Abstract
In late December 2019, coronavirus disease 2019 (COVID‐19) first broke out in Wuhan, China, and has now become a global pandemic. However, there is no specific antiviral treatment for COVID‐19. This study enrolled 33 COVID‐19 patients in the nineth hospital of Nanchang from 27th January to 24th February 2020. Clinical indexes of patients upon admission/discharge were examined. Patients were divided into two groups according to different treatment plans (danoprevir and lopinavir/ritonavir). The days to achieve negative nucleic acid testing and the days of hospital stays were counted and statistically analyzed. COVID‐19 patients treated with danoprevir or lopinavir/ritonavir were all improved and discharged. Indexes like blood routine, inflammation and immune‐related indexes were significantly recovered after treatment. Additionally, under the circumstance that there was no significant difference in patients' general information between the two groups, we found that the mean time to achieve both negative nucleic acid testing and hospital stays of patients treated with danoprevir were significantly shorter than those of patients with lopinavir/ritonavir. Collectively, applying danoprevir is a good treatment plan for COVID‐19 patients.
Keywords: COVID‐19, danoprevir, lopinavir/ritonavir, time to achieve negative nucleic acid testing
Highlight
-
1.
The average time to achieve negative nucleic acid testing of COVID‐19 patients treated with danoprevir was obviously shorter than that of patients treated with lopinavir/ritonavir.
-
2.
The average time of hospital stays of COVID‐19 patients treated with danoprevir was significantly shorter than that of patients treated with lopinavir/ritonavir.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is caused by a novel severe acute respiratory syndrome coronavirus (SARS‐CoV‐2), a causative agent of a potentially fatal disease, and it has become a great global public health concern. 1 From late 2019 to early 2020, the novel coronavirus SARS‐CoV‐2 suddenly broke out among ordinary people in Wuhan, China, and rapidly spread in a short period of time. According to the data released by the World Health Organization (WHO), more than 2 540 000 cases were reported worldwide by 23 April 2020, among which 175 000 patients died. The massive outbreak of COVID‐19 has caused numerous casualties and a huge economic loss. Therefore, it is vital to make effective treatment plans as soon as possible and speed up the coordination of prevention and treatment, so as to protect people's health and reduce economic loss.
The symptoms of COVID‐19 infections occur after an average incubation period of approximately 5.2 days. 2 The most common clinical symptoms include fever, dry cough, myalgia, fatigue, dyspnea, and diarrhea, 3 which are generally similar to the symptoms caused by β‐coronavirus. 4 Studies have indicated that clinical indexes such as patients' blood indexes are important indicators essential for the diagnosis and treatment of COVID‐19. In patients with COVID‐19, there is an increase in white blood cell (WBC) count and the plasma pro‐inflammatory cytokine level. For example, a prior study showed that the C‐reactive protein (CRP) of a COVID‐19 patient was 16.16 mg/L, which was above the normal range (0‐10 mg/L), with a high erythrocyte sedimentation rate (ESR) and D‐dimer level. 5 Besides this, studies found that the lymphocyte count and its percentage were significantly decreased in severe COVID‐19 patients relative to those in mild patients. 4 , 6 Moreover, the levels of cytokines and chemokines in the blood of COVID‐19 patients were significantly elevated, while some severe COVID‐19 patients showed high levels of pro‐inflammatory cytokines, such as IL2, IL7, IL10, GCSF, IP10, MCP1, MIP1α, and TNF‐α. 4 Collectively, screening out the indicators with significant differences is of great reference value for the diagnosis and treatment of COVID‐19 patients.
At present, there have been no specific antiviral drugs or vaccines available for treatment of patients with COVID‐19. Therefore, it is desirable to develop appropriate and effective therapeutic drugs. A study has revealed that broad‐spectrum antiviral drugs and HIV protease inhibitors could attenuate the viral infection. 7 Antiviral therapies have been applied in patients, currently. For example, patients in a study were given 75 mg oseltamivir, 500 mg lopinavir, 500 mg ritonavir and 0.25 g ganciclovir twice a day for 3 to 14 days. 8 The in vitro experiments done by Wang et al 9 found that Remdesivir and Chloroquine play inhibitory roles in the growth of SARS‐CoV‐2, and preliminary clinical treatment in COVID‐19 patients showed that chloroquine was an effective drug in improving clinical efficacy and potentiating virus clearance. 10 Danoprevir is a potent hepatitis C virus (HCV) protease inhibitor (NS3/4A, IC50 = 0.29 nM), and was approved and marketed in China in 2018 for the treatment of patients with chronic hepatitis C. The latest research suggested that patients (n = 11) who underwent 4 to 12 days treatment of danoprevir and ritonavir were all discharged. 11 In addition, studies indicated that lopinavir/ritonavir (LPV/r) manifested a favorable effect in fighting against COVID‐19 virus and relieving clinical symptoms, 12 and the combined treatment of traditional Chinese medicine and western medicine could protect COVID‐19 patients from severe kidney damage. 13 However, the efficacy of danoprevir and LPV/r in the treatment of COVID‐19 has not been compared.
In this study, on the basis of the treatment plan and the recovery of 33 COVID‐19 patients in the nineth hospital of Nanchang, we analyzed the clinical conditions of patients treated with danoprevir and LPV/r and made a comparative study concerning the time to achieve negative nucleic acid testing (NAT) and hospital stays between the two groups, so as to determine the efficacy of the two therapeutic drugs.
2. MATERIALS AND METHODS
2.1. Research Object
A total of 33 COVID‐19 patients in the nineth hospital of Nanchang from 27 January to 24 February 2020 were involved in this study, including 11 males and 22 females aged between 18 and 71. Nucleic acid samples were collected from the respiratory tract of patients and were confirmed to be positive by quantitative PCR. The information is detailed in Table 2.
Table 2.
General information of patients in the two groups
| Danoprevir | LPV/r | P value | |
|---|---|---|---|
| Cases | 5 | 28 | |
| Demographic information | |||
| Age (median) | 44 | 43 | .7620 |
| Gender (%) | |||
| Male | 2 (40.0) | 9 (32.1) | 1.0000 |
| Female | 3 (60.0) | 19 (67.9) | |
| Clinical features | |||
| Asymptomatic (%) | |||
| Yes | 3 (60.0) | 10 (35.7) | .3600 |
| No | 2 (40.0) | 18 (64.3) | |
| Fever (%) | |||
| Yes | 1 (20.0) | 13 (46.4) | .3662 |
| No | 4 (80.0) | 15 (53.6) | |
| Cough (%) | |||
| Yes | 1 (20.0) | 12 (42.9) | .6253 |
| No | 4 (80.0) | 16 (57.1) | |
| Chest distress (%) | |||
| Yes | 0 (0.0) | 5 (17.9) | / |
| No | 5 (100.0) | 23 (82.1) | |
| Dyspnoea (%) | |||
| Yes | 0 (0.0) | 1 (3.6) | / |
| No | 5 (100.0) | 27 (96.4) | |
| Fatigue (%) | |||
| Yes | 0 (0.0) | 1 (3.6) | / |
| No | 5 (100.0) | 27 (96.4) | |
| Underlying disease | |||
| Diabetes (%) | |||
| Yes | 0 (0.0) | 4 (14.3) | / |
| No | 5 (100.0) | 24 (85.7) | |
| Hypertension (%) | |||
| Yes | 1 (20.0) | 3 (10.7) | .4996 |
| No | 4 (80.0) | 25 (89.3) | |
| Hepatitis B (%) | |||
| Yes | 0 (0.0) | 5 (17.9) | / |
| No | 5 (100.0) | 23 (82.1) | |
| Clinical classification on admission | |||
| Severity (%) | |||
| Mild | 1 (20.0) | 9 (32.1) | / |
| Moderate | 4 (80.0) | 18 (64.3) | |
| Severe | 0 (0.0) | 1 (3.6) | |
Abbreviation: LPV/r, lopinavir/ritonavir.
2.2. Clinical classification and treatment regimen
The severity of disease was distinguished according to the clinical features of COVID‐19 patients: (1) mild type: the clinical symptoms are mild, and no signs of pneumonia are found on imaging; (2) moderate type: having fever and respiratory symptoms, and signs of pneumonia can be observed on imaging; (3) severe type: adults who meet any of the following criteria: respiratory rate ≥30 times/min, oxygen saturation in resting state ≤93%; arterial partial pressure of oxygen (PaO2)/concentration of oxygen (FiO2) ≤300 mm Hg; pulmonary imaging shows significant progression of lesion >50% within 24 to 48 hours.
All patients were classified into mild, moderate and severe types, and then grouped into the danoprevir group and LPV/r group according to the therapeutic regimen based on the actual situation of patients. The specific treatment regimen was designed as below: danoprevir sodium tablets, 100 mg twice daily, by oral; ribavirin tablets, 1000 mg (weight < 75 kg) or 1200 mg (weight ≥ 75 kg) daily, taken two times, by oral; LPV/r oral solution (80 of 20 mg), adult, 5 ml (400 of 100 mg) twice daily or 10 ml (800 of 200 mg) once daily, with meal. All drugs were given until patients were discharged.
2.3. Evaluation criteria
-
(1)
Blood routine index: WBC (4‐10 × 109/L); lymphocyte count (0.8‐4.0 × 109/L); eosinophilic granulocyte count (EOC, 0.05‐0.5 × 109/L); ferritin (male: 16‐220 ng/mL, female: 10‐124 ng/mL); D‐dimer (0‐0.6 mg/L).
-
(2)
Inflammation‐related indexes: CRP (<10 mg/L); ESR (0‐15 mm/h).
-
(3)
Immune‐related indexes: immunoglobulin G (IgG, 7‐16 g/L); interleukin‐4 (IL‐4, 0‐2.8 pg/mL); interleukin‐6 (IL‐6, 0‐5.3 pg/mL); interleukin‐10 (IL‐10, 0‐4.91 pg/mL); CD8 + % (15‐44); CD4 + % (27‐51); CD16 + %CD56 + % (7‐40).
-
(4)
Negative NAT detected by PCR.
-
(5)
The hospital days of patients.
2.4. Statistical analysis
All data were processed by Graphpad Prism 6.0 software (Graphpad Prism, San Diego, CA). Enumeration data of patients in the two groups were analyzed by Fisher exact test, while measurement data were presented as mean ± standard deviation and were analyzed by t test. P < .05 was considered statistically significant.
3. RESULTS
3.1. Comparison of clinical indexes upon admission and discharge
In this study, we enrolled 33 COVID‐19 cases in the nineth hospital of Nanchang, and nucleic acid samples were collected from the respiratory tract of patients and confirmed to be positive. We statistically analyzed the blood routine and immune‐related indexes of the patients on admission/discharge for the purpose of direct comparison of therapeutic outcomes. As shown in Table 1, the abnormal proportions (the percentage of patients with abnormal clinical indexes in total number of patients) of clinical indexes were evidently lower in patients on discharge than those in patients on admission. Besides this, several indexes (WBC count, lymphocyte count, CRP, IL‐6, IL‐10, and CD4 + %) of patients on admission and discharge showed a significant difference (P < .05). Blood routine and some immune indexes are commonly used for disease diagnosis, as they can effectively determine the patients' physical conditions. Based on the clinical data, it turned out that most indexes of patients recovered to normal after treatment, suggesting that our treatment had favorable outcomes. Collectively, most blood routine and immune‐related indexes recovered to normal in patients who could be discharged.
Table 1.
Comparison of clinical indexes upon admission and discharge
| Indexes | Admission | Discharge | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Case | Normal | Abnormal | Abnormal proportion | Case | Normal | Abnormal | Abnormal proportion | ||
| WBC count | 33 | 20 | 13 | 0.3939 | 32 | 28 | 4 | 0.1250 | .0227* |
| Lymphocyte count | 33 | 19 | 14 | 0.4242 | 32 | 28 | 4 | 0.1250 | .0116* |
| ESO count | 33 | 8 | 25 | 0.7576 | 33 | 14 | 19 | 0.5758 | .1912 |
| CRP | 26 | 9 | 17 | 0.6538 | 16 | 14 | 2 | 0.1250 | .0012* |
| Ferritin | 20 | 13 | 7 | 0.3500 | 13 | 13 | 0 | 0.0000 | / |
| ESR | 23 | 2 | 21 | 0.9130 | 10 | 4 | 6 | 0.6000 | .0534 |
| IgG | 22 | 13 | 9 | 0.4091 | 14 | 7 | 7 | 0.5000 | .7343 |
| D‐dimer | 27 | 10 | 17 | 0.6296 | 19 | 8 | 11 | 0.5789 | .7670 |
| IL‐4 | 31 | 0 | 31 | 1.0000 | 18 | 5 | 13 | 0.7222 | / |
| IL‐6 | 31 | 4 | 27 | 0.8710 | 18 | 9 | 9 | 0.5000 | .0074* |
| IL‐10 | 31 | 3 | 28 | 0.9032 | 18 | 7 | 11 | 0.6111 | .0255* |
| CD8 + % | 20 | 18 | 2 | 0.1000 | 16 | 15 | 1 | 0.0625 | 1.0000 |
| CD4 + % | 22 | 6 | 16 | 0.7273 | 16 | 13 | 3 | 0.1875 | .0025* |
| CD16 + %CD56 + % | 17 | 11 | 6 | 0.3529 | 14 | 10 | 4 | 0.2857 | 1.0000 |
Note: Normal reference value: WBC count: (4‐10) × 109/L; lymphocyte count: (0.8‐4.0) × 109/L; ESO count: (0.05‐0.5) × 109/L; ferritin: male (16‐220) ng/mL, female (10‐124) ng/mL; ESR: (0‐15) mm/h; IgG: (7‐16) g/L; D‐dimer: (0‐0.6) mg/L; IL‐4: (0‐2.8) pg/mL; IL‐6: (0‐5.3) pg/mL; IL‐10: (0‐4.91) pg/mL; CD8 + %: 15‐44; CD4 + %: 27‐51; CD16 + %CD56 + %: 7‐40. Above or below the normal reference value is considered as abnormal.
Abbreviations: CRP, C‐reactive protein; ESO, eosinophil count; ESR, erythrocyte sedimentation rate; IgG, immunoglobulin G; IL‐4, interleukin‐4.
P < .05 was considered statistically significant.
3.2. Comparison of general information between the two groups
The 5 cases of 33 COVID‐19 patients were given danoprevir, and 28 cases of 33 COVID‐19 patients were given LPV/r. Our study included demographic information, clinical features on admission, underlying medical disease and clinical classification on admission (Table 2). Most COVID‐19 patients showed some clinical features upon admission, among which fever (58%) and cough (61%) were the most common symptoms. Symptoms like chest distress, dyspnoea and fatigue mainly appeared in patients over 40 years. On the basis of clinical diagnosis, patients were divided into mild type, moderate type and severe type. The moderate cases were the main subject of our study. To compare the efficacy of danoprevir and LPV/r in treating COVID‐19, we performed statistical analysis on the general information of the patients in the two groups. In terms of significance, there was no significant difference (P > .05) in patients' general information between the two groups, which is conducive to the subsequent assessment of the efficacy of two drugs.
3.3. Comparison of the time to achieve negative NAT and hospital stays between the two groups
Finally, we analyzed the time to achieve negative NAT and hospital stays of patients in the two groups. All patients in the two groups were monitored until they were discharged. The time for two consecutive negative NAT of throat swab and hospital stays of COVID‐19 patients were analyzed and compared. As illustrated in Table 3, it was found that in the danoprevir group, the first patient had a negative NAT on the fourth day, and all patients had a negative result within 13 days, with a median time of 7 days and average time of 8 days. However, in the LPV/r group, the first patient had a negative NAT result on the fifth day, and all patients had a negative result within 21 days, with a median time of 12 days and average time of 12.5 days. The results suggested that there was no marked difference (P > .05) in the median time to achieve negative NAT between the two groups, while the average time showed a significant difference (P < .05). After being treated with the two different drugs, the percentage of patients with a positive NAT was significantly decreased (P < .05) in the danoprevir group relative to that in the LPV/r group (Figure 1A). Similarly, the hospital stays of patients in the danoprevir group ranged from 7 to 21 days, with a median time of 9 days and an average of 11.4 days. However, in the LPV/r group, the hospital stays ranged from 8 to 23 days, with the median and average time of 17 days and 16.7 days, respectively. There was no marked difference (P > .05) in the median hospital stays of patients in the two groups, while the average time showed a significant difference (P < .05). Moreover, the discharge rate of patients in the danoprevir group was higher than that in the LPV/r group as shown in Figure 1B (P < .05). Taken together, our data demonstrated that the efficacy of danoprevir was better than that of LPV/r in the treatment of COVID‐19.
Table 3.
Comparison of time to achieve negative NAT and hospital stays of COVID‐19 patients in the two groups
| Danoprevir | LPV/r | P value | |
|---|---|---|---|
| Cases | 5 | 28 | |
| Negative NAT, d | |||
| Median | 7.0 | 12.0 | .7903 |
| Average | 8.0 | 12.5 | .0388* |
| Hospital stays, d | |||
| Median | 9.0 | 17.0 | .6802 |
| Average | 11.4 | 16.7 | .0324* |
Abbreviations: COVID‐19, coronavirus disease 2019; LPV/r, lopinavir/ritonavir; NAT, nucleic acid testing.
P < .05 was considered statistically significant.
Figure 1.
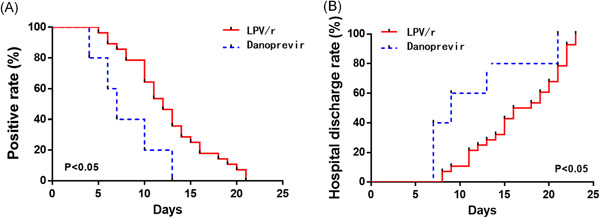
Comparison of time to achieve negative nucleic acid testing (NAT) and hospital stays between the two groups. The rate of positive results (A) and the hospital discharge rate (B) of patients in the danoprevir group and LPV/r group were compared; P <.05 was considered statistically significant
4. DISCUSSION
Coronaviruses are enveloped single‐stranded RNA viruses that are widely distributed in humans and animals throughout the world. 4 Coronaviruses are one of the major pathogens targeting the human respiratory system. In the past two decades, there were two events similar to COVID‐19 pandemic caused by cross‐infection between animals with β‐coronavirus and humans. The first instance was SARS emerging in 2002‐2003, which was caused by a new β‐coronavirus (SARS‐CoV) originating from bats. SARS‐CoV was transmitted to humans with palm civet cats as intermediate hosts in Guangdong province, resulting in a total of 8422 infections in Chinese mainland and Hongkong and 916 deaths with a mortality of 11%. 14 Another one was the Middle East Respiratory Syndrome (MERS) in 2012 caused by MERS‐CoV, which is also derived from bats. It emerged in Saudi Arabia with camels as intermediate hosts and caused 2494 infections and 858 deaths with a mortality of 34% (https://www.who.int/emergencies/mers-cov/en/. Accessed 16 February 2020). SARS‐CoV and MERS‐CoV are both recognized to be major threats to public health. SARS‐CoV‐2, which also originated from bats, manifests variable clinical features from asymptomatic status to acute respiratory distress syndrome and multiple organ dysfunction. The epidemiological and clinical features of COVID‐19 suggest a higher transmissivity and a lower mortality rate compared to those of SARS. 8 , 15 Despite the overall mortality rate of COVID‐19 being lower than SARS or MERS, the mortality rate of severe cases with COVID‐19 is rather worrying. 16 In today's global pandemic, strict prevention and effective treatment of COVID‐19 allow of no delay.
Based on the experience accumulated in treating SARS and MERS, antiviral drugs such as ribavirin, LPV/r, arbidol and remdeswir (a broad‐spectrum anti‐RNA drug developed for Ebola) have been applied in treatment of COVID‐19 patients. 17 , 18 In the present study, 33 cases were given danoprevir or LPV/r and grouped. The abnormal proportions of clinical indexes of patients upon discharge were significantly decreased compared to those of patients on admission, suggesting the improved condition of patients. These two kinds of treatment plan had a favorable effect on COVID‐19 patients.
Danoprevir is an HCV protease inhibitor which can be used in the treatment of chronic hepatitis C. Ritonavir is a CYP3A4 inhibitor that increases blood concentration of Danoreivir. In a study on ritonavir, 11 COVID‐19 patients met the following criteria and could be discharged: (1) normal body temperature for at least 3 days; (2) significantly improved respiratory symptoms; (3) lung imaging showed obvious absorption and recovery of acute exudative lesions; (4) two consecutive negative tests of SARS‐CoV‐2 nucleic acid. 11 However, there have been no comparisons in the therapeutic effect between danoprevir and other drugs in the clinical treatment of COVID 19. LPV, a human immunodeficiency virus 1 (HIV‐1) protease inhibitor, usually extends its half‐life by inhibiting cytochrome P4507 in combination with ritonavir. Studies have indicated that LPV at a high dose (9.6 g/mL) or a low dose (5.5 g/mL) can inhibit SARS‐CoV, and it also plays an inhibitory role in the replication cycle of MERS‐CoV. 19 , 20 Although most studies focus on the efficacy of LPV, we also need to be aware of the adverse reactions like diarrhea, nausea and weakness, which are common in patients. Also, there was a report revealing that some patients exhibited elevated total bilirubin, triglyceride and liver enzyme levels after the treatment with LPV. 21
To better compare the efficacy between danoprevir and LPV/r in clinical practice and to provide guidance for clinical treatment of COVID‐19 and other pneumonia, we analyzed 33 COVID‐19 patients and divided them into two groups (danoprevir group and LPV/r group). Before that, we first analyzed the general information of patients in the two groups to exclude the interference of age, gender, underlying diseases, clinical symptoms and other factors (Table 2). Our findings illustrated that the time to achieve two consecutive negative NAT and hospital stays of patients in the danoprevir group were shorter than those of patients in the LPV/r group, especially the average negative result time and hospital stays (P < .05).
Although many drugs including protease inhibitors and RNA‐dependent RNA polymerase (RDRP) inhibitors are undergoing clinical trials in China and the rest of the world, yet no drug has been approved for the treatment of COVID‐19 by the regulatory authorities of major countries. In this study, by analyzing the clinical data of 33 cases (10 mild cases, 22 moderate cases, and 1 severe case) in two different treatment groups, we found that danoprevir produced a superior efficacy to LPV/r. In conclusion, our study suggests that it is a correct choice to use danoprevir in the treatment of patients with COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
ACKNOWLEDGEMENT
This study was supported by the funds from Science and Technology Bureau of Nanchang City (202033).
Zhang Z, Wang S, Tu X, et al. A comparative study on the time to achieve negative nucleic acid testing and hospital stays between danoprevir and lopinavir/ritonavir in the treatment of patients with COVID‐19. J Med Virol. 2020;92:2631–2636. 10.1002/jmv.26141
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are included within the article. The data and materials in the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. 10.1016/j.jaut.2020.102433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382:1199‐1207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu YC, Chen CS, Chan YJ. Overview of the 2019 novel coronavirus (2019‐nCoV): The pathogen of severe specific contagious pneumonia (SSCP). J Chin Med Assoc. 2020;83:217‐220. 10.1097/JCMA.0000000000000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lei J, Li J, Li X, Qi X. CT Imaging of the 2019 novel coronavirus (2019‐nCoV) pneumonia. Radiology. 2020;295(18):18. 10.1148/radiol.2020200236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen X, Yang Y, Huang M, et al. Differences between COVID‐19 and suspected then confirmed SARS‐CoV‐2‐negative pneumonia: a retrospective study from a single center. J Med Virol. 2020. 10.1002/jmv.25810 [DOI] [PubMed] [Google Scholar]
- 7. Lu H. Drug treatment options for the 2019‐new coronavirus (2019‐nCoV). Biosci Trends. 2020;14:69‐71. 10.5582/bst.2020.01020 [DOI] [PubMed] [Google Scholar]
- 8. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30:269‐271. 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72‐73. 10.5582/bst.2020.01047 [DOI] [PubMed] [Google Scholar]
- 11. Chen H, Zhang Z, Wang, et al. First clinical study using HCV protease inhibitor danoprevir to treat naïve and experienced COVID‐19 patients. medRxiv. 2020. 10.1101/2020.03.22.20034041 [DOI] [PMC free article] [PubMed]
- 12. Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64‐68. 10.5582/bst.2020.01030 [DOI] [PubMed] [Google Scholar]
- 13. Li L. The novel coronavirus SARS‐CoV‐2: high pathogenecity and its prevention and therapy. Basic Clin Med. 2020;40, 10.16352/j.issn.1001-6325.20200420.002 [DOI] [Google Scholar]
- 14. Chan‐Yeung M, Xu RH. SARS: epidemiology. Respirology. 2003;8(suppl):S9‐S14. 10.1046/j.1440-1843.2003.00518.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395:514‐523. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li T, Lu H, Zhang W. Clinical observation and management of COVID‐19 patients. Emerg Microbes Infect. 2020;9:687‐690. 10.1080/22221751.2020.1741327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singhal T. A review of coronavirus disease‐2019 (COVID‐19). Indian J Pediatr. 2020;87:281‐286. 10.1007/s12098-020-03263-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929‐936. 10.1056/NEJMoa2001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yao TT, Qian JD, Zhu WY, Wang Y, Wang GQ. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus‐A possible reference for coronavirus disease‐19 treatment option. J Med Virol. 2020. 10.1002/jmv.25729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Wilde AH, Jochmans D, Posthuma CC, et al. Screening of an FDA‐approved compound library identifies four small‐molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875‐4884. 10.1128/AAC.03011-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim UJ, Won EJ, Kee SJ, Jung SI, Jang HC. Combination therapy with lopinavir/ritonavir, ribavirin and interferon‐alpha for Middle East respiratory syndrome. Antivir Ther. 2016;21:455‐459. 10.3851/IMP3002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article. The data and materials in the current study are available from the corresponding author on reasonable request.


