1.
To the Editor,
As well as age, co‐morbidities including cardiovascular diseases and hypertension, malignancies and diabetes, and other host‐related factors are adverse prognostic factors for COVID‐19 disease. 1 Asthma is a heterogenous condition, characterized by a type 2 eosinophilic inflammation in more than 50% of those with a formal asthma diagnosis. Despite a prevalence of 4.2%, surprisingly to us, asthma was not listed in co‐morbidities in a Chinese study on 140 hospitalized patients. 2 These findings are consistent with our observation of a low occurrence of asthmatics among admitted COVID‐19 cases (3 out of 275 individuals, one requiring ICU) in Prato (Italy), a city with 200 000 inhabitants of whom at least 10 000 are expected to be asthmatic. Further, none of the 2500 asthmatic patients referring to our Allergy Unit has been hospitalized. To date, there are no published reports of other type 2 conditions associated with severe COVID‐19.
Herein, we share some clues supporting the hypothesis that type 2 conditions do not represent a risk factor, despite the most morbidity occurring due to SARS‐CoV‐2 induced lung damage.
Immune responses to viruses are characterized by initial activation of innate immunity and production of type I and III Interferons (IFN‐α/β and ‐λ, respectively), crucial to control propagation. 3 Upon stimulation with viruses, plasmacytoid dendritic cells (pDCs) are the predominant source of IFN‐α produced in the peripheral blood, despite representing at most 0.2%‐0.8% of the circulating mononuclear cells. 3 , 4 Intriguingly, a defective production of IFNs by pDC and epithelial cells has been described in severe atopic patients 4 , 5 with a consequent delayed and inefficient antiviral defence. IFNs serve as negative regulators of Th2 development, differentiation and function. 6 A negative link exists between IgE and IFN‐α, since IgE cross‐linking down‐regulates Toll‐like receptor (TLR) 9 expression and dampens TLR‐7 signalling, abrogating viral‐induced type I and III IFN production from pDCs. Of note, the magnitude of the IFN‐α responses after ex vivo viral challenge of pDCS is inversely related to serum IgE levels. Furthermore, pDCs are sensitive to histamine through H2 receptors, which also enhance this negative regulatory pathway 4 (Figure 1). This suggests that pDC antiviral responses may be suppressed in a similar way in atopy. Indeed, asthmatics have a greater susceptibility to respiratory viral infections which may be a trigger for exacerbations. 7 However, the Th2‐dominant environment might be also protective, able to down‐regulate the late phase hyper‐inflammation which typically marks severe respiratory viral diseases, when the viral load decreases but immunopathologic events are the hallmarks of tissue damage. This seems to be particularly the case in SARS‐CoV infections.
FIGURE 1.
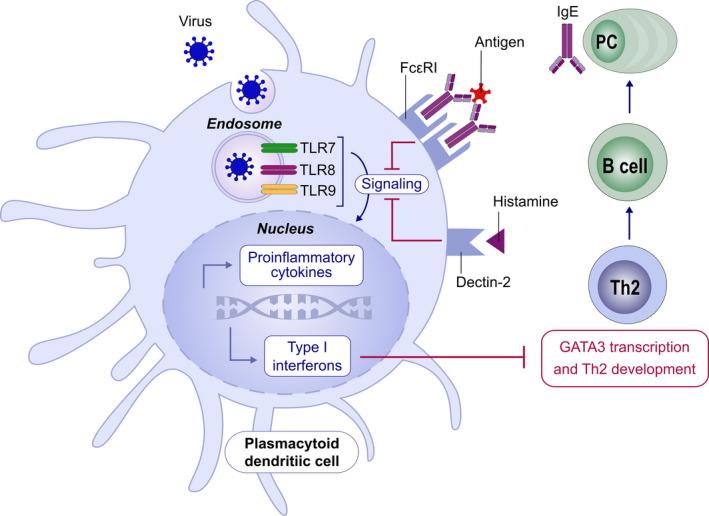
Counterregulation Between Allergic and IFN‐α Pathways
In SARS‐CoV‐1 infection, poor outcomes were linked to an early robust type I IFN expression together with high levels of IFN‐related chemokines (ie CXCL‐10) and IFN‐ɣ. 8 This dysregulated and hyperactivated innate response may preclude a regular switch towards protective adaptive immune responses as speculated by Richardson in COVID‐19 patients 9 and demonstrated in a mouse model of SARS‐CoV infection where early (or absent/low) IFN signalling appeared beneficial in preventing monocyte/macrophage lung infiltration, vascular leakage, cytokine storm and impaired T‐cell responses. 3 Thus, timing and robustness of type I IFN production is able to affect the outcome.
Further, the role of eosinophils, foes in asthma but possibly friends in COVID‐19 infected lungs, needs to be established. Eosinophils are reduced in peripheral blood of SARS‐CoV‐2‐infected patients. 2 It is tempting to speculate that increased numbers of eosinophils in the airways of asthmatic patients might be protective against the exaggerated inflammatory responses of the severe COVID‐19 phenotype.
There is reason to suggest here that antiviral and immunomodulatory activities of inhaled asthma medications (with particular focus on steroids) should be investigated, expanding previous evidence from past coronaviruses. 10 , 11
Although our knowledge on COVID‐19 pathogenesis and risk factors is “work in progress” and currently mainly based on preliminary data, it is striking to us that there is currently no evidence of an increased risk of poor/fatal outcome in asthmatics and particularly in severe/uncontrolled patients. Like other respiratory viral infections, coronaviruses might exacerbate asthma symptoms, particularly in severe or uncontrolled patients but we suggest that a Th2‐skewed immunity may be protective against severe COVID‐19 disease, owing to the cross‐regulation between allergic and interferon‐mediated immune responses (Figure 2).
FIGURE 2.
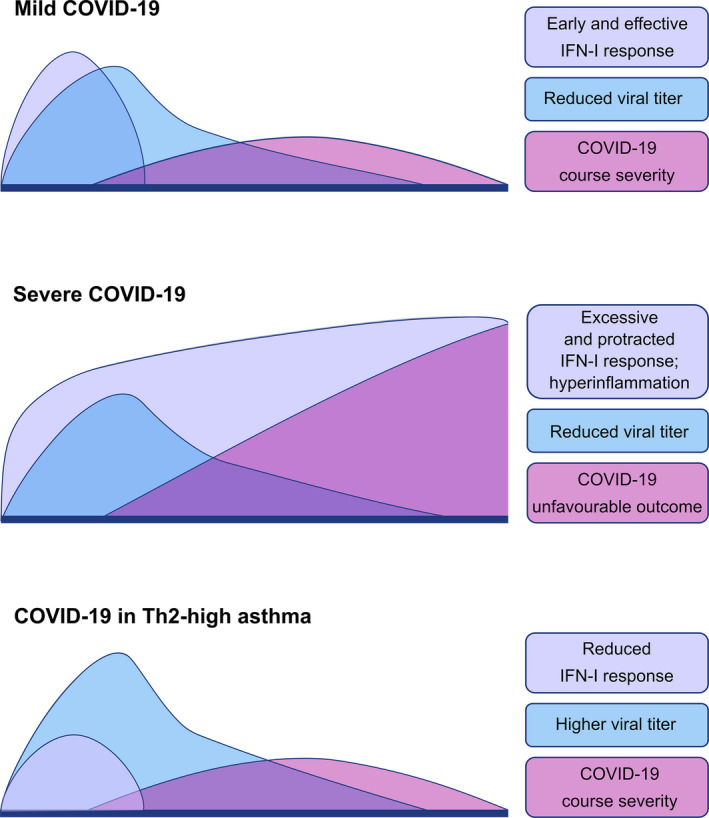
Suggested Type I Interferon responses in SARS‐Cov‐2 infection in non‐asthmatic and Th2 high asthmatic patients
CONFLICTS OF INTEREST
Dr Carli, Dr Cecchi, Prof. Parronchi and Dr Farsi have nothing to disclose. Prof. Stebbing's conflicts of interest can be found at https://www.nature.com/onc/editors and none are relevant here.
REFERENCES
- 1. W‐jie G, W‐hua L, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid‐19 in China: A Nationwide Analysis. Eur Respir J. 2020;55(5):2000547. 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75:1730‐1741. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 3. Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated Type I interferon and inflammatory monocyte‐macrophage responses cause lethal Pneumonia in SARS‐CoV‐Infected Mice. Cell Host Microbe. 2016;19(2):181‐193. 10.1016/j.chom.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gonzales‐van Horn SR, Farrar JD. Interferon at the crossroads of allergy and viral infections. J Leukoc Biol. 2015;98(2):185‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lebre MC, van Capel TM, Bos JD, Knol EF, Kapsenberg ML, de Jong EC. Aberrant function of peripheral blood myeloid and plasmacytoid dendritic cells in atopic dermatitis patients. J Allergy Clin Immunol. 2008;122(5):969‐976. [DOI] [PubMed] [Google Scholar]
- 6. Maggi E, Parronchi P, Manetti R, et al. Reciprocal regulatory effects of IFN‐gamma and IL‐4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992;148(7):2142‐2147. [PubMed] [Google Scholar]
- 7. Gern . JE . How rhinovirus infections cause exacerbations of asthma. Clin Exp Allergy. 2015;45:32‐42. [DOI] [PubMed] [Google Scholar]
- 8. Cameron MJ, Ran L, Xu L, et al. Interferon‐mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J Virol. 2007;81(16):8692‐8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richardson PJ, Corbellino M, Stebbing J. Baricitinib for COVID‐19: a suitable treatment? – Authors' reply. Lancet Infect Dis. 2020;19:1473‐3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamaya M, Nishimura H, Deng X, et al. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV‐229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Resp Investig. 2020;58(3):155‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lam KP, Chu YT, Lee MS, et al. Inhibitory effects of albuterol and fenoterol on RANTES and IP‐10 expression in bronchial epithelial cells. Pediatr Allergy Immunol. 2011;22(4):431‐439. [DOI] [PubMed] [Google Scholar]
ACKNOWLEDGEMENTS
We thank Dr Massimo Edoardo Di Natale and Dr Pamela Lotti who contributed to data acquisition about COVID‐19 admitted patients in Prato.


