Abstract
A healthy patient presented to Klinikum Altmühlfranken Weißenburg Hospital, Germany, with two morning attacks of painful muscle spasm in the left upper and lower limbs, without altered consciousness. Full examinations, radiological imaging, electroencephalography, lumbar puncture, and autoimmune profile were either normal or not consistent with patient's complaint. Subsequent epileptic episodes were observed on admission day and the following days; thus, the patient was diagnosed with focal epilepsy. The patient started to develop a fever and severe cough on day 4, and SARS‐coronavirus‐2 was confirmed through a nasopharyngeal swap. She received anticonvulsants and symptomatic treatments and completely recovered. This report emphasizes the potential nervous system involvement in severe acute respiratory syndrome‐coronavirus‐2 pathogenesis.
Keywords: case report, coronavirus, COVID‐19, epilepsy, infection, outbreak, pandemic
Highlights
Coronaviruses are serious pathogens that infect respiratory, gastrointestinal, and central nervous systems.
SARS‐CoV‐2 may have neuroinvasive and neurotropic potential as some patients have demonstrated neurologic symptoms.
Neurologic manifestations may occur early in the infection course of SARS‐CoV‐2.
1. INTRODUCTION
Coronaviruses (CoVs) are serious pathogens that infect respiratory, gastrointestinal, and central nervous systems. 1 Severe acute respiratory syndrome‐CoV‐2 (SARS‐CoV2), or COVID‐19 has spread across 213 countries and territories. Undoubtedly, SARS‐CoV‐2 is now much more hazardous than expected compared to previous outbreaks such as the H1N1 pandemic which caused 12 429 deaths over a year while SARS‐CoV‐2 caused more than 13 000 over 5 weeks in the United States. 2 In addition, there are no available vaccines or specific antiviral drugs for COVID‐19 patients. Consequently, the current treatment protocols depend mainly on supportive treatment as well as involve some previously used drugs of SARS‐ and MERS‐CoV due to genetic similarity. 3 , 4 , 5 , 6 SARS‐CoV‐2 may have neuroinvasive and neurotropic potential as some patients have demonstrated neurologic symptoms such as nausea, vomiting, headache, acute cerebrovascular problems, and ataxia. 7 , 8 , 9 This report discusses the clinical, radiological, and laboratory features, and the outcome for the first case of SARS‐CoV‐2 presenting with focal epilepsy.
2. CASE PRESENTATION
On 17 March 2020, a 73‐year‐old female presented to the Emergency Department of Klinikum Altmühlfranken Weißenburg Hospital, Germany, complaining of two‐morning separate attacks of painful muscle stiffening and twitching in the left arm and leg. Each episode persisted for few seconds with no associated symptoms. Furthermore, she reported a 2‐day‐history of fatigue, mild night dry cough, and back pain at the cervical and thoracic regions. Apart from her well‐controlled arterial hypertension, she had no other pre‐existing conditions, no history of seizures, and no significant habits. Upon complete assessment, the patient was awake and oriented without any neurological deficits or electrocardiography abnormalities. She was subfebrile (37.4°C) with a blood pressure of 177/84 mm Hg, oxygen saturation of 100%, respiratory rate of 15 breaths/minute, and heart rate of 99 beats/minute. At this stage, the patient developed a similar cramp attack at 0:12 pm that lasted for 15 seconds (Figure 1). Further neurologic consultation excluded meningitis and stroke, while focal epilepsy was considered in the differential diagnosis. Noncontrast cranial computed tomography (CT) scan showed mild dilatation of the lateral ventricles with prominent fissures and sulci. Scattered deep white matter hypodensities were noted (suggestive of chronic small vessel ischemia). We did not identify any obstructive lesions. Moreover, no evidence of intracerebral hemorrhage, brain ischemia, or infarction was present. CT carotid angiography revealed proximal left internal carotid artery plaques with focal calcification (slightly stenotic). Magnetic resonance imaging (MRI) of the brain showed a dilated ventricular system with a patent and prominent aqueduct of Sylvius. The callosal angle measured 66 degrees. No visible cerebrospinal fluid (CSF) occlusion (findings suggestive of normal pressure hydrocephalus). Acute or subacute ischemia, infarction, and intracerebral bleeding were excluded (Figures S1‐3). The patient, thereafter, was transferred to the stroke unit, where she experienced another identical episode that lasted for 30 seconds at 06:20 pm. Laboratory findings are detailed in Table 1.
Figure 1.
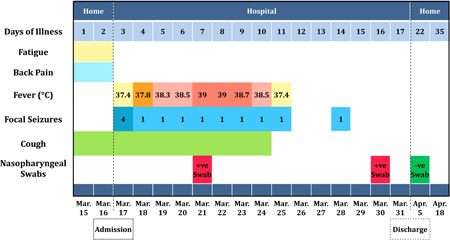
Symptoms and maximum body temperatures based on the day of illness and day of hospitalization, from 15 March to 18 April 2020
Table 1.
Clinical laboratory findings of the patient
| Measure | Reference | 17 March—Admission day | 20 March | 24 March | 30 March—One day before discharge |
|---|---|---|---|---|---|
| Leukocytes | 4‐9 ×109/L | 6.33 | 4.19 | 3.42 | 6.25 |
| RBCs | 4‐5.2 million/mL | 5.01 | 4.77 | 4.67 | 4.74 |
| Hemoglobin | 12‐16 g/dL | 15.4 | 14.6 | 14.1 | 14.2 |
| Hematocrit | 36%‐46% | 45.7 | 43.3 | 42.3 | 43.4 |
| MCV | 83‐103 fl | 91.2 | 90.8 | 90.6 | 91.6 |
| MCH | 28‐34 pg | 30.7 | 30.6 | 30.2 | 30 |
| MCHC | 32‐36 g/dL | 33.7 | 33.7 | 33.3 | 32.7 |
| Platelets | 150‐350 109/L | 173 | 138 | 161 | 347 |
| Neutrophils | 50%‐70% | 61 | … | … | … |
| Lymphocytes | 25%‐40% | 17 | … | … | … |
| Monocytes | 2%‐8% | 18 | … | … | … |
| Basophils | 0%‐1% | 1 | … | … | … |
| CRP | < 0.5 mg/dL | 0.85 | 3.19 | 4 | |
| Procalcitonin | <0.05 ng/ml | 0.065 | … | … | |
| Blood glucose | 70‐115 mg/dL | 168.4 | … | … | … |
| Serum creatinine | 0.5‐1.4 mg/dL | 0.89 | 0.75 | … | … |
| GFR‐ CKD‐ EPI | mL/min per 1.73 m2 | 64.4 | 79.8 | … | … |
| Urea | 10‐50 mg/d | 25 | 35 | … | … |
| Sodium | 136‐145 mmol/L | 134 | 135 | … | … |
| Potassium | 3.5‐5.1 mmol/L | 3.46 | 3.82 | … | … |
| Calcium | 2.1‐2.5 mmol/L | 2.45 | … | … | … |
| Chloride | 94‐107 mmol/L | 96 | … | … | … |
| Magnesium | 0.7‐1.1 mmol/L | ‐ | 0.9 | … | … |
| ALT | 7‐35 U/L | 22 | 33 | … | … |
| AST | 13‐35 U/L | 26 | 36 | … | … |
| Total bilirubin | <1 mg/dL | 0.33 | 0.22 | … | … |
| LDH | 135‐214 U/L | 219 | 264 | 389 | 350 |
| Alkaline phosphatase | 35‐104 U/L | 37 | 32 | … | … |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CRP, c‐reactive protein; LDH, lactate dehydrogenase; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; RBC, red blood cell.
On 18 March, the temperature started to rise, reaching 37.9°C, and a mild night dry cough was also reported. Cervical spine MRI showed diffuse degenerative changes with multiple disc bulge/protrusion associated with moderate to severe neural foraminal stenosis at C4‐5 and C5‐6 on the left side, and mild foraminal stenosis at C4‐5 on the right side. No significant spinal stenosis or evidence of myelopathy was detected. On the same‐day evening, the patient had another attack of tonic‐clonic seizure in the left arm, hand, and leg without any neurologic deficits or loss of consciousness.
On 19 March, her temperature overrode 38°C, a mild night dry cough was reported, and the patient developed another comparable cramp episode. The cerebrospinal fluid sample was clear with slightly elevated leukocytes (0.5/mm3), and the magnesium level was normal. The blood culture was obtained and found to be sterile. Electroencephalography (EEG) was performed and was found to be normal. In addition, autoimmune testing including antinuclear antibody, cytoplasmic antineutrophil cytoplasmic antibodies (ANCA), and perinuclear ANCA were negative. Furthermore, ultrasound (US) imaging of the abdomen showed cholecystolithiasis, yet the US pelvis was normal. Echocardiography showed mild hypertrophy of the left ventricle while the carotid duplex showed mild carotid stenosis. The chest X‐ray showed increased interstitial markings bilaterally, with suspected right middle‐inferior lobe infiltrate (Figure 2). The patient received three doses of 400 mg magnesium, levetiracetam 1,000 mg was continued, and lacosamide 50 mg and clobazam 10 mg twice daily were added.
Figure 2.
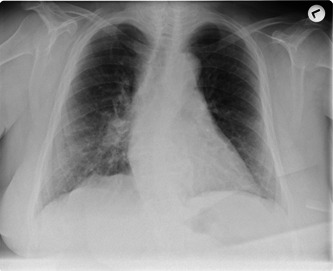
Posteroanterior chest radiographs, 19 March 2020 (hospital day 3)
On 20 March, the patient developed a noticeable cough, fever (38°C‐38.5°C), and a similar cramp episode. In addition, C‐reactive protein was noted to be increased; thus, the nasopharyngeal swab was obtained. On the following day, the swap result came positive for SARS‐CoV‐2.
Between 22 March and 25 March, the patient had a persistent fever (38°C‐39°C), severe cough, and one cramp episode per day. The muscle cramp was the main patient's complaint, and she described it as extremely painful. Besides this, she even avoided standing and walking as she was afraid of cramp recurrence. Along with the antiepileptic regimen, the patient was symptomatically managed with normal saline, paracetamol, ambroxol, acetylcysteine, and metamizole.
Between 26 March and 30 March, all symptoms were obviously relieved, and the patient started to mobilize. However, the patient had another similar cramping on 28 March, and her swap was still positive on 30 March. The patient was then discharged home for self‐isolation on 31 March. A home nasopharyngeal swap was obtained on 5 April, which was negative. On a recent follow‐up (18 April), the patient declared no recurrent episodes.
3. DISCUSSION
In this report, we showed the clinical, radiological, and laboratory features, and the outcome for the first case of SARS‐CoV‐2 presenting with recurrent focal seizures. Neurological complications involving central (CNS) and peripheral nervous systems (PNS) are not uncommon following CoVs infection. 7 , 10 Previous reports confirmed that SARS‐CoV could present in the CSF 11 , 12 and brain 13 of SARS autopsies.
Recent studies have demonstrated an association between SARS‐CoV‐2 infection and neurological problems such as nausea, vomiting, headache, encephalitis, acute cerebrovascular problems, ataxia, decreased level of consciousness, seizures, and Guillain‐Barré syndrome. 7 , 14 , 15 Noteworthily, a recent study observed that neurological problems had occurred early in the infection with a 24 to 48 hours median period to hospital admission. 7 Of note, respiratory failure in some infected individuals may be partially attributed to SARS‐CoV‐2 neuroinvasion. 8 Patients with severe SARS‐CoV‐2 may have hypoxia, metabolic and electrolyte imbalance, and multiorgan failure. 3 Thus, it is expected that subsequent clinical or subclinical seizures and status epilepticus may develop in these patients. 16 Therefore, it is substantial to monitor patients with a critical condition using continuous EEG to avoid delayed diagnosis of seizures.
Mao et al found that 36.4% of SARS‐CoV‐2 patients had several neurologic manifestations. They also observed that patients with severe infection, compared to nonsevere patients, were more likely to develop neurologic problems, especially acute cerebrovascular disease, conscious disturbance, and skeletal muscle injury. 7 In addition, a recent report from Iran found a case of generalized convulsions following SARS‐CoV‐2 infection. 17
Indeed, the angiotensin‐converting enzyme 2 (ACE2), which has been known as the functional receptor for SARS‐CoV‐2, is present in multiple human organs, including the nervous system, skeletal muscles, and intestine. 18 This may explain how SARS‐CoV‐2 could gain access to the brain tissue and induce neuronal damage. 19 Zayet et al 20 proposed that the viral load might be correlated with neurological features as the viral load of two encephalitis patients was higher than cases with no neurologic features. Several other theories may explain the neuroinvasive potential of SARS‐CoV‐2. First, SARS‐COV‐2 could invade the CNS through the hematogenous or retrograde neuronal route like other respiratory viruses. The hematogenous route entails virus access to CNS through affecting the endothelial cells of blood‐brain‐barrier or infecting the choroid plexus epithelial cells. 21 , 22 The retrograde neuronal route through the olfactory bulb, leading to inflammation and demyelination, may be supported by the fact that some cases had sinusitis and smell impairment. 23 Second, the toxins produced by SARS‐CoV‐2 and inflammatory cytokines by the brain 24 could provoke the inflammatory cascade leading to neuronal hyperexcitability via activation of glutamate receptors and consequently resulting in acute seizures. 25 , 26 Third, the SARS‐CoV‐2‐mediated harmful immune response may lead to neurological problems. 7 The authors observed that the lymphocyte counts were lower in patients with neurological symptoms than without neurological symptoms. It was also shown that individuals with severe infection had higher D‐dimer concentrations compared to patients with nonsevere infection. Thus, severely infected patients may be more likely to develop cerebrovascular abnormalities. 7 Healthcare professionals are on a learning phase of SARS‐CoV‐2 clinical findings. As the present case has developed obvious SARS‐CoV‐2 symptoms on day 4 of admission, this may explain the delayed nasopharyngeal swap and emphasize on the value of earlier nervous system presentation.
It is noteworthy that we did not perform a PCR for the CSF due to the insufficient understanding of neuroinvasive potential at the early SARS‐CoV‐2 pandemic, similar to previous studies. 7 , 27 , 28 However, other findings could confirm the association between SARS‐CoV‐2, our observed manifestations, and its neuroinvasive potential.
4. CONCLUSIONS
Neurologic manifestations are not uncommon and may occur early in the infection course of SARS‐CoV‐2. Thus, clinicians should pay attention to SARS‐CoV‐2 as a differential diagnosis to avoid delayed or misdiagnosis and prevention of the present awful transmission.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUATIONS
SE and MEM shared in the development of the idea and study design. SE managed the patient. MGK, SG, and AK prepared the figures. All authors wrote and approved the manuscript.
ETHICS STATEMENT
All the methods were performed in accordance with the relevant approved guidelines, regulations, and declaration of Helsinki. Written informed consent was obtained from the patient to have her details and accompanying images published.
Supporting information
Supporting information
Elgamasy S, Kamel MG, Ghozy S, Khalil A, Morra ME, Islam SMS. First case of focal epilepsy associated with SARS‐coronavirus‐2. J Med Virol. 2020;92:2238–2242. 10.1002/jmv.26113
Contributor Information
Mostafa E. Morra, Email: mostafamorra.stu.6@azhar.edu.eg.
Sheikh M. S. Islam, Email: shariful.islam@deakin.edu.au.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boëlle PY, Ansart S, Cori A, Valleron AJ. Transmission parameters of the A/H1N1 (2009) influenza virus pandemic: a review. Influenza Other Respir Viruses. 2011;5(5):306‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan JF, Yuan S, Kok K‐H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morra ME, Van Thanh L, Kamel MG, et al. Clinical outcomes of current medical approaches for Middle East respiratory syndrome: a systematic review and meta‐analysis. Rev Med Virol. 2018;28(3):e1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li X, Zai J, Zhao Q, et al. Evolutionary history, potential intermediate animal host, and cross‐species analyses of SARS‐CoV‐2. J Med Virol. 2020;92(6):602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may be at least partially responsible for the respiratory failure of COVID‐19 patients. J Med Virol. 2020;92:552‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yashavantha Rao HC, Jayabaskaran C. The emergence of a novel coronavirus (SARS‐CoV‐2) disease and their neuroinvasive propensity may affect in COVID‐19 patients. J Med Virol. 2020;92(7):786–790. 10.1002/jmv.25918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dubé M, Le Coupanec A, Wong AH, Rini JM, Desforges M, Talbot PJ. Axonal transport enables neuron‐to‐neuron propagation of human coronavirus OC43. J Virol. 2018;92(17):e00404‐e00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arabi YM, Balkhy HH, Hayden FG, et al. Middle East respiratory syndrome. New Engl J Med. 2017;376(6):584‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Desforges M, Favreau DJ, Brison É, et al. Human coronaviruses: respiratory pathogens revisited as infectious neuroinvasive, neurotropic, and neurovirulent agents. 2013. 10.1201/b13908 [DOI]
- 13. Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim J‐E, Heo J‐H, Kim H‐o, et al. Neurological complications during treatment of Middle East respiratory syndrome. Journal of Clinical Neurology. 2017;13(3):227‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma K, Tengsupakul S, Sanchez O, Phaltas R, Maertens P. Guillain–Barré syndrome with unilateral peripheral facial and bulbar palsy in a child: a case report. SAGE Open Med Case Rep. 2019;7:2050313. 2050313X19838750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miró JM, de Terán FD, Singer PA, Prior MA‐A. Emergency electroencephalogram: usefulness in the diagnosis of nonconvulsive status epilepticus by the on‐call neurologist. Neurología. 2018;33(2):71‐77. [DOI] [PubMed] [Google Scholar]
- 17. Karimi N, Sharifi Razavi A, Rouhani N. Frequent Convulsive Seizures in an Adult Patient with COVID‐19: a Case Report. Iran Red Crescent Med J. 2020;22(3). 10.5812/ircmj.102828 [DOI] [Google Scholar]
- 18. Hussain M, Jabeen N, Raza F, et al. Structural variations in human ACE2 may influence its binding with SARS‐CoV‐2 spike protein. J Med Virol. 2020. 10.1002/jmv.25832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID‐19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995‐998. [DOI] [PubMed] [Google Scholar]
- 20. Zayet S, Abdallah YB, Royer PY, Toko‐Tchiundzie L, Gendrin V, Klopfenstein T. Encephalopathy in patients with COVID‐19: ‘causality or coincidence?’. J Med Virol. 2020. 10.1002/jmv.26027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paniz‐Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). J Med Virol. 2020;92(7):699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2020;12(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bohmwald K, Galvez N, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Libbey JE, Fujinami RS. Neurotropic viral infections leading to epilepsy: focus on Theiler's murine encephalomyelitis virus. Future Virol. 2011;6(11):1339‐1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singhi P. Infectious causes of seizures and epilepsy in the developing world. Dev Med Child Neurol. 2011;53(7):600‐609. [DOI] [PubMed] [Google Scholar]
- 26. Libbey JE, Kirkman NJ, Smith MC, et al. Seizures following picornavirus infection. Epilepsia. 2008;49(6):1066‐1074. [DOI] [PubMed] [Google Scholar]
- 27. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain‐Barré syndrome associated with SARS‐CoV‐2 infection: causality or coincidence? The Lancet Neurology. 2020;19(5):383‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yin R, Feng W, Wang T, et al. Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019. J Med Virol. 2020. 10.1002/jmv.25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


