Abstract
While several studies from China have reported COVID‐19‐related liver injury, there are currently no data on liver dysfunction in hospitalized COVID‐19 patients in Europe. The aim of this study was to describe the prevalence and predictive value of abnormal liver function in patients hospitalized with COVID‐19. This was a retrospective cohort study of confirmed COVID‐19 patients hospitalized in two referral hospitals in France. Clinical, biological and radiological data were collected and analysed. In all, 234 patients confirmed to have COVID‐19 by RT‐PCR were included. Liver function was abnormal in 66.6% of patients on admission. In multivariate logistic regression, abnormal liver test on admission were associated with in‐hospital aggravation (OR = 4.1, 95% CI 1.5‐10.8; P = .004) and mortality (OR 3.3; 95% CI = 1.04‐10.5; P = .04). This study of liver tests in a European COVID‐19 population confirms a high prevalence of abnormal liver tests on admission that are predictive of severe disease course and higher in‐hospital mortality.
Keywords: abnormal liver tests, Coronavirus disease 2019, in‐hospital aggravation, in‐hospital mortality, prognosis, SARS‐CoV‐2
Abbreviations
- ACE‐2
angiotensin‐converting enzyme 2
- ALP
alkaline phosphatase
- ALT
alanine transaminase
- BMI
body mass index
- CEI
angiotensin‐converting enzyme inhibitors
- CI
confidence interval
- COVID‐19
Coronavirus disease 2019
- CRP
C‐reactive protein
- CT
computed tomography
- CU
intensive care unit
- DILI
drug‐induced liver injury
- GGT
gamma‐glutamyl transferase
- OR
odds ratio
- RB
angiotensin receptor blockers
- RT‐PCR
reverse transcription polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus‐2
- SD
standard deviation
- ST
aspartate aminotransferase
- TBil
total bilirubin
- ULN
upper limit of normal
Key points.
14% to 76.3% of Coronavirus disease 2019 (COVID‐19) patients have abnormal liver function tests.
Patients with abnormal liver tests on admission were more likely to need ICU admission, intubation and mechanical ventilation.
Abnormal liver tests on admission were associated with severe disease upon admission and inhospital mortality.
ACEI/ARB exposure was not associated with abnormal liver tests on admission nor with clinical outcomes.
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) mainly causes acute respiratory disease, but it can also cause multiple organ failure. 14% to 76.3% of Coronavirus disease 2019 (COVID‐19) patients have abnormal liver function tests, 1 , 2 , 3 , 4 , 5 , 6 , 7 and these patients have been reported to be more likely to progress to severe pneumonia. However, these data come from Chinese populations, and there are no specific data on COVID‐19 patients in Europe. This knowledge gap prompted us to study the prevalence of abnormal liver function tests and the relationship between abnormal liver function and clinical outcome in a multicentre cohort study of patients hospitalized with COVID‐19 in France.
2. METHODS
We retrospectively studied a cohort of COVID‐19 patients hospitalized in two COVID‐19 referral hospitals in France (CHU Montpellier and CH Narbonne). All consecutive patients admitted to both hospitals from 10 March to 18 April 2020 with confirmed SARS‐CoV‐2 infection, according to WHO guidelines 8 by positive result of reverse transcription polymerase chain reaction (RT‐PCR) in nasopharyngeal samples, were included. Clinical, biological and chest computed tomography (CT) data were collected. On admission, every patient was screened for comorbidities, antihypertensive therapy, immunosuppression status, underlying liver disease, elapsed time from symptoms onset to hospitalization, laboratory findings including platelets, C‐reactive protein, liver function tests, serum albumin, procalcitonin, D‐dimer and lymphocyte count. During hospitalization, data on need for mechanical intubation, initial hospitalization service, need for intensive care unit hospitalization, length of hospital stay, COVID‐specific drug treatment were collected. Clinical outcomes included type of first admission unit, invasive mechanical ventilation and status deceased or alive.
Abnormal liver function was defined as any parameter over the upper limit of normal (ULN): alanine transaminase (ALT) >41 U/L, aspartate transaminase (AST) >40 U/L, alkaline phosphatase (ALP) >130 U/L, gamma‐glutamyl transferase (GGT) >60 U/L and total bilirubin (TBil) >21 µmol/L. All patients were classified into severe or non‐severe COVID‐19 pneumonia at the time of admission according to current guidelines. 8 Severe cases were defined by fever or suspected respiratory infection, plus one of the following: respiratory rate >30 breaths/min; severe respiratory distress; or SpO2 < 93% on room air. In‐hospital aggravation was defined by any of the following characteristics established in hospital but not present on admission: (a) oxygen saturation at rest <93%; (b) need for mechanical ventilation; (c) acute respiratory distress syndrome (PaO2/FiO2 < 300, >30 breaths/min) and (d) any organ failure or shock requiring intensive care management.
The ethics committee of the University Hospital of Montpellier granted ethical approval and patients gave informed consent.
Statistical analyses were performed using XLSTAT 2019.3.2. Continuous variables were expressed as mean and standard deviation (SD). Differences in non‐Gaussian distributions were assessed with Mann‐Whitney U test and normal distributions with Student's t test. Categorical variables were expressed as percentages and analysed using chi‐square test. Survival data were analysed with Kaplan‐Meier plots and log‐rank tests, taking time‐to‐event data into account. Multivariate logistic regression was performed to identify risk factors associated with in‐hospital aggravation and death. Variables included in the model were as follows: abnormal liver test, CRP, comorbidities and age after their validation in univariate analysis. Before entering these variables, multicollinearity was excluded. The contribution of each variable to the risk of developing the endpoint is reported as odds ratio (OR) with 95% confidence interval (CI). A P value <.05 was considered significant.
3. RESULTS
During the study period, 300 patients were admitted in COVID‐19 units of the two referral hospitals. In all, 234 patients were included in the final analysis after being confirmed positive for SARS‐CoV‐2 by RT‐PCR in nasopharyngeal samples (Figure S1). In total, 149 patients (63.7%) were men, and the mean (SD) age was 67 (14) years (Table 1). In all, 156 (66.6%) patients had abnormal liver function tests on admission, and 22 (9.4%) patients presented with ALT > 2‐fold ULN or TBil > ULN. In all, 175 (74.8%) patients had at least one comorbidity. In total, 108 (46.2%) patients had arterial hypertension and 68 (29%) were treated with either angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs). Current treatment with ACEIs or ARBs did not impact disease severity or clinical outcomes (Table S1). In a univariate analysis, patients with abnormal liver tests on admission were more likely to need ICU admission, intubation and mechanical ventilation. Also, abnormal liver tests on admission were associated with severe disease upon admission and in‐hospital mortality (Table 1). The survival probability 25 days after hospital admission was significantly worse in patients with abnormal liver tests on admission compared to patients with normal liver tests (Figure 1). In a multivariate logistic regression analysis, abnormal liver tests on admission were strongly associated with in‐hospital aggravation (OR = 4.1, 95% CI 1.5‐10.8; P = .004) together with older age (OR 1.037, 95% CI 1.007‐1.067, P = .007) and higher CRP (OR 1.037, 95% CI 1.003‐1.011, P = .001) but not comorbidities (P > .05) (Table S2). Also abnormal liver function tests on admission were independently associated with in‐hospital mortality (OR 3.3; 95% CI = 1.04‐10.5; P = .04) together with older age (OR 1.14; 95% CI 1.08‐1.21; P = .001) and higher CRP (OR 1.007, 95% CI 1.002‐1.013; P = .009) (Table S3).
TABLE 1.
Clinical and biological characteristics and outcomes of patients with COVID‐19
| Characteristics | Total (n = 234) | Normal liver function (n = 78) | Abnormal liver function (n = 156) | P value |
|---|---|---|---|---|
| Age, mean ± SD, y | 67 ± 14 | 69 ± 14 | 66.5 ± 14 | .21 |
| Sex | ||||
| Male, N (%) | 149 (63.7) | 40 (26.8) | 109 (73.2) | |
| Comorbidities, N (%) | ||||
| Arterial hypertension | 108 (46.2) | 38 (48.7) | 70 (44.9) | .5 |
| Cardiovascular disease | 80 (33.6) | 23 (29.5) | 57 (36.5) | .28 |
| Diabetes | 64 (27.4) | 25 (32.1) | 39 (25) | .25 |
| Chronic liver disease | 9 (3.8) | 3 (3.8) | 6 (3.8) | .6 |
| Malignancy | 30 (12.8) | 12 (15.4) | 18 (11.5) | .4 |
| Immunosuppression | 16 (6.8) | 4 (5.1) | 12 (7.7) | .5 |
| Active smoking | 13 (5.6) | 3 (3.8) | 10 (6.4) | .44 |
| Chronic alcohol consumption | 12 (5.1) | 5 (6.4) | 7 (4.5) | .5 |
| Any | 59 (25.2) | 16 (20.5) | 43 (27.6) | .24 |
| Antihypertensive therapy on admission | ||||
| ACEI/ARB, N (%) | 68 (29.4) | 20 (25.6) | 48 (30.7) | .055 |
| Other (β‐blockers, diuretics, calcium channel blocking agent, etc), N (%) | 35 (15) | 17 (21.8) | 18 (11.5) | |
| BMI (kg/m2), mean ± SD | 23 (4.5) | 22.1 (4) | 23.5 (4) | .11 |
| Typical symptoms on admission, N (%) (fever, cough, dyspnoea) | 228 (97.4) | 75 (96.2) | 153 (98.1) | .38 |
| Severe disease on admission, N (%) | 114 (49) | 25 (32) | 89 (57) | .0001 |
| Non‐severe disease on admission, N (%) | 120 (51) | 53 (68) | 67 (43) | |
| Time between symptoms onset and hospitalization, d, mean ± SD | 7 (4.8) | 6.5 (5.1) | 7.2 (4.6) | .34 |
| Typical chest CT during hospitalization, a N (%) | 132 (56.4) | 43 (55.1) | 89 (57.1) | .13 |
| Initial hospitalization in ICU, N (%) | 33 (14.1) | 2 (2.5) | 31 (19.9) | .0001 |
| Need for ICU during hospitalization, N (%) | 82 (35) | 14 (17.9) | 68 (43.6) | .001 |
| Intubation and mechanical ventilation during hospitalization, N (%) | 50 (21.4) | 6 (7.7) | 44 (28.2) | .0003 |
| Laboratory tests on admission, mean ± SD | ||||
| AST (U/L) | 45 ± 32 | 24 ± 7 | 57 ± 37.4 | .0001 |
| ALT (U/L) | 37 ± 29 | 19.3 ± 7.8 | 47.2 ± 31.7 | .0001 |
| ALP (U/L) | 79.3 ± 50.5 | 63 ± 20.5 | 87.6 ± 57.5 | .0001 |
| GGT (U/L) | 82 ± 94.8 | 31.8 ± 13 | 106.6 ± 106.4 | .0001 |
| TBil (µmol/L) | 10.4 ± 10.2 | 7.9 ± 3.2 | 11.6 ± 12.1 | .008 |
| CRP (mg/dL) | 116 ± 93.5 | 80.4 ± 65.1 | 135 ± 100 | .0001 |
| Lymphocyte count (×109/L) | 1.09 ± 0.61 | 1.20 ± 0.62 | 1.04 ± 0.60 | .03 |
| D‐dimer (µg/mL) | 1274 ± 1108 | 1233.8 ± 1121.6 | 1292.1 ± 1111.2 | .49 |
| Procalcitonin (ng/ml) | 0.76 ± 1.9 | 0.47 ± 1.5 | 0.89 ± 2.0 | .001 |
| Albumin (g/L) | 34 ± 8.8 | 35 ± 11 | 33 ± 5 | .24 |
| Platelets (×109/L) | 222.7 ± 97 | 232 ± 109.2 | 217 ± 90.6 | .27 |
| ICU length of stay, mean ± SD, d | 7.2 ± 5 | 5.3 ± 5.6 | 7.7 ± 4.8 | .59 |
| Clinical outcomes, N (%) | ||||
| In‐hospital aggravation | 54 (23.1) | 7 (9) | 47 (30.1) | .001 |
| Discharged alive | 149 (63.7) | 52 (66.7) | 97 (62.2) | .09 |
| In‐hospital death | 37 (15.8) | 6 (7.7) | 31 (19.9) | .04 |
Statistically significant values are given in bold.
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ALP, alkaline phosphatase; ALT, alanine transaminase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; BMI, body mass index; CRP, C‐reactive protein; GGT, gamma‐glutamyl transferase; ICU, intensive care unit; max, maximum; min, minimum; SD, standard deviation; TBil, total bilirubin.
According to current guidelines. 9
FIGURE 1.
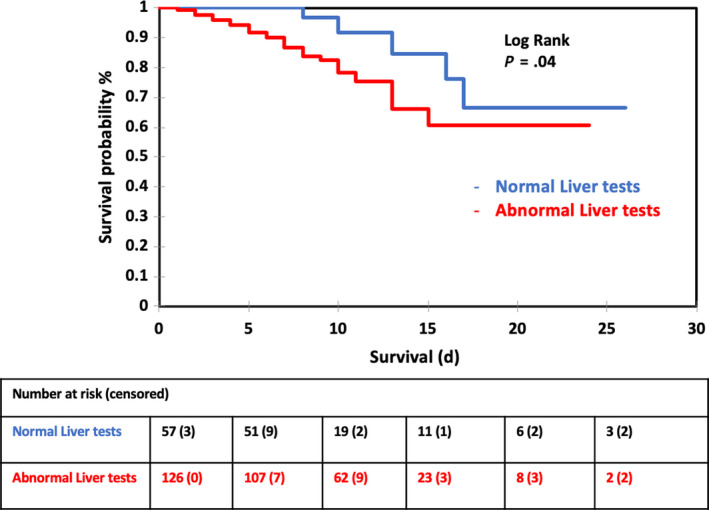
Kaplan‐Meier survival curves comparing COVID‐2 patients with normal and abnormal liver function tests on admission
During hospitalization, specific COVID‐19 drug treatment was prescribed in 125 (53.4%) patients: 86 (36.7%) received hydroxychloroquine, 27 (11.5%) lopinavir/ritonavir and 12 (5.1%) remdesivir respectively. Of the 30 patients discharged and who had normal liver tests on admission, 16 (53.4%) had abnormal liver tests at discharge; 8 (50%) had received hydroxychloroquine and 14 (87.5%) had received antibiotics such as azithromycin, amoxicillin‐clavulanic acid or quinolones. By the end of the study period, 186 (79.5%) patients were discharged, and 48 (20.5%) remained hospitalized.
4. DISCUSSION
Since the start of the COVID‐19 pandemic, several studies have described the disease characteristics including different organ injuries and particularly liver injury. 1 , 2 , 3 , 4 , 5 , 6 , 7 Several mechanisms have been suggested, one being direct liver toxicity through angiotensin‐converting enzyme 2 (ACE‐2) receptors expressed in cholangiocytes. 10 Also, sepsis induced by COVID‐19 may promote cytokine storm and more specifically Interleukin‐6 production which regulates hepatic homeostasis and liver regeneration. 11 Ultimately, COVID‐19‐specific drugs may induce liver injury.
To our best knowledge, this is the first study exploring liver abnormalities in European COVID‐19 patients. In our study, two‐thirds of patients had abnormal liver tests on admission which is slightly higher than reported previously. 1 , 2 , 4 , 6 This is likely to be due to the different and older, more comorbid population who had a higher prevalence of chronic liver disease, diabetes, cardiovascular diseases and arterial hypertension compared to previous Chinese studies. 1 , 12 Although our patients did not have a higher body mass index (BMI) than Chinese cohort, 1 we observed a higher prevalence of severe pneumonia upon admission in our study which may explain higher prevalence of liver tests abnormalities on admission. Only a few of our patients (3.8%) had underlying liver disease, and there were no significantly different comorbidities (alcohol consumption or metabolic syndrome) between the two groups of patients with or without abnormal liver tests on admission, suggesting that liver changes may be COVID‐19 related and not due to pre‐existing liver disease. However, we had little information about viral status (hepatitis B and C viruses) in the whole population, since viral screening does not currently form part of the management. Also drug‐induced liver injury (DILI) could be ruled out as abnormal liver tests pre‐existed to specific COVID‐19 medication. Whether SARS‐CoV‐2 directly infects liver cells is unclear and further studies including liver biopsy specimens may be needed. Patients with abnormal liver test on admission were more frequently directly admitted to ICU, which might suggest that these patients had more severe COVID‐19 disease at presentation. One may expect that it was related to time delay of disease evolution, but we failed to find any difference in elapsed time from symptoms onset to admission to the hospital between the two groups. Nevertheless, abnormal liver tests on admission, together with CRP and age, were independent predictive risk factors for in‐hospital aggravation also reported by Cai et al. 1 Furthermore, abnormal liver tests on admission were independently associated with in‐hospital mortality that underlines once again its reliability to the disease severity. Several studies have linked the disease severity to SARS‐CoV‐2 viral load 13 , 14 but further studies are needed to confirm this hypothesis.
The severity of liver damage was not the main clinical issue in this cohort since the biological abnormalities were moderate in our study; however, these liver abnormalities could be a surrogate marker for the severity of the viral infection and the risk of death. The mechanisms of liver injury in COVID‐19 require further study. We could observe that ACEI/ARB exposure was not associated with abnormal liver tests on admission nor with clinical outcomes which supports actual recommendations to continue them during COVID‐19 pandemic. 15
Our study is limited by the relatively small sample size and its retrospective nature. Information regarding the potential liver toxicity due to COVID‐19 experimental drugs was not assessed. Nevertheless, this preliminary study conducted in a European population confirms a high prevalence of abnormal liver parameters on admission reflecting severe disease and higher in‐hospital mortality in COVID‐19 patients.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest that pertain to this work.
Supporting information
Supplementary Material
Meszaros M, Meunier L, Morquin D, et al. Abnormal liver tests in patients hospitalized with Coronavirus disease 2019: Should we worry?. Liver Int. 2020;40:1860–1864. 10.1111/liv.14557
Handling Editor: Jian Sun
REFERENCES
- 1. Cai Q, Huang D, Yu H, et al. Characteristics of liver tests in COVID‐19 patients. J Hepatol. 2020. 10.1016/j.jhep.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan W‐J, Ni Z‐Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qi X, Liu C, Jiang Z, et al. Multicenter analysis of clinical characteristics and outcome of COVID‐19 patients with liver injury. J Hepatol. 2020;;S0168‐8278(20)30222‐1. 10.1016/j.jhep.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang C, Shi L, Wang F‐S. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D, Hu BO, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . Laboratory testing for coronavirus disease (COVID‐19) in suspected human cases: interim guidance, 19 March 2020. World Health Organization; 2020. https://apps.who.int/iris/handle/10665/331501 [Google Scholar]
- 9. Simpson S, Kay FU, Abbara S, et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID‐19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. J Thorac Imaging. 2020;2(2):e200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019‐nCoV infection. bioRxiv. 10.1101/2020.02.03.931766 [DOI] [Google Scholar]
- 11. Bode JG, Albrecht U, Häussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins – regulation by IL‐6‐ and IL‐1‐type cytokines involving STAT3 and its crosstalk with NF‐κB‐dependent signaling. Eur J Cell Biol. 2012;91(6‐7):496‐505. [DOI] [PubMed] [Google Scholar]
- 12. Lei F, Liu Y‐M, Zhou F, et al. Longitudinal association between markers of liver injury and mortality in COVID‐19 in China. Hepatology. 2020. 10.1002/hep.31301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu T, Chen C, Zhu Z, et al. Clinical features and dynamics of viral load in imported and non‐imported patients with COVID‐19. Int J Infect Dis. 2020;94:68‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sriram K, Insel PA. Risks of ACE inhibitor and ARB usage in COVID‐19: evaluating the evidence. Clin Pharmacol Ther. 2020. 10.1002/cpt.1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


