrom, R. , MaCurdy, T. E. , Ali, F. , Sholley, C. , Worrall, C. , & Kelman, J. A. (2010). Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. Journal of the American Medical Association, 304(4), 411–418. 10.1001/jama.2010.920 Epub 2010 Jun 28 [DOI] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395(10223), 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S. , Goplen, N. P. , Zhu, B. , Cheon, I. S. , Son, Y. , Wang, Z. , … Sun, J. (2019). Macrophage PPAR‐γ suppresses long‐term lung fibrotic sequelae following acute influenza infection. PLoS ONE, 14(10), e0223430 10.1371/journal.pone.0223430 PMID: 31584978; PMCID: PMC6777801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iffland, K. , & Grotenhermen, F. (2017). An update on safety and side effects of cannabidiol: A review of clinical data and relevant animal studies. Cannabis and Cannabinoid Research, 2, 139–154. 10.1089/can.2016.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, H. I. , Toyang, N. J. , & McLaughlin, W. (2017). Potential of cannabidiol for the treatment of viral hepatitis. Pharmacognosy Research, 9(1), 116–118. 10.4103/0974-8490.199780 PMID: 28250664; PMCID: PMC5330095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam, J. E. , Keshamouni, V. G. , Phan, S. H. , Hu, B. , Gangireddy, S. R. , Hogaboam, C. M. , … Reddy, R. C. (2008. May). PPAR‐γ agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin‐induced pulmonary fibrosis. American Journal of Physiology. Lung Cellular and Molecular Physiology, 294(5), L891–L901. 10.1152/ajplung.00333.2007 Epub 2007 Dec 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar, S. A. , Stone, N. L. , Bellman, Z. D. , Yates, A. S. , England, T. J. , & O'Sullivan, S. E. (2019. Sep). A systematic review of cannabidiol dosing in clinical populations. British Journal of Clinical Pharmacology, 85(9), 1888–1900. 10.1111/bcp.14038 Epub 2019 Jul 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, F. H. , Li, S. K. , Lee, Y. C. , & Ma, J. K. F. (2020). Temporal changes in computed tomography of COVID‐19 pneumonia with perilobular fibrosis. Hong Kong Medical Journal. 10.12809/hkmj208490 [DOI] [PubMed] [Google Scholar]
- O'Sullivan, S. E. , & Kendall, D. (2010). Cannabinoid activation of peroxisome proliferator‐activated receptors: Potential for modulation of inflammatory disease. Immunobiology, 215, 611–616. 10.1016/j.imbio.2009.09.007 [DOI] [PubMed] [Google Scholar]
- Reznik, S. E. , Gardner, E. L. , & Ashby, C. R. Jr. (2016). Cannabidiol: A potential treatment for post Ebola syndrome? International Journal of Infectious Diseases, 52, 74–76. 10.1016/j.ijid.2016.09.020 [DOI] [PubMed] [Google Scholar]
- Ribeiro, A. , Almeida, V. I. , Costola‐de‐Souza, C. , Ferraz‐de‐Paula, V. , Pinheiro, M. L. , Vitoretti, L. B. , … Palermo‐Neto, J. (2015). Cannabidiol improves lung function and inflammation in mice submitted to LPS‐induced acute lung injury. Immunopharmacology and Immunotoxicology, 37(1), 35–41. 10.3109/08923973.2014.976794 Epub 2014 Oct 30 [DOI] [PubMed] [Google Scholar]
- Ribeiro, A. , Ferraz‐de‐Paula, V. , Pinheiro, M. L. , Vitoretti, L. B. , Mariano‐Souza, D. P. , Quinteiro‐Filho, W. M. , … Palermo‐Neto, J. (2012). Cannabidiol, a non‐psychotropic plant‐derived cannabinoid, decreases inflammation in a murine model of acute lung injury: Role for the adenosine A(2A) receptor. European Journal of Pharmacology, 678(1–3), 78–85. 10.1016/j.ejphar.2011.12.043 Epub 2012 Jan 12 [DOI] [PubMed] [Google Scholar]
- Vuolo, F. , Abreu, S. C. , Michels, M. , Xisto, D. G. , Blanco, N. G. , Hallak, J. E. , … Pizzichinni, E. (2019). Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. European Journal of Pharmacology, 843, 251–259. 10.1016/j.ejphar.2018.11.029 Epub 2018 Nov 24 [DOI] [PubMed] [Google Scholar]
- Wang, B. , Kovalchuk, A. , Dongping, L. , Ilnytskyy, Y. , Kovalchuk, I. , & Kovalchuk, O. (2020). In search of preventative strategies: Novel antiinflammatory high‐CBD Cannabis sativa extracts modulate ACE2 expression in COVID‐19 gateway tissues Preprints, 2020040315. 10.20944/preprints202004.0315.v1 [DOI] [PMC free article] [PubMed]


