Abstract
In less than 6 months, the severe acute respiratory syndrome‐coronavirus type 2 (SARS‐CoV‐2) has spread worldwide infecting nearly 6 million people and killing over 350,000. Initially thought to be restricted to the respiratory system, we now understand that coronavirus disease 2019 (COVID‐19) also involves multiple other organs, including the central and peripheral nervous system. The number of recognized neurologic manifestations of SARS‐CoV‐2 infection is rapidly accumulating. These may result from a variety of mechanisms, including virus‐induced hyperinflammatory and hypercoagulable states, direct virus infection of the central nervous system (CNS), and postinfectious immune mediated processes. Example of COVID‐19 CNS disease include encephalopathy, encephalitis, acute disseminated encephalomyelitis, meningitis, ischemic and hemorrhagic stroke, venous sinus thrombosis, and endothelialitis. In the peripheral nervous system, COVID‐19 is associated with dysfunction of smell and taste, muscle injury, the Guillain‐Barre syndrome, and its variants. Due to its worldwide distribution and multifactorial pathogenic mechanisms, COVID‐19 poses a global threat to the entire nervous system. Although our understanding of SARS‐CoV‐2 neuropathogenesis is still incomplete and our knowledge is evolving rapidly, we hope that this review will provide a useful framework and help neurologists in understanding the many neurologic facets of COVID‐19. ANN NEUROL 2020;88:1–11 ANN NEUROL 2020;88:1–11
The novel coronavirus, now called severe acute respiratory syndrome‐coronavirus type 2 (SARS‐CoV‐2), is the agent of coronavirus disease 2019 (COVID‐19), that was first diagnosed on December 8, 2019, in a patient in the city of Wuhan in central China. Common symptoms of COVID‐19 include fever, cough, fatigue, and shortness of breath. Whereas most affected individuals have no or minor symptoms, some go on to develop pneumonia, acute respiratory distress syndrome (ARDS), and succumb from multiple organ failure. On January 30, 2020, the World Health Organization (WHO) declared it a Public Health Emergency of international concern. It has been estimated that the number of infected individuals during the early epidemic doubled every 2.4 days, and the R0 value, or number of people that can be infected by a single individual, may be as high as 4.7 to 6.6. 1 After spreading throughout China, the disease took hold in Europe and the United States, and in view of this alarming development and the rapid growth of cases, public health officials in many jurisdictions ordered people to shelter in place beginning with the state of California on March 19, 2020. As of May 29, 2020, there have been 5.88 million confirmed cases in 188 countries and 363,000 reported deaths, and most countries are in various phases of relaxing quarantine requirements while continuing some social distancing measures.
What are coronaviruses and what makes SARS‐CoV‐2 so contagious? Coronaviruses, which have a diameter of approximately 100 nm, are named after their crown‐like appearance on electron microscopy. They infect many animal species and are part of the family of Coronaviridae that contain four distinct Genera. Coronaviruses are positive strand, single stranded ribonucleic acid (+ss‐RNA) viruses. They have the largest genome of all RNA viruses, approximately 30 kilobases in length. The full sequence of SARS‐CoV‐2 was published on January 7, 2020, and revealed that it is was a β‐coronavirus, similar to other human coronaviruses that are responsible for 15% of all cases of acute viral nasopharyngitis, also known as “common cold.” 2 However, SARS‐CoV‐2 contains unique sequences, including a polybasic cleavage site in the spike protein, which is a potential determinant of increased transmissibility. 3
Coronaviruses have caused deadly outbreaks in the past. The first one caused by SARS‐CoV, occurred in China in 2003 and affected approximately 8,000 people, with a 10% mortality rate. The Middle‐East Respiratory Syndrome (MERS) outbreak began in Saudi Arabia in 2012, and affected 2,500 individuals with a 35% mortality rate. SARS‐CoV‐2 has approximately 80% sequence homology with SARS‐CoV, but 96% homology with a bat coronavirus and 92% with a pangolin coronavirus, suggesting it arouse in animals and then spread between species to humans. The spike protein of SARS‐CoV‐2 binds to its cellular receptor, the angiotensin converting enzyme 2 (ACE2), which also acts as receptor for SARS‐CoV. Viral entry occurs after proteolytic cleavage of the spike protein by the transmembrane protease TMPRSS2. ACE2 is expressed abundantly in lung alveolar cells, but also in many cell types and organs in the body, including the cerebral cortex, digestive tract, kidney, gallbladder, testis, and adrenal gland. 4
Experience with the neurological complications of MERS and SARS provides a framework for considering both reported and potential neurological complications with SARS‐CoV‐2 and COVID‐19. 5 , 6 , 7 , 8 , 9 , 10 In both MERS and SARS, significant neurological complications were fortunately extremely rare. Reported cases of neurological disease suggests a minimum incidence of ~1:200 cases (MERS) ‐1:1,000 cases (SARS). It is important to recognize, however, that the total number of confirmed cases of MERS and SARS together is only ~10,500 cases. It is likely that the sheer numeracy of COVID‐19 compared to MERS and SARS, with nearly 6 million cases reported worldwide to date, will bring out a broader spectrum of neurological manifestations. In MERS and SARS neurological disease could be considered in three major categories: (1) the neurological consequences of the associated pulmonary and systemic diseases, including encephalopathy and stroke, (2) direct central nervous system (CNS) invasion by virus, including encephalitis, and (3) postinfectious and potentially immune‐mediated complications, including Guillain‐Barre syndrome (GBS) and its variants and acute disseminated encephalomyelitis (ADEM).
Neurological Complications of Systemic COVID‐19
In a review of 214 patients hospitalized in 3 dedicated COVID‐19 hospitals in Wuhan, China, 36% of patients had nerurologic. 11 These were further subdivided into those thought to reflect CNS, peripheral nervous system (PNS), and skeletal muscle injury. Overall, 25% of patients had symptoms considered as evidence of CNS dysfunction, including dizziness (17%), headache (13%), impaired consciousness (7.5%), acute cerebrovascular disease (3%), ataxia (0.5%), and seizures (0.5%). Confirming this low incidence of seizures, no cases of status epilepticus or new onset seizures were reported in a large cohort of over 304 hospitalized patients with COVID‐19 in Hubei Province, China, 12 although there have been isolated case reports describing seizures at presentation in both adult and pediatric patients with COVID‐19. 13 , 14
In the series by Mao and colleagues, 11 the patients were subdivided based on the severity of their pneumonia and pulmonary impairment, and among those with “severe” disease (n = 88) the incidence of CNS symptoms was higher (31%) compared to the non‐severe group (21%), although the results were not statistically significant (p = 0.09). Although all the categorized CNS symptoms occurred more frequently in patients with severe disease compared to non‐severe disease, only impaired consciousness (15% in severe vs 2% in non‐severe, p < 0.001) and acute cerebrovascular disease (5.7% vs 0.8%; p = 0.03) were significantly different between the two groups. Diagnostic studies were limited, but the impairment of consciousness seems most consistent with encephalopathy. Not surprisingly, when compared to those with non‐severe disease, the severe cohort were older (58 ± 15 years vs 49 ± 15 years), and more likely to have comorbidities, including hypertension, diabetes, malignancy, cardiac, cerebrovascular, or kidney disease (48% vs 33%; p = 0.03). The severe group also had more evidence of systemic inflammation, including elevated C‐reactive protein (CRP; median 37 mg/L) and D‐dimer (median 0.9 mg/L) compared to non‐severe cases, and were also more likely to have evidence of hepatic (elevated alanine and aspartate aminotransferases) and renal (elevated BUN and creatinine) dysfunction.
A second survey of 58 hospitalized patients (median age 63 years) with COVID‐19 ARDS at Strasbourg University Hospital found that 69% of patients had agitation, 67% had corticospinal tract signs, and 36% had a “dysexecutive” syndrome with difficulty in concentration, attention, orientation, and following commands. 15 All patients studied (11/11) had evidence of frontal hypoperfusion on arterial spin label and dynamic susceptibility‐weighted perfusion magnetic resonance imaging (MRI). Only seven patients had a cerebrospinal fluid (CSF) examination, none had a pleocytosis, and none had SARS‐CoV‐2 RNA detected by reverse transcriptase‐polymerase chain reaction (RT‐PCR). One patient did have elevated immunoglobulin G (IgG) levels and “mildly” elevated total protein. CSF specific oligoclonal bands (OCBs) were not detected, but one patient had “mirror pattern” OCBs in CSF and serum.
In a study of MRI abnormalities in patients in the intensive care unit (ICU) with COVID‐19, 21% (50/235) of patients developed neurological symptoms. 16 In this group of neurologically symptomatic patients, only 27 had MRIs performed, and of these 44% (12/27) had new acute findings. Surprisingly, 56% (15/27) had no new MRI changes. The most common new abnormalities were multifocal areas of cortical fluid‐attenuated inversion recovery (FLAIR) signal (10/12), accompanied in three patients by areas of increased FLAIR signal in the subcortical and deep white matter. One patient each had new transverse sinus thrombosis and acute middle cerebral artery infarction. Five of the 10 patients with cortical FLAIR abnormalities had a CSF examination, and none of these patients had a pleocytosis elevated IgG index, or OCBs (0/3 tested), although 4 patients had an elevated protein (mean 80 mg/dl; range = 60–110). RT‐PCR for SARS‐CoV‐2 was negative in all 5 cases tested. In another MRI series of critically ill patients on mechanical ventilation, many were found to have confluent T2 hyperintensities and restricted diffusion in the deep and subcortical white matter, in some cases, accompanied by punctate microhemorrhages in the juxtacortical and callosal white matter that resembled findings seen in delayed post‐hypoxic leukoencephalopathy. 17
The mechanism of encephalopathy in COVID‐19 remains to be determined. From available studies, COVID‐19 encephalopathy seems to be more common in patients with more severe disease, associated comorbidities, evidence of multi‐organ system dysfunction, including hypoxemia, and renal and hepatic impairment, and elevated markers of systemic inflammation. Virus is not detected in CSF by RT‐PCR and pleocytosis is usually absent. Some patients may have altered perfusion detectable by MRI, others have leukoencephalopathy with or without punctate microhemorrhages. This group needs to be distinguished from patients with encephalitis (who have a pleocytosis) and postinfectious immune‐mediated encephalitis (see below).
In a series of five consecutive patients with COVID‐19 with delayed awakening post‐mechanical ventilation for ARDS, MRI showed enhancement of the wall of basal skull arteries without enlargement of the vessel wall or stenosis. Toxic‐metabolic derangements and seizures were ruled out, CSF SARS‐CoV‐2 RT‐PCR was negative in all and they showed marked improvement in alertness 48 to 72 hours after treatment with methylprednisolone 0.5 g/days iv for 5 days. These findings suggest that an endothelialitis rather than a vasculitis was responsible for the encephalopathy. 18 Direct infection of endothelial cells by SARS‐CoV‐2 and associated endothelial inflammation has been demonstrated histologically in postmortem specimens from a variety of organs, which did not include the brain. 19
However, in an autopsy series, including examination of the brain, of 20 patients with COVID‐19, six had microthrombi and acute infarctions and two focal parenchymal infiltrates of T‐lymphocytes, whereas the others mainly had minimal inflammation and slight neuronal loss without acute hypoxic–ischemic changes in most cases. There was no evidence of meningoencephalitis, microglial nodules, or viral inclusions, including in the olfactory bulbs and brainstem, and no demyelination. ACE2 was expressed in lung and brain capillaries. All cases had evidence of systemic inflammation. 20
A second major manifestation of systemic COVID‐19 disease is acute cerebrovascular disease. In the study by Mao and colleagues, 11 this was present in 6 of the 214 (3%) hospitalized cases, but 5 of the 6 events occurred in those with severe disease (incidence 6%; p = 0.03 vs non‐severe disease). 11 Five of the six reported events were ischemic strokes, and one was hemorrhagic. In the review of cases at Strasbourg University Hospital, 15 3 of 13 (23%) had cerebral ischemic stroke. In a single center retrospective study from China of 221 patients hospitalized with COVID‐19, 13 had acute strokes, including 11 ischemic, 1 hemorrhagic, and 1 venous sinus thrombosis. 21 The stroke patients were older, had more comorbidities, including diabetes, hypertension, and a prior stroke, and elevated inflammatory markers, including D‐dimer and CRP. Another review of six consecutive patients with COVID‐19 admitted to the National Hospital in Queen Square with stroke, noted that occlusions typically involved large vessels and often occurred in multiple vascular territories. 22 In 5 of 6 cases, the strokes occurred 8 to 24 days after onset of COVID‐19 symptoms. All patients had a highly prothrombotic state with very high D‐dimer levels and elevated ferritin. Five of the six patients had detectable lupus anticoagulant, suggesting another potential prothrombotic mechanism for stroke in COVID‐19. Anticardiolipin IgA and antiphospholipid IgA and IgM antibodies directed against β2‐glycoprotein‐1 were also found in three patients with COVID‐associated multiple territory large vessel infarctions. 23 Finally, a postmortem MRI study showed subcortical micro‐ and macro‐bleeds (two decedents), cortico‐subcortical edematous changes evocative of posterior reversible encephalopathy syndrome (PRES; one decedent), and nonspecific deep white matter changes (one decedent). 24
Although initial reports emphasized acute cerebrovascular disease in older patients with COVID‐19, a recent report described five cases of large vessel stroke as a presenting feature of COVID‐19 in younger individuals, two of whom lacked classic stroke risk factors. 25 These patients ranged in age from 33 to 49 years. Two of the five patients had diabetes, one of whom had had a mild prior stroke, and one had hypertension and dyslipidemia. The infarcts involved large vessel territories, including the middle cerebral artery (3), posterior cerebral artery (1), and internal carotid artery (1). Two patients had preceding COVID‐19 symptoms, including fever, chills, cough, and headache; one patient had only lethargy. Surprisingly, two of the five patients had no COVID‐19‐related symptoms preceding their stroke presentation. These five patients had elevated prothrombin (range = 12.8–15.2 seconds) and activated partial thromboplastin times (range = 25–42.7 seconds), elevated fibrinogen (range = 370–739 mg/dl), D‐dimer (range = 52–13,800 ng/ml) and ferritin (range = 7–1,564 ng/ml) consistent with a hypercoagulable state and the presence of disseminated intravascular coagulation (DIC).
COVID‐19 cerebrovascular disease seems to be predominantly ischemic and to involve large vessels. In older individuals, it reflects the underlying severity of systemic disease as well as the hyperinflammatory state, whereas in younger patients, it seems to be due to hypercoagulopathy. Children with a Kawasaki disease‐like multisystem inflammatory syndrome (MIS) have recently been described. 26 , 27 Patients with Kawasaki disease can develop cerebral vasculopathy and forms of neurological involvement, and in one series of 10 COVID‐19 associated cases of MIS, two patients had meningeal symptoms. 27 As noted, in addition to hypercoagulable states, SARS‐CoV‐2 can infect and injure endothelial cells. However, it remains to be determined whether virus‐induced injury to endothelial cells (a vasculopathy) or even true vasculitis contributes to COVID‐19 related cerebrovascular syndromes, and this determination will require additional detailed vessel imaging and neuropathological analyses. Similarly, the number of cases is too small to determine the comparative therapeutic benefit, if any, of antiplatelet or anticoagulant drugs or immunomodulatory therapies in COVID‐19 associated neurovascular syndromes.
Neuroinvasion by SARS‐CoV‐2
In contrast to encephalopathy, in which evidence for direct invasion by virus of the CNS is absent, encephalitis occurs when direct invasion of the CNS by virus produces tissue injury and neurological dysfunction. Evidence for direct invasion of the CNS was seen in patients with SARS. Xu and colleagues described a fatal case in a 39‐year‐old man with delirium that progressed to somnolence and coma. 10 At postmortem, the SARS‐CoV antigen was detected in brain tissue by immunohistochemistry (IHC) and viral RNA by in situ hybridization (ISH). SARS‐CoV virions were seen by transmission electron microscopy of brain tissue inoculated cell culture. In a postmortem analysis of four patients with SARS, low level infection of cerebral neurons with SARS‐CoV (1–24% of cells) was seen in the cerebrum in all four cases by IHC and ISH, although none of the cases had virus detected in the cerebellum. 28
By definition, encephalitis is an inflammatory process, with supportive evidence, including the presence of a CSF pleocytosis and elevated protein. However, in studies of transgenic mice expressing the human SARS‐CoV receptor, ACE2, infection with SARS‐CoV was associated with viral entry into the CNS, spread within the CNS, and neuronal injury with relatively limited inflammation. 29 This suggests the possibility that, in some cases of SARS‐CoV‐2 CNS invasion, that signs of inflammation could be modest or even absent. Regardless of the presence or absence of inflammation, diagnostic studies may show evidence of either a generalized or focal CNS process, including areas of attenuation on computed tomography (CT), hyperintense signal on FLAIR, or T2‐weighted sequences on MRI, and focal patterns, including seizures on electroencephalogram (EEG). Definitive evidence supporting direct viral invasion would include a positive CSF RT‐PCR for SARS‐CoV‐2, demonstration of intrathecal synthesis of SARS‐CoV‐2‐specific antibodies, or detection of SARS‐CoV‐2 antigen or RNA in brain tissue obtained at biopsy or autopsy.
Cases meeting strict criteria for encephalitis resulting from direct SARS‐CoV‐2 are currently extremely rare, although several plausible case reports have now surfaced. Moriguchi et al described a 24‐year‐old man with COVID‐19 disease who developed nuchal rigidity, progressively decreased consciousness (Glasgow Coma Scale [GCS] = 6), and generalized seizures. 30 CSF showed a slight mononuclear predominant pleocytosis (12 cells/μl 3 ) and elevated opening pressure (>320 mm H20). Neuroimaging showed hippocampal and mesial temporal increased FLAIR signal and the CSF RT‐PCR was positive for SARS‐CoV‐2. Unfortunately, studies to exclude other viral etiologies of encephalitis were limited. A second case involved a 41‐year‐old woman with headache, fever, a new onset seizure, and photophobia and nuchal rigidity, followed by hallucinations and disorientation. A head CT scan was normal and MRI was not performed. An EEG showed generalized slowing. The CSF examination showed a lymphocytic pleocytosis (70 cells/μl; 100% lymphocytes), and elevated protein (100 mg/dl), and a positive SARS‐CoV‐2 RT‐PCR. 31 , 32
Several cases have emerged in which patients had inflammatory features consistent with encephalitis, but who did not have evidence of direct viral CNS invasion. Bernard‐Valnet et al reported on two patients with “meningoencephalitis concomitant to SARS‐CoV2.” 33 These patients had nuchal rigidity, altered mental status, mild CSF lymphocytic pleocytosis (17–21 cells/μl3 on initial lumbar puncture [LP]), and mildly elevated CSF protein (46–47 mg/dl). However, in both patients, the MRI was normal and neither patient had a positive CSF RT‐PCR for SARS‐CoV‐2. Similarly, Pilotto et al describe a 60‐year‐old man with COVID‐19 who developed confusion, irritability, and then apathy progressing to “akinetic mutism” with nuchal rigidity. 34 The CSF showed a mild lymphocytic pleocytosis (18 cells/μl3) and elevated protein (70 mg/dl). An EEG showed generalized slowing with an anterior predominance. The CT and MRI were normal, and CSF RT‐PCR was negative twice for SARS‐CoV‐2. Although treated with a wide variety of medications, this patient showed improvement coincident to administration of high dose methylprednisolone. 34 Another study reported on six critically ill patients with severe ARDS, elevated inflammatory markers, and depressed consciousness and/or agitation, who were considered to have “autoimmune meningoencephalitis.” 35 No patient had a CSF pleocytosis but five had elevated CSF protein (52–131 mg/dL) and three had an MRI that showed cortical hyperintensities with sulcal effacement. There were no controls but patients were felt to have responded to plasma exchange. In one report, a patient with neuropsychiatric symptoms and COVID‐19 had a “hematic” CSF tap with 960 “red and white blood cells” and an elevated protein (65 mg/dL) and detectable N‐methyl‐D‐aspartate (NMDA) receptor antibodies. This currently isolated case also raises the possibility that COVID‐19 may trigger auto‐antibody production. 36
The available studies suggest that SARS‐CoV‐2 can rarely produce a true encephalitis or meningoencephalitis with associated evidence of direct viral invasion of the CNS. The failure to detect virus in CSF in the other reported cases, despite evidence of inflammation as evidenced by CSF pleocytosis and elevated protein, raises the possibility that some cases of COVID‐19 encephalitis may occur in the absence of direct virus invasion, and could potentially result from immune‐mediated inflammatory mechanisms (see below). It is important to realize that techniques, including detection of intrathecal SARS‐CoV‐2 antibody synthesis or of viral antigen or nucleic acid in brain tissue, may establish evidence for viral invasion when CSF RT‐PCR studies are negative. For example, detection of intrathecal antibody synthesis is significantly more sensitive than CSF nucleic acid amplification tests for diagnosis of both West Nile Virus neuroinvasive disease and Enterovirus (EV)‐D68 associated acute flaccid myelitis (AFM). 37 , 38 , 39 In the case of EV‐D68‐associated AFM, nasopharyngeal and throat swabs are frequently positive for virus by RT‐PCR when obtained early after disease onset, yet, CSF RT‐PCR tests are only positive in a small minority (<3%) of cases. 40 The sensitivity of SARS‐CoV‐2 RT‐PCR in properly performed nasopharyngeal swabs for detection of acute COVID‐19 is high, but data are currently too limited to evaluate sensitivity of this technique in CSF in patients with neurological disease.
Post‐Infectious and Immune‐Mediated Complications of SARS‐CoV‐2
The identification of postinfectious complications of SARS‐CoV‐2 would be expected to temporally lag behind those resulting from acute infection. Occasional cases of GBS and its variants and of ADEM were reported after MERS and SARS. 5 , 7 , 9 Reports are now emerging of similar associations with COVID‐19 and GBS, and with GBS variants, including the Miller‐Fisher syndrome. 41 , 42 , 43 , 44 , 45 , 46 The largest series to date, describes five patients. 47 In this series, all patients developed GBS 5 to 10 days following COVID‐19 symptom onset. The clinical presentation included bilateral multi‐limb flaccid weakness with areflexia. Three patients had associated respiratory failure and two had associated facial weakness. MRI showed caudal root nerve enhancement in two cases and enhancement of the facial nerve in a third case. The CSF was normocellular in all five cases, and had an elevated protein consistent with albuminocytological dissociation in three cases. Electrophysiological studies showed reduced compound motor amplitudes and prolonged distal latencies, and the overall pattern was felt to be consistent with demyelination in two cases and axonal neuropathy in three cases. Fibrillation potentials were seen by electromyography (EMG) acutely in three patients and later in a fourth patient. None of the patients had SARS‐CoV‐2 detected in the CSF by RT‐PCR. Antiganglioside antibodies were absent in the three tested patients. All patients received intravenous immunoglobulin (ivIG) and one plasma exchange, although improvement was noted in only two cases (one “mild improvement” only).
Cases of acute necrotizing encephalopathy (ANE) have been reported in COVID‐19. 48 , 49 One patient was a 50‐year‐old woman with COVID‐19 confirmed by nasopharyngeal RT‐PCR who developed altered mental status and MRI and CT findings typical of ANE, including bilateral thalamic lesions. Unfortunately, CSF studies were limited and CSF RT‐PCR testing for SARS‐CoV‐2 was not performed. A second case occurred in a 59‐year‐old woman with aplastic anemia who developed seizures and reduced consciousness 10 days after onset of her COVID‐19 symptoms. 49 The mechanism behind ANE remains unknown, and either direct viral or postinfectious inflammatory processes have been postulated to play a role, and many cases have been reported after upper respiratory infections, including influenza. Some patients have mutations in RAN binding protein‐2 (RANBP2), indicating that host genetic factors may also play a role in susceptibility.
Rare cases of ADEM were associated with MERS. 6 The first case of “COVID‐19 associated disseminated encephalomyelitis” was reported in a 40‐year‐old woman. 50 This individual had COVID‐19 symptoms followed 11 days later by dysarthria, dysphagia, facial weakness, and a gaze preference. A chest X‐ray showed pneumonia and nasopharyngeal RT‐PCR was positive for SARS‐CoV‐2. Head CT showed multiple areas of patchy hypoattenuation and an MRI showed areas of increased FLAIR and T2 signal in the subcortical and deep white matter that were felt to be consistent with demyelination. Her CSF was normal. A second reported case was in a 54‐year‐old woman who developed seizures and neurological deterioration (GCS = 12) and had chest X‐ray lesions consistent with COVID‐19 and a positive nasopharyngeal RT‐PCR for SARS‐CoV‐2. 51 Her MRI showed multiple periventricular T2 hyperintense, nonenhancing, lesions in the white matter of the cerebrum, brainstem, and spinal cord consistent with multifocal demyelination. Her CSF studies were unremarkable, including a negative CSF RT‐PCR for SARS CoV‐2. She was treated with high dose dexamethasone and her symptoms gradually resolved. A single case of acute flaccid myelitis has also been described in COVID‐19. 52 This patient developed upper limb weakness and a flaccid areflexic lower limb paralysis, urinary and bowel incontinence, and a T10 sensory level. Unfortunately, neither spine imaging nor CSF studies were available so the mechanism remains unknown. The most convincing example of ADEM‐like pathology associated with COVID‐19 was in a 71‐year‐old man who developed symptoms immediately following coronary bypass graft surgery that progressed to respiratory failure and a hyperinflammatory state. A postmortem examination showed brain swelling and disseminated hemorrhagic lesions and subcortical white matter pathology with perivenular myelin injury but also necrotic blood vessels and perivascular inflammation. The lesions had features of both acute hemorrhagic leukoencephalitis and of acute disseminated encephalomyelitis. 53
The rarity of postinfectious potentially immune‐mediated cases following COVID‐19 other than GBS and its variants, and the general paucity of details, makes their status unclear. The cases of ADEM‐like illness are hard to distinguish from some of the patients with acute encephalopathy and associated MRI white matter lesions, but can be differentiated from cases of encephalitis by the absence of CSF pleocytosis. GBS is a common neurological disease even in the absence of COVID‐19, and identifying the magnitude of the COVID‐19 risk and association will require better epidemiological data. However, the 5 cases of GBS occurring in a population of 1,000 to 1,200 patients with COVID‐19 seen over a 1 month period by Toscano et al in Northern Italy suggest an incidence that is much higher than that can be expected in the general population (~1/100,000 person‐years). 54 The mechanism of pathogenesis will need to be identified, and the efficacy of conventional therapies, including ivIG and plasma exchange, evaluated.
Other COVID‐19 Related Neurological Disorders
One of the more striking reported symptom manifestations in patients with COVID‐19 is loss or perturbation of smell (anosmia or hyposmia) and/or taste (dysgeusia). The frequency of these symptoms, their specificity as a potential diagnostic clue for COVID‐19 infection as opposed to influenza or other symptomatologic similar diseases, and their implication for understanding viral pathogenesis all remain uncertain. In the Wuhan COVID‐19 series, impairment of smell was noted in 5% and of taste in 6% of the 214 hospitalized patients. 11 It is likely that the frequency was under‐represented due to incomplete evaluations in these hospitalized sick patients. A later study of 31 patients, suggested that disorders of taste occurred in 81% of COVID‐19 cases (46% anosmia, 29% hyposmia, and 6% dysosmia) and disorders of taste in 94% (ageusia 45%, hypogeusia 23%, and dysgeusia 26%). 55 The average duration of smell and taste disorders in the COVID‐19 cases was 7.1 ± 3.1 days. A multicenter European study of 417 cases with “mild‐to‐moderate” COVID‐19 disease found a similarly high frequency of olfactory dysfunction (86%), with 80% of those affected having anosmia and 20% hyposmia. 56 Approximately 70% of patients had recovered within 8 days of symptom onset. It has been suggested that olfactory and/or gustatory dysfunction may be indicative of neuro‐invasion and provide a route from the nasopharynx or oropharynx to cardiorespiratory centers in the medulla, based on studies of transgenic mice expressing the human SARS virus receptor (ACE2) and infected with SARS‐CoV, however, no evidence supporting host entry via this pathway yet exists in man. 29 The transient nature of the dysfunction in most patients would seem to make direct viral infection and subsequent killing of olfactory or gustatory neurons unlikely. MRI of the olfactory bulb was normal in one RT‐PCR confirmed patient with anosmia. 57
In the Wuhan COVID‐19 series, 11% of patients were reported to have evidence of skeletal muscle injury (defined as a creatine kinase [CK] >200 U/L and skeletal muscle pain). 11 Injury was significantly more common in patients with “severe” disease (19%) compared to non‐severe disease (5%; p < 0.001). Unfortunately, almost no clinical details were provided beyond the presence of associated muscle pain. Subsequently two reports have emerged of rhabdomyolysis as either a presenting feature or a late complication of COVID‐19. 58 , 59 One patient had limb pain and weakness with a peak CK of ~12,000 U/L and myoglobulin >12,000 μg/L, and the other had a peak CK of 13,581 U/L. Neither patient had muscle biopsy performed. The mechanism of injury remains to be determined.
Immunopathogenesis of SARS‐CoV‐2 and Implication for Management and Treatment of Neurologic Manifestations
One of the most puzzling features of SARS‐CoV‐2 infection is that it is asymptomatic or associated with minor symptoms in approximately 80% of patients, especially children and young adults, whereas 20% will develop COVID‐19 with various degrees of severity. Can knowledge gathered on SARS‐CoV inform us about the immunopathogenesis of SARS‐CoV‐2? A successful production of type I interferon (IFN) response is a key first line defense for suppressing replication of many neurotropic viruses at the site of entry and dissemination. SARS‐CoV suppresses type I IFN response and downstream signaling using multiple strategies, and this dampening is closely associated with disease severity. 60
Because SARS‐CoV‐2 shares an overall genomic similarity of 80% with SARS‐CoV and uses the same receptor, it is reasonable to expect that the innate immune mechanisms involved in pathogenesis will be similar for the two viruses. SARS‐CoV has developed multiple strategies to evade the innate immune response in order to optimize its replication capacity. 61 It seems likely that SARS‐CoV‐2 uses the same strategy. The magnitude of the immune response against SARS‐CoV‐2 needs to be precisely calibrated to control viral replication without triggering immunopathogenic injury. A hyperinflammatory response likely plays a major role in ARDS and, in a subset of children, may contribute to the development of a Kawasaki‐like multisystem inflammatory disorder. 20 In a mouse model of SARS, rapid SARS‐CoV replication and delay in IFN‐I signaling led to inflammatory monocyte–macrophage accumulation, resulting in elevated lung cytokine/chemokine levels and associated vascular leakage and lethal pneumonia. This “cytokine storm,” in turn, was associated with a decrease in T cell counts and suboptimal T cell responses to SARS‐CoV infection. 62
The same pattern is found in 522 patients with COVID‐19, where the number of total T cells, CD4+ and CD8+ T cells, were dramatically reduced, especially in those requiring ICU care, and T cell numbers were negatively correlated to serum IL‐6, IL‐10, and TNF‐α concentration. Conversely, patients in the disease resolution period showed reduced IL‐6, IL‐10, and TNF‐α levels and restored T cell counts. 63 These data were corroborated by other groups who also noticed a decrease in type 1 interferon response in severely affected patients. 64 , 65 It has been suggested that reduced and delayed IFN gamma production (“too little and too late”) in the lungs and depletion of both CD4+ and CD8+ T cells may combine to potentiate viral injury, by reducing control of viral replication and enhancing the upregulation of pro‐inflammatory cytokines, including TNF‐α, IL‐6, and IL‐10 (“cytokine storm”), and that it may be the immune dysregulation as much or more than the direct viral infection that results in pulmonary epithelial cell injury, and similar mechanisms could be operative in the CNS. 66
What are the possible mechanisms for the apparent immune dysregulation seen in those patients and could they have a role in the neuropathogenesis of COVID‐19? The source of cytokines found in the serum in unclear, but they could be produced by lung macrophages. IL‐6 could also come from infected neurons, as seen in a transgenic mouse model of SARS‐Cov. 29 A high level of circulating cytokines, in turn, could lead to lymphocytopenia. TNF‐α, a pro‐inflammatory cytokine, may cause T cell apoptosis via interacting with its receptor, TNFR1, which expression is increased in aged T cells. 67 , 68 IL‐6, that has both pro‐inflammatory and anti‐inflammatory properties, contributes to host defense in response to infections. However, continual synthesis of IL‐6 has been shown to play a pathological role in chronic inflammation and infection. 69 , 70 IL‐10, an inhibitory cytokine that prevents T cell proliferation, can also induce T cell exhaustion. Interestingly, patients with COVID‐19 have high levels of the PD‐1 and Tim‐3 exhaustion markers on their T cells. 63 In turn, decreased numbers of CD4+ and CD8+ T lymphocytes will considerably weaken the cellular immune response to SARS‐CoV‐2 in severe cases, allowing further viral replication. This can be compounded by the use of corticosteroids. Of note, a study in convalescent patients with SARS‐CoV showed that CD8+ T cell responses were more frequent and had a greater magnitude of response than CD4+ T cells. 71 Finally, one autopsy series of patients with COVID‐19 showed histological features suggestive of secondary hemophagocytic lymphohistiocytosis (sHLH), also known as macrophage activation syndrome. This syndrome is characterized by an imbalance of innate and adaptive immune responses with aberrant activation of macrophages, and a blunted adaptive immune response. 20
This dysregulated immune response may have a role in the pathogenesis of the COVID‐19 encephalopathy. High levels of circulating pro‐inflammatory cytokines can cause a confusion and alteration of consciousness, whereas a weakened T cell response may be unable to eliminate virus‐infected cells in the brain causing further neurologic dysfunction. Careful studies of the CSF cytokine profile and T cell response to SARS‐CoV‐2 as well as postmortem studies, including CNS and muscle tissues, are urgently needed to better understand the neuropathogenesis of COVID‐19. These will help inform whether therapeutic strategies aimed at blocking pro‐inflammatory cytokines, including the IL‐6 inhibitors tocilizumab and sarilumab, could have a beneficial effect on encephalopathy or whether corticosteroids that dampened the adaptive cellular immune response to viruses are contra‐indicated. As we strive to find medications to counter the deleterious inflammatory state triggered by SARS‐CoV‐2, lessons can also be learned from COVID‐19 outcomes in patients with neurological diseases, such as multiple sclerosis or myasthenia gravis, treated with immunomodulatory therapies.
Although we are only starting to grasp the complexity of SARS‐CoV‐2 biology, it is already apparent that COVID‐19 causes a global threat to the entire nervous system, both through its worldwide distribution and multifactorial pathogenic mechanisms (Fig). As we hope for a vaccine or a cure, neurologists will play an important role in diagnosing, investigating, and treating the many neurologic manifestations of COVID‐19 (Table). 72
TABLE 1.
Neurologic Conditions Associated with SARS‐CoV‐2 Infection
| Disease entity | Presentation | Supportive Neurodiagnostic testing | Pathogenesis |
|---|---|---|---|
| Encephalopathy | Altered mental status |
MRI: non‐specific EEG: abnormal (slow) CSF: nl cells and Pro CSF SARS‐CoV‐2 RT‐PCR: NEG |
Multiple organ failure Hypoxemia Systemic Inflammation Endothelialitis |
| Encephalitis | Altered mental status and CNS dysfunction |
MRI: non‐specific (? WM changes) EEG: abnormal (slow, +focal) CSF: pleocytosis & elev. Pro CSF SARS‐CoV‐2 RT‐PCR: NEG |
CNS inflammation |
| Viral encephalitis | Altered mental status and CNS dysfunction |
MRI: new abnormality EEG: abnormal (slow, ±focal) CSF: Pleocytosis and elev. Pro CSF SARS‐CoV‐2 RT‐PCR: POS Brain Tissue: POS (Ag or RNA) |
Brain parenchymal neuro‐invasion |
| Viral meningitis | Headache, nuchal rigidity |
MRI: meningeal enhancement, CSF: pleocytosis & elev. Pro CSF SARS‐CoV‐2 RT PCR: POS |
Subarachnoid invasion |
| Stroke | Focal motor or sensory deficit | MRI: ischemia or bleed, abnormal coagulation factors, increased inflammatory markers | Coagulopathy |
| Anosmia/ageusia | Olfactory or taste dysfunction | Abnormal smell/taste tests | ? Peripheral vs central neuro‐invasion |
| ADEM | Headache, acute neurologic symptoms | MRI: hyperintense FLAIR lesions with variable enhancement | Postinfectious |
| Guillain‐Barre syndrome | Flaccid muscle weakness |
CSF: increased protein, nl WBC CSF SARS‐CoV‐2 RT‐PCR: NEG EMG/NCS: abnormal |
Postinfectious |
| Muscle injury | Myalgia | CK elevated | Myopathy or myositis? |
ADEM = acute disseminated encephalomyelitis; CNS = central nervous system; CK= creatinine kinase; CSF = cerebrospinal fluid; EEG = electroencephalogram; EMG = electromyogram; FLAIR = fluid‐attenuated inversion recovery; MRI = magnetic resonance imaging; NCS = nerve conduction study; NEG = negative; POS = positive; pro = protein; RT‐PCR = reverse transcriptase‐polymerase chain reaction; SARS‐CoV‐2 = severe acute respiratory syndrome‐coronavirus type 2; WBC = white blood cell; WM = white matter.
FIGURE 1.
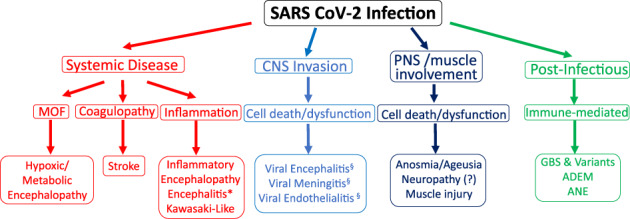
Mechanisms of severe acute respiratory syndrome‐coronavirus type 2 (SARS‐CoV‐2) neuropathogenesis. SARS‐CoV‐2 pathogenic effects on the nervous system are likely multifactorial, including manifestations of systemic disease, direct neuro‐invasion of the central nervous system (CNS), involvement of the peripheral nervous system (PNS) and muscle, as well as through a postinfectious, immune‐mediated mechanism. MOF = multi‐organ failure; GBS = Guillain‐Barre syndrome. *CNS inflammation (CSF pleocytosis and proteinorrachia) with no evidence of direct viral infection of CNS; §direct evidence of viral invasion (reverse transcriptase‐polymerase chain reaction positive [RT‐PCR+], biopsy); ADEM = acute disseminated encephalomyelitis; ANE = acute necrotizing encephalopathy. [Color figure can be viewed at www.annalsofneurology.org]
Potential Conflicts of Interest
Nothing to report.
Acknowledgment
We thank Jeffrey R. Clark for editorial assistance.
References
- 1. Sanche S, Lin YT, Xu C, et al. The novel coronavirus, 2019‐nCoV, is highly contagious and more infectious than initially estimated. medRxiv 2020. 10.1101/2020.02.07.20021154. [DOI] [Google Scholar]
- 2. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020;579:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andersen KG, Rambaut A, Lipkin WI, et al. The proximal origin of SARS‐CoV‐2. Nat Med 2020;26:450–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uhlén M, Fagerberg L, Hallström BM, et al. Tissue‐based map of the human proteome. Science 2015;347:1260419. [DOI] [PubMed] [Google Scholar]
- 5. Algahtani H, Subahi A, Shirah B. Neurological complications of Middle East respiratory syndrome coronavirus: a report of two cases and review of the literature. Case Rep Neurol Med 2016;2016:1–6. 10.1155/2016/3502683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arabi YM, Harthi A, Hussein J, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS‐CoV). Infection 2015;43:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim JE, Heo JH, Kim HO, et al. Neurological complications during treatment of Middle East respiratory syndrome. J Clin Neurol 2017;13:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lau KK, Yu WC, Chu CM, et al. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis 2004;10:342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsai LK, Hsieh ST, Chao CC, et al. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol 2004;61:1669–1673. [DOI] [PubMed] [Google Scholar]
- 10. Xu J, Zhong S, Liu J, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis 2005;41:1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;6683–6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu L, Xiong W, Liu D, et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia 2020;1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dugue R, Cay‐Martinez KC, Thakur K, et al. Neurologic manifestations in an infant with COVID‐19. Neurology 2020. 10.1212/wnl.0000000000009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sohal S, Mossammat M. COVID‐19 presenting with seizures. IDCases 2020;20:e00782. 10.1016/j.idcr.2020.e00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med 2020;382:2268–2270. 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kandemirli SG, Dogan L, Sarikaya ZT, et al. Brain MRI findings in patients in the intensive care unit with COVID‐19 infection. Radiology 2020;201697. 10.1148/radiol.2020201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Radmanesh A, Derman A, Lui YW, et al. COVID‐19 ‐associated diffuse leukoencephalopathy and microhemorrhages. Radiology 2020;202040. 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pugin D, Vargas M‐I, Thieffry C, et al. Covid‐19‐related encephalopathy responsive 18 to high doses glucocorticoids. Neurology 2020; in press. [DOI] [PubMed] [Google Scholar]
- 19. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet 2020;395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bryce C, Grimes Z, Pujadas E, et al. Pathophysiology of SARS‐CoV‐2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID‐19 autopsy experience. medRxiv 2020. 10.1101/2020.05.18.20099960. [DOI] [Google Scholar]
- 21. Li YWM, Zhou Y, Jiang C, et al. Acute cerebrovascular disease following COVID‐19: a single center, retrospective, observational study. Lancet 2020. in press. 10.2139/ssrn.3550025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID‐19. J Neurol Neurosurg Psychiatry 2020;jnnp‐2020‐323586. 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med 2020;382:e38. 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coolen T, Lolli V, Sadeghi N, et al. Early postmortem brain MRI findings in COVID‐19 non‐survivors. medRxiv 2020. 10.1101/2020.05.04.20090316. [DOI] [PubMed] [Google Scholar]
- 25. Oxley TJ, Mocco J, Majidi S, et al. Large‐vessel stroke as a presenting feature of Covid‐19 in the young. N Engl J Med 2020;382:e60. 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jones VG, Mills M, Suarez D, et al. COVID‐19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr 2020;10:537–540. 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 27. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. The Lancet 2020;395:1771‐1778. 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS‐CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol 2004;203:622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Netland J, Meyerholz DK, Moore S, et al. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 2008;82:7264–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS‐Coronavirus‐2. Int J Infect Dis 2020;94:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duong L, Xu P, Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID‐19 infection in downtown Los Angeles, early April 2020. Brain Behav Immun 2020. 10.1016/j.bbi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang YH, Jiang D, Huang JT. SARS‐CoV‐2 detected in cerebrospinal fluid by PCR in a case of COVID‐19 encephalitis. Brain Behav Immun 2020. 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bernard‐Valnet R, Pizzarotti B, Anichini A, et al. Two patients with acute meningo‐encephalitis concomitant to SARS‐CoV‐2 infection. Eur J Neurol 2020. 10.1111/ene.14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pilotto A, Odolini S, Masciocchi S, et al. Steroid‐responsive severe encephalopathy in SARS‐CoV‐2 infection. Ann Neurol 2020. (Epub ahead of print). 10.1002/ana.25783. [DOI] [Google Scholar]
- 35. Dogan L, Kaya D, Sarikaya T, et al. Plasmapheresis treatment in COVID‐19‐related autoimmune meningoencephalitis: case series. Brain Behav Immun 2020. 10.1016/j.bbi.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Panariello A, Bassetti R, Radice A, et al. Anti‐NMDA receptor encephalitis in a psychiatric Covid‐19 patient: a case report. Brain Behav Immun 2020. 10.1016/j.bbi.2020.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA 2013;310:308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mishra N, Ng TFF, Marine RL, et al. Antibodies to enteroviruses in cerebrospinal fluid of patients with acute flaccid myelitis. mBio 2019;10:e01903–e01919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schubert RD, Hawes IA, Ramachandran PS, et al. Pan‐viral serology implicates enteroviruses in acute flaccid myelitis. Nat Med 2019;25:1748–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hixon AM, Frost J, Rudy MJ, et al. Understanding enterovirus D68‐induced neurologic disease: a basic science review. Viruses 2019;11:821. 10.3390/v11090821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alberti P, Beretta S, Piatti M, et al. Guillain‐Barre syndrome related to COVID‐19 infection. Neurol Neuroimmunol Neuroinflamm 2020;7:e741. 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Camdessanche JP, Morel J, Pozzetto B, et al. COVID‐19 may induce Guillain‐Barre syndrome. Rev Neurol 2020;176:516–518. 10.1016/j.neurol.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gutierrez‐Ortiz C, Mendez A, Rodrigo‐Rey S, et al. Miller fisher syndrome and polyneuritis cranialis in COVID‐19. Neurology 2020. 10.1212/wnl.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 44. Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID‐19 infection: a case report. J Clin Neurosci 2020;76:233–235. 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Virani A, Rabold E, Hanson T, et al. Guillain‐Barre syndrome associated with SARS‐CoV‐2 infection. IDCases 2020;20:e00771. 10.1016/j.idcr.2020.e00771:e00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao H, Shen D, Zhou H, et al. Guillain‐Barre syndrome associated with SARS‐CoV‐2 infection: causality or coincidence? Lancet Neurol 2020;19:383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Toscano G, Palmerini F, Ravaglia S, et al. Guillain‐Barre syndrome associated with SARS‐CoV‐2. N Engl J Med 2020. 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Poyiadji N, Shahin G, Noujaim D, et al. COVID‐19‐associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 2020;201187. 10.1148/radiol.2020201187:201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dixon L, Varley J, Gontsarova A, et al. COVID‐19‐related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflam 2020;7:e789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang T, Rodricks MB, Hirsh E. COVID‐19‐associated acute disseminated encephalomyelitis: a case report. medRxiv 2020. 10.1101/2020.04.16.20068148. [DOI] [Google Scholar]
- 51. Zanin L, Saraceno G, Panciani PP, et al. SARS‐CoV‐2 can induce brain and spine demyelinating lesions. Acta Neurochir 2020. 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao K, Huang J, Dai D, et al. Acute myelitis after SARS‐CoV‐2 infection: a case report. medRxiv 2020. 10.1101/2020.03.16.20035105. [DOI] [Google Scholar]
- 53. Reichard RR, Kashani KB, Boire NA, et al. Neuropathology of COVID‐19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)‐like pathology. Acta Neuropathol 2020. 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain‐Barré syndrome: a systematic review and meta‐analysis. Neuroepidemiology 2011;36:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Beltran‐Corbellini A, Chico‐Garcia JL, Martinez‐Poles J, et al. Acute‐onset smell and taste disorders in the context of Covid‐19: a pilot multicenter PCR‐based case‐control study. Eur J Neurol 2020. 10.1111/ene.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol 2020. 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Galougahi MK, Ghorbani J, Bakhshayeshkaram M, et al. Olfactory bulb magnetic resonance imaging in SARS‐CoV‐2‐induced anosmia: the first report. Acad Radiol 2020;27:892–893. 10.1016/j.acra.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Suwanwongse K, Shabarek N. Rhabdomyolysis as a presentation of 2019 novel coronavirus disease. Cureus 2020;12:e7561. 10.7759/cureus.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID‐19. Emerg Infect Dis 2020;26. 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017;39:529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nelemans T, Kikkert M. Viral innate immune evasion and the pathogenesis of emerging RNA virus infections. Viruses 2019;11:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte‐macrophage responses cause lethal pneumonia in SARS‐CoV‐infected mice. Cell Host Microbe 2016;19:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID‐19). Front Immunol 2020;11:827. 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis 2020. 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Trouillet‐Assant S, Viel S, Gaymard A, et al. Type I IFN immunoprofiling in COVID‐19 patients. J Allergy Clin Immunol 2020. 10.1016/j.jaci.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pedersen SF, Ho YC. SARS‐CoV‐2: a storm is raging. J Clin Invest 2020;130:2202–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Aggarwal S, Gollapudi S, Gupta S. Increased TNF‐alpha‐induced apoptosis in lymphocytes from aged humans: changes in TNF‐alpha receptor expression and activation of caspases. J Immunol 1999;162:2154–2161. [PubMed] [Google Scholar]
- 68. Gupta S, Bi R, Kim C, et al. Role of NF‐kappaB signaling pathway in increased tumor necrosis factor‐alpha‐induced apoptosis of lymphocytes in aged humans. Cell Death Differ 2005;12:177–183. [DOI] [PubMed] [Google Scholar]
- 69. Gabay C. Interleukin‐6 and chronic inflammation. Arthritis Res Ther 2006;8:S3. 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jones SA, Jenkins BJ. Recent insights into targeting the IL‐6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol 2018;18:773–789. [DOI] [PubMed] [Google Scholar]
- 71. Li CK, Wu H, Yan H, et al. T cell responses to whole SARS coronavirus in humans. J Immunol 2008;181:5490–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McArthur JC. The path forward: academic neurology responds to COVID‐19. Ann Neurol 2020;87:789–793. 10.1002/ana.25773. [DOI] [PMC free article] [PubMed] [Google Scholar]


