Abstract
To recognize the period of exaggerated cytokine response in patients with coronavirus disease 2019 (COVID‐19) pneumonia, and to describe the clinical outcomes of using tocilizumab as a treatment option. The data of 12 adult COVID‐19 pneumonia patients who were followed in the inpatient clinics of Biruni University Medical Faculty Hospital (Istanbul, Turkey) were retrospectively analyzed. Diagnostic tests, laboratory examinations, clinical findings, and computed tomography of the thorax imaging results were evaluated. A dramatic laboratory and clinical improvement was observed in 83% (10 out of 12) of patients after tocilizumab. In 17% (2 out of 12) of our patients, short‐term ventilator support was required in the intensive care unit. The longest hospital stay was 18 days. However, in the end, all of our patients were discharged home with good health. Although arterial oxygen saturations (87.58 ± 3.12%) dropped in room air in the pre‐tocilizumab period, post‐tocilizumab they normalized in all patients (94.42 ± 1%). None of them had fever after tocilizumab treatment and the levels of C‐reactive protein (13.08 ± 12.89) were almost within normal limits. Eosinophil values were quite low at the time of diagnosis (10 ± 17.06), but increased significantly post‐tocilizumab (155.33 ± 192.69). There is currently no proven treatment for COVID‐19 induced by novel coronavirus SARS‐CoV‐2. Based on our experience with twelve adult COVID‐19 pneumonia patients, we can say that tocilizumab, an IL‐6 inhibitor, is more beneficial in preventing the damage caused by excessive cytokine response in the body if administered at the right time and provides clinical and radiological recovery.
Keywords: COVID‐19, Pneumonia, SARS‐Cov‐2, Tocilizumab
1. INTRODUCTION
The world began to receive news of a novel coronavirus that was unlike its previous forms in the Wuhan region of China in late December 2019. 1 Although this virus, which caused severe acute respiratory syndrome, was originally named 2019‐nCov, later its name was changed to SARS‐CoV‐2, due to its similarities with the virus that caused the severe acute respiratory syndrome (SARS) epidemic in 2003. 2 , 3 This respiratory disease caused by SARS‐CoV‐2 started to be referred to as coronavirus disease 2019 (COVID‐19). 4
The epidemic had now engulfed the whole world and on 10 March 2020, the World Health Organization declared a pandemic. 5 In the process since the isolation of SARS‐CoV‐2, no effective drug has yet been found to treat it completely. Many researchers around the world are investigating the effectiveness of dozens of drugs on COVID‐19 and trying to find a solution to this disease. Antiviral agents such as lopinavir, ritonavir, 6 remdesivir, 7 antibacterial drugs such as macrolides, 8 antiparasitic drugs such as ivermectin, 9 antimalarial drugs such as hydroxychloroquine, 10 corticosteroids, and drugs with anti‐inflammatory activities such as colchicine 11 are being investigated and their effectiveness is being discussed.
Tocilizumab, a recombinant humanized anti‐human interleukin‐6 (IL‐6) receptor monoclonal antibody, is also thought to be useful in COVID‐19 treatment. 12 COVID‐19 is clinically classified as a mild, moderate, severe, and critical disease. 13 When the disease has reached the modarete level, lung involvement is observed at various stages. 13 The reason why tocilizumab, a blocker of IL‐6, is considered a promising treatment in COVID‐19, is that SARS‐CoV‐2 causes a condition similar to the cytokine storm syndrome, and it is known that IL‐6 plays a proinflamatory role in this inflammatory cascade. 12 Therefore, tocilizumab has begun to take its place in the treatment guidelines of several countries like China and Italy. 14
Since December 2019, the process of outbreak was being followed very closely by the Republic of Turkey, Ministry of Health. Precautions were taken against COVID‐19 and preparations were made for the possibility of being involved in the pandemic. The first case in our country was reported on 10 March 2020. In our center, COVID‐19 patients started to be admitted since 18 March 2020. We followed the treatment guidelines of our Ministry of Health, which were recommended and updated regularly by the National COVID‐19 Scientific Committee, to treat our patients. In our country, as in China and Italy, tocilizumab was presented as a treatment option, considering that a cytokine storm was introduced during COVID‐19. As of 18 March 2020, we started tocilizumab treatment to 12 patients who were admitted with COVID‐19 pneumonia and thought to be in a cytokine storm. By sharing our experience in this clinical series, we aimed to guide at which point we should choose tocilizumab and what were the clinical, laboratory, and radiological outcomes.
2. METHODS
2.1. Patients
Twelve patients who were followed and treated with the diagnosis of COVID‐19 pneumonia between 18 March and 8 April 2020 at Biruni University Faculty of Medicine Hospital (Istanbul, Turkey), and who received tocilizumab treatment because it was considered that they entered the cytokine storm during this period, were included in the study. Approval was obtained from the Biruni University Medical Research Ethics Committee for the study. Informed consent was obtained from all patients in that they agreed to publish their data in this case series. We will protect patients' privacy and comply with the Helsinki Declaration.
2.2. Diagnosis
Computed tomography (CT) of the thorax was done at the time of admission to all patients included in the study. As suggested in the guidelines of our Ministry of Health, an oropharyngeal sample was first taken with a swab, then a nasal sample was taken using the same swab and placed in the same transport medium for diagnosis. 15 Samples were tested by real‐time reverse‐transcriptase‐polymerase chain reaction (RT‐PCR) assay developed from the virus sequence. As the drugs used in the treatment (especially azithromycin and hydroxychloroquine) may cause prolonged QT duration, the patients' electrocardiograms (ECGs) were taken.
2.3. Laboratory tests‐vital signs
The biochemical parameters such as complete blood counts (CBCs), the levels of creatinine, sodium, potassium, alanine aminotransferase (ALT), aspartate aminotransaminase (AST), lactate dehydrogenase (LDH), D‐dimer, ferritin, troponin, and C‐reactive protein (CRP) were measured with fasting blood samples by routine methods with commercial kits. All laboratory tests and vital signs (temperature, systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), arterial oxygen saturation (SpO2), respiratory rate (RR)) of our patients retrospectively on the day of diagnosis, the day before tocilizumab application and 2 days (48 hours) after the second tocilizumab application.
2.4. Treatment—tocilizumab administration decision
Clinical syndromes associated with COVID‐19 have been classified by the World Health Organization (WHO) as mild disease, pneumonia, severe pneumonia, acute respiratory distress syndrome, sepsis, and septic shock. 15 Indication for hospitalization begins at the stage of pneumonia. In addition to fever or suspected respiratory infection, the presence of one of these criteria were the criteria used to diagnose severe pneumonia; respiratory rate> 30 breaths/min, or severe respiratory distress, or SpO2 ≤ 93% on room air. In the next step, patients are recommended to evaluate the indication of intensive care in accordance with the specified criteria.
After the examinations for the diagnosis, we hospitalized our 12 patients that were diagnosed with COVID‐19 pneumonia to our inpatient special COVID clinic (non‐intensive care unit). According to the WHO classification, all of them had severe pneumonia (Table 1). As suggested in the COVID‐19 guidelines of our Ministry of Health, initially all of our patients were treated with hydroxychloroquine (first day, 2 × 400 mg loading + 4 days, 2 × 200 mg maintenance), oseltamivir (2 × 75 mg, 5 days), azytromycin (1 × 500 mg, number of days depending on the disease), or moxifloxacin (1 × 400 mg, depending on the condition of the disease) 16 (Table 1). We also provided nasal oxygen support to all of our patients according to their needs.
Table 1.
Hospitalization times and treatment features of our patients
| Length of hospital stay, d | Standard treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case number | Sex | Age | Date of hospitalization | Stage of illness | CS | ICU | H | O | A | M | Hospitalization day of tocilizumab application |
| Case 1 | M | 58 | 3/18/20 | Severe pneumonia | 12 | ✓ | ✓ | ✓ | 8‐9 | ||
| Case 2 | F | 76 | 3/20/20 | Severe pneumonia | 10 | ✓ | ✓ | ✓ | 6‐7 | ||
| Case 3 | F | 47 | 3/20/20 | Severe pneumonia | 11 | ✓ | ✓ | ✓ | 5‐6 | ||
| Case 4 | M | 67 | 3/20/20 | Severe pneumonia | 12 | ✓ | ✓ | ✓ | 2‐3 | ||
| Case 5 | F | 69 | 3/20/20 | Severe pneumonia | 10 | 8 | ✓ | ✓ | ✓ | 3‐4 | |
| Case 6 | M | 79 | 3/23/20 | Severe pneumonia | 11 | 7 | ✓ | ✓ | ✓ | 4‐5 | |
| Case 7 | M | 47 | 3/23/20 | Severe pneumonia | 10 | ✓ | ✓ | ✓ | 6‐7 | ||
| Case 8 | M | 77 | 3/24/20 | Severe pneumonia | 10 | ✓ | ✓ | ✓ | 6‐7 | ||
| Case 9 | M | 63 | 3/24/20 | Severe pneumonia | 14 | ✓ | ✓ | ✓ | 9‐10 | ||
| Case 10 | F | 70 | 3/24/20 | Severe pneumonia | 12 | ✓ | ✓ | ✓ | 9‐10 | ||
| Case 11 | F | 78 | 3/30/20 | Severe pneumonia | 10 | ✓ | ✓ | ✓ | 5‐6 | ||
| Case 12 | F | 59 | 3/31/20 | Severe pneumonia | 7 | ✓ | ✓ | ✓ | 2‐3 | ||
Abbreviations: A, azytromycin; CS, covid service; H, hydroxychloroquine; ICU, intensive care unit; M, moxifloxacin.
Despite the standard treatment used, we concluded that the patients with increased acute phase reactants, persistent fever, decreased SpO2 and deepened respiratory distress, progress to the cytokine storm period. We decided to use tocilizumab, which can regulate the immune system overreaction when patients are on this threshold of intensive care.
2.5. Tocilizumab application
A volume of liquid equal to the tocilizumab concentration calculated for the patient under aseptic conditions (10 mL for 200 mg, 20 mL for 400 mg, and 40 mL for 800 mg) was taken from sterile 100 mL of isotonic sodium chloride (0.9%) infusion solution. The amount of tocilizumab concentrate to be applied is drawn from the vial and added to a 100 mL infusion bag. The final fluid volume in the infusion bag was adjusted to be 100 mL and the solution in the bag was slowly turned upside down and mixed without foaming. It was administered intravenously within 1 hour.
As suggested in the guidelines of our Ministry of Health, when the first dose of tocilizumab was administered as 400 mg, a dose of 400 mg was repeated within 24 hours, taking into account the changes in clinical and laboratory findings. 16
2.6. Data collection
Data on patients' gender, age, concomitant diseases, clinical symptoms, treatments used, swab sample and presence of thorax CT findings, laboratory findings and vital signs on the day of diagnosis pre‐tocilizumab and post‐tocilizumab, hospital stay, and discharge methods were recorded.
2.7. Statistical analysis
Statistical analyses were performed with the Number Cruncher Statistical System (NCSS) 2020 Statistical Software (Utah) package program. In addition to descriptive statistical methods in the evaluation of the data, the distribution of variables was examined with the Shapiro‐Wilk normality test. One‐way analysis of variance paired in time comparisons of normally distributed variables, Newman‐Keuls multiple comparison test in subgroup comparisons, Friedman test in time comparisons of variables that do not have normal distribution, Dunn's multiple comparison test in subgroup comparisons, and in the comparison of qualitative data, Stuart‐Maxwell test, and McNemar's test were used. The results were evaluated at the significance level of P < .05.
3. RESULTS
3.1. Demographic characteristics and clinical presentations
There were a total of 12 cases, half of whom were male (50%, n = 6) and half were female (50%, n = 6) (Table 2). The average age of the cases was 65.83 ± 11.30 years, and ranged between 47 and 79 years. All of the patients complained of cough (12 out of 12, 100%). Fatigue and fever were also present in most patients (11 out of 12, 92%). The most frequent complaints were, respectively, loss of smell after‐taste (9 out of 12, 75%) and dyspnea (8 out of 12, 67%). Two of our patients had diarrhea (2 out of 12, 17%) (Table 2). As for the concomitant diseases of the patients, diabetes mellitus (7 out of 12, 58%) and hypertension (7 out of 12, 58%) were in the first place (Table 2).
Table 2.
Demographic features, clinical presentations and comorbidities of patients
| Characteristic | Patients (n = 21) | Patients, % |
|---|---|---|
| Sex | 12 | 100 |
| Male | 6 | 50 |
| Female | 6 | 50 |
| Diagnostic method | ||
| RT‐PCR | 11 | 92 |
| Thorax CT | 12 | 100 |
| Complaints | ||
| Cough | 12 | 100 |
| Fever | 11 | 92 |
| Fatigue | 11 | 92 |
| Taste‐smell loss | 9 | 75 |
| Dyspnea | 8 | 67 |
| Diarrhea | 2 | 17 |
| Comorbidities | ||
| Diabetes mellitus | 7 | 58 |
| Hypertension | 7 | 58 |
| Atrial fibrillation | 4 | 33 |
| Chronic obstructive pulmonary disease and/or asthma | 3 | 25 |
| Malignancy | 2 | 16 |
| Chronic renal failure | 1 | 8 |
Abbreviations: CT, computed tomography; RT‐PCR, real‐time reverse‐transcriptase polymerase chain reaction.
3.2. Laboratory examinations
RT‐PCR was positive in 11 out of 12 patients (92%) in the swab taken from the oropharyngeal and nasal samples of the patients (Table 2).
In the comparisons between the data, the values of white blood cell count (WBC), neutrophils (Neut), lymphocytes (Lymp), eosinophil, hemoglobin (Hb), hematocrit (Htc), platelet (Plt), mean platelet volume (MPV), CRP, LDH, D‐dimer were statistically significant on the day of patients' admission, pre‐tocilizumab and post‐tocilizumab and advanced multiple variance tests were applied (Table 3).
Table 3.
Single variance analysis of laboratory and clinical findings
| Diagnosis day | Pre‐tocilizumab a | Post‐tocilizumab b | P | |
|---|---|---|---|---|
| WBC, cells/μL | 6103.33 ± 2077.85 | 5435.83 ± 2643.56 | 5542.5 ± 2196.73 | .01‡ |
| Neut, cells/μL | 4310.83 ± 1816.85 | 3731.67 ± 2252.28 | 3208.33 ± 1472.57 | .087‡ |
| Lymp, cells/μL | 1096.67 ± 376.89 | 1142.5 ± 408.59 | 1390.83 ± 775.41 | .235‡ |
| Eosinophil, cells/μL | 10 ± 17.06 | 39.17 ± 31.75 | 155.33 ± 192.69 | .0001* |
| Hb, g/dL | 13.83 ± 1.34 | 13.08 ± 1.67 | 12.75 ± 1.42 | .038‡ |
| Htc, % | 40.08 ± 3.5 | 38.25 ± 4.81 | 37.75 ± 4.14 | .009‡ |
| Plt, K/µL | 180.08 ± 56.01 | 219.25 ± 107.33 | 345.67 ± 99.76 | .0001‡ |
| MPV, fL | 10.25 ± 0.62 | 10.08 ± 0.79 | 9.25 ± 0.62 | .001‡ |
| ALT, U/L | 33.25 ± 18.93 | 38.42 ± 19.27 | 65 ± 70.93 | .233* |
| AST, U/L | 39.83 ± 30.5 | 40.92 ± 19.7 | 41.75 ± 25.02 | .640* |
| CRP, mg/L | 54.25 ± 44.82 | 109.83 ± 55.78 | 13.08 ± 12.89 | .001* |
| LDH, U/L | 259.58 ± 88.19 | 360.33 ± 92.29 | 343.25 ± 121.8 | .012‡ |
| Ferritin, ng/mL | 387.83 ± 320.35 | 639.25 ± 487.53 | 577.17 ± 422.22 | .138* |
| D‐dimer, ng/mL | 599.17 ± 270.53 | 1028.67 ± 327.24 | 1027.5 ± 557.64 | .003‡ |
| Troponin, ng/L | 4.88 ± 5.54 | 8.75 ± 15.06 | 15.92 ± 19.98 | .186* |
| Crea, mg/dL | 1.59 ± 2.37 | 2.16 ± 2.8 | 0.91 ± 0.31 | .761* |
| SBP, mm Hg | 125 ± 14.46 | 132.5 ± 16.58 | 117.5 ± 15.45 | .042 ‡ |
| DBP, mm Hg | 78.33 ± 9.37 | 82.5 ± 9.65 | 71.67 ± 8.35 | .013‡ |
| HR, beats/minute | 94.33 ± 10.78 | 94.25 ± 4.67 | 84 ± 7.76 | .011‡ |
| Sat, % | 92 ± 4.47 | 87.58 ± 3.12 | 94.42 ± 1 | .0001‡ |
| RR, breaths/minute | 27 ± 21.38 | 25.5 ± 1.93 | 16.17 ± 1.34 | .001‡ |
| Temperature | 11 (92%) | 12 (100%) | 0 (0%) | .001ǂ |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransaminase; CRP, C‐reactive protein; DBP, diastolic blood pressure; Hb, hemoglobin; HR, heart rate; Htc, hematocrit; Lymp, lymphocytes; MPV, mean platelet volume; Neut, neutrophils; Plt, platelet; RR, respiratory rate; Sat, saturations; SBP, systolic blood pressure; WBC, white blood cell count.
‡ Paired One Way Variance Analysis. ǂ Stuart Maxwell Test. * Friedman Test.
The day before tocilizumab application.
48 h after tocilizumab.
Initially, eosinophil values (10 ± 17.06), which were significantly low in almost all patients, increased (155. 33 ± 192.69) significantly after tocilizumab treatment (Figure 1). A statistically significant difference was observed between the eosinophil averages on the day of diagnosis, before the tocilizumab, and after the tocilizumab (P = .0001). Averages of eosinophils on the day of diagnosis were found to be statistically significantly lower than before the tocilizumab and after the tocilizumab (P = .012 and .003), and averages before the tocilizumab were statistically significantly lower than the post‐tocilizumab eosinophil averages (P = .041).
Figure 1.
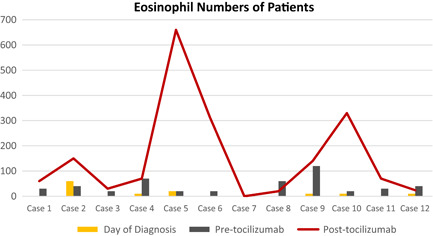
Eosinophil numbers of patients
CRP values, which were moderately high at baseline, showed significant increases in the pre‐tocilizumab period, while decreasing significantly in the post‐tocilizumab period. A statistically significant difference was observed between the the day of diagnosis, before the tocilizumab, and after the tocilizumab CRP averages (P = .0001). Post‐tocilizumab CRP averages were found to be statistically significantly lower than the day of diagnosis and pre‐tocilizumab CRP averages (P = .019 and .003).
No statistically significant difference was observed between the averages of Neut, Lymp, ALT, AST, ferritin, troponin, creatinine on the day of diagnosis, before the tocilizumab, after the tocilizumab, respectively (P = .087, .235, .233, .640, .138, .186, and .761).
3.3. Clinical findings
Although patients' SBP and DBP values and heart rates tended to increase compared to the day of admission on the day of pre‐tocilizumab, they regressed below the day of admission after treatment with tocilizumab (Table 3).
The most critical change in patients' fever was recorded on the day after tocilizumab. As high fever on the diagnosis day and pre‐tocilizumab dropped dramatically after treatment, this was one of the most important outcomes of treatment. A statistically significant difference was observed between the day of diagnosis, pre‐tocilizumab, and post‐tocilizumab fever distributions (P = .01). Post‐tocilizumab, the presence of fever was found statistically significantly lower than the diagnosis day (P = .002), and no statistically significant difference was observed between the presence of fever on the day of admission and pre‐tocilizumab (P = .999).
SpO2 on room air values, which were relatively low at the time of admission in all patients, decreased in the pre‐tocilizumab period significantly and exceeded the initial values after treatment (Table 3 and Figure 2).
Figure 2.
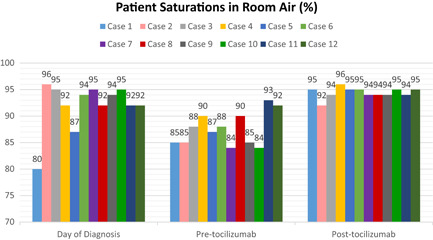
Patient saturations in room air
When the patient group was evaluated for the development of clinically significant arrythmias, no untoward rhythm disturbances or significant QTc prolongations (>500 milliseconds) attributable to tocilizumab use could be detected. Including these cardiological conditions, no additional adverse events were seen in patients after tocilizumab administration
3.4. Thorax CT features
All of our patients were hospitalized with the diagnosis of COVID‐19 pneumonia. Therefore, the tomography features of all patients were abnormal at the time of admission. All had ground‐glass involvement compatible with COVID‐19 in thorax CT (Table 2). The admission time of our patients range from 18 March to 31 March 2020 (Table 1). As we had only one patient who was hospitalized for the 3‐week period that was required to see the radiological improvement, we were able to share the thorax CT images of this patient before and after tocilizumab (Figure 3).
Figure 3.
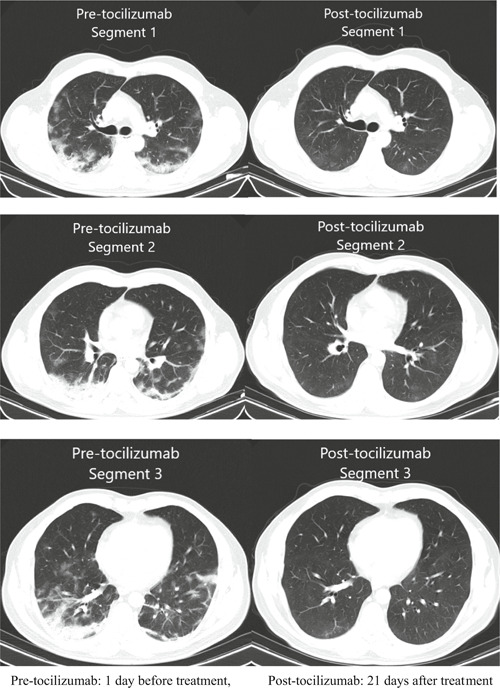
Thorax CT images of one of our patients before and after tocilizumab
4. DISCUSSION
COVID‐19 is a brutal disease that affects the whole world, causing morbidity and mortality, and collapse in the healthcare system and economy. SARS‐CoV‐2 is concerning for scientists as it can be transmitted from one person to another easily due to its high virulence, it might cause an infection in a short time and the need for mechanical ventilation in severe illness can exceed the number of intensive care unit beds present globally. 17
Unfortunately, there is currently no clear‐proven treatment for COVID‐19 or a vaccine developed to prevent transmission. For this reason, the primary goal is to reduce contact with the virus. However, when the person is infected and the disease develops, treatments that will prevent the hospitalization of the patient or shorten the duration of hospitalization and reduce the need for intensive care should be applied. This will save the lives of patients, reduce the burden of health systems and reduce costs.
We retrospectively analyzed the laboratory and clinical findings of 12 patients who received tocilizumab. According to the disease classification of WHO, all of the patients we found to be classified as severe pneumonia had ground‐glass appearance in thorax CT, while RT‐PCR was positive in 11 of 12 patients.
We applied the initial treatment to all of our patients, as suggested in the guidelines of our Ministry of Health. Some of the drugs used in the treatment scheme recommended in our country were applied in the earlier stages of the disease, unlike those recommended from other parts of the world. For this reason, our country's healthcare workers who undertook the COVID‐19 treatment always had an advantage. However, despite this standard treatment, all of our 12 patients exhibited a clinical deterioration graph, such as increases in acute phase reactants, fever, heart rate, and respiratory rate, and deepening of low arterial saturation. As in every other part of the world, our Ministry of Health's guidelines are updated frequently by the National COVID‐19 Scientific Committee. The recommendation for tocilizumab therapy was also available in updates before 22nd March. However, it was not a standard treatment and was left to the discretion of the physician in patients deemed appropriate.
In this interim period, we thought of using tocilizumab before going into intensive care for our patients, who we think had entered the macrophage activation syndrome, namely cytokine storm. However, in later updates, 18 tocilizumab was taken to the standard treatment of the patients who were hospitalized in the intensive care unit.
After tocilizumab treatment, 10 patients (83%) had dramatic improvements both in the laboratory tests and clinical conditions. Although two of our patients (17%) needed ventilator support in the intensive care unit for a short period of time, all of our patients were discharged home with health without the need for oxygen support. The immune system response to the SARS CoV‐2 infection becomes excessive, a cytokine storm develops, which causes significant organ damage in the lungs and other organs. 19 To suppress this excessive response out of control, it has been thought many times that stopping IL‐6, which started the uprisings, would benefit COVID‐19 treatment. 20 , 21 , 22
Our experience with 12 patients allows us to conclude that recognizing this inflammatory response from the very beginning and blocking IL‐6 will increase the success of tocilizumab treatment. A study by Luo et al, evaluating the data of 15 patients, whose severity was classified as mildly, moderately, seriously, and critically ill showed that 46.7% of the patients included in the study were seriously ill, and 40% were critically ill. In other words, the majority of patients were critical. In the end, three patients had died and two had clinical deterioration. 22 In another study by Xu et al, 19 patients of totally 21 patients could be discharged. In this study, 4 of the patients were critically ill and 17 were severe. As a result, 19 patients (90.5%) recovered including 2 critical patients. In another study evaluating 25 patients who were treated with tocilizumab with the diagnosis of COVID‐19, all patients needed treatment in intensive care unit, 3 (12%) of them died. 23 The reason why our treatment results were better than theirs is that the patients were given tocilizumab later than necessary in their series. The most valuable conclusion from the results of these three studies and our own experience is that the use of IL‐6 inhibitor tocilizumab in the earliest period possible when the cytokine storm has just begun is crucial. Applying tocilizumab at a point where cytokine response is very advanced in the patient would not be as beneficial.
In the three studies that shared their experiences before ours, an evaluation was made regarding the limited laboratory parameters. 21 , 22 , 23 We found that eosinophil values were very low at baseline, even zero in six of our patients (50%), and there were serious increases in the post‐tocilizumab period. This raised the question as to whether Covid‐19's initial low eosinophil level may be predictive for the course of the disease toward severe pneumonia. The fact that the serious increase in eosinophil levels accompanied the dramatic good clinical outcome after tocilizumab treatment also supported this idea.
We applied two doses of tocilizumab to our patients at 24‐hour intervals. However, there are studies in the literature that try different routes of administration, such as the subcutaneous route, 24 or administration of one or three doses. 21 , 22 , 23
A meta‐analysis reviewing studies evaluating COVID‐19 patients treated with tocilizumab, a total of 29 cases from 11 studies were examined. Similar to our study, a significant decrease was detected in CRP values after tocilizumab treatment. Among these cases, six patients (20.7%) died but in our study we did not have any exitus. 25
Two cases with acute elevated inflammatory biomarkers consistent with acute pancreatitis and acute hypertriglyceridemia have been reported after the application of tocilizumab with the diagnosis of COVID‐19. 26 In our study, tocilizumab treatment in severe COVID‐19 emerged as a treatment option without acute side effects.
There are some limitations to be considered in this study. First of all, the number of patients is low. However, COVID‐19 is a rapidly spreading disease that has not yet been cured and it is highly mortal. For this reason, sharing experiences, even in a small number of patients, is very important to protect patients' lives. In addition, the IL‐6 level was not examined, as this is not a routine examination due to its high cost and low availability. To predict the onset of cytokine storm, we believe that the increase in acute phase reactants and routine clinical evaluation of the patient are sufficient and allow using the time wisely and decreasing health costs.
5. CONCLUSION
The virus, SARS‐CoV‐2, whose origin is still an enigma, has led to an outbreak that resulted in a pandemic. COVID‐19 across the world is a catastrophic problem with many puzzles, such as lack of approved specific treatment methods, whether it provides immunity after recovery or whether there will be re‐infection or latent infection. All scientists fighting COVID‐19 are trying to find answers to all these puzzles, but are also trying to save time in this war. With our experience from our patients, we have seen that with the use of tocilizumab, patients who would have needed intensive care in the near future could regain their health without the need for intensive care or even if they needed intensive care, the duration of the ICU treatment was shortened. Considering all these benefits, the use of tocilizumab at early stages for the right patients would save precious time in the treatment of COVID‐19, decrease morbidity and mortality, and could be a strong tool available for this fight.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
All authors contributed equally to this paper.
Borku Uysal B, Ikitimur H, Yavuzer S, et al. Tociluzumab challenge: A series of cytokine storm therapy experiences in hospitalized COVID‐19 pneumonia patients. J Med Virol. 2020;92:2648–2656. 10.1002/jmv.26111
REFERENCES
- 1.Wuhan Municipal Health Commission. Report of clustering pneumonia of unknown etiology in Wuhan City (published 31 December 2019), 2020.
- 2.World Health Organization. Coronavirus disease 2019 (COVID‐19): Situation Report—48, 2020.
- 3. Gorbalenya AE, Baker SC, Baric RS, et al. Severe acute respiratory syndrome‐related coronavirus—the species and its viruses, a statement of the Coronavirus Study Group. BioRxiv. 2020. [Google Scholar]
- 4. World Health Organization . Coronavirus disease 2019 (COVID‐19): Situation Report—22, 2020.
- 5. World Health Organization . Coronavirus disease 2019 (COVID‐19): Situation Report—51, 2020.
- 6. Cao B, Wang Y, Wen D, et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382:1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid‐19. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohe M, Shida H, Jodo S, et al. Macrolide treatment for COVID‐19: will this be the way forward? Biosci Trends. 2020;14:159‐160. [DOI] [PubMed] [Google Scholar]
- 9. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA‐approved drug ivermectin inhibits the replication of SARS‐CoV‐2 in vitro. Antiviral Res. 2020;178:104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keshtkar‐Jahromi M, Bavari S. A call for randomized controlled trials to test the efficacy of chloroquine and hydroxychloroquine as therapeutics against novel coronavirus disease (COVID‐19). Am J Trop Med Hyg. 2020;102:932‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deftereos SG, Siasos G, Giannopoulos G, et al. The GReek study in the Effects of Colchicine in COVID‐19 complications prevention (GRECCO‐19 study): rationale and study design. Hellenic J Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID‐19 and interleukin‐6 receptor (IL‐6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization . Report of the WHO‐China joint mission on coronavirus disease 2019 (COVID‐19), 2020.
- 14. Sarzi‐Puttini P, Giorgi V, Sirotti S, et al. COVID‐19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 2020;38(2):337‐342. [PubMed] [Google Scholar]
- 15. World Health Organization . Clinical management of severe acute respiratory infection (SARI) when COVID‐19 disease is suspected: interim guidance, 13 March 2020, Geneva, 2020 [28/03/2020].
- 16. Turkish Ministry of Health . COVID‐19 adult patient management and treatment, 22 March 2020. Turkish Ministry of Health, 2020.
- 17. World Health Organization . Modes of transmission of virus causing COVID‐19: implications for IPC precaution recommendations: scientific brief, 27 March 2020. World Health Organization, 2020.
- 18. Turkish Ministry of Health . COVID‐19 (SARS‐CoV‐2 Infection) Guideline, 14 April 2020. Ankara: Turkish Ministry of Health; 2020.
- 19. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020. [DOI] [PubMed] [Google Scholar]
- 20. Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID‐19? J Transl Med. 2020;18(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970‐10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: a single center experience. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alattar R, Ibrahim TBH, Shaar SH, et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mazzitelli M, Arrighi E, Serapide F, et al. Use of subcutaneous tocilizumab in patients with COVID‐19 pneumonia. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Antwi‐Amoabeng D, Kanji Z, Ford B, Beutler BD, Riddle MS, Siddiqui F. Clinical outcomes in COVID‐19 patients treated with tocilizumab: an individual patient data systematic review. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morrison AR, Johnson JM, Ramesh M, Bradley P, Jennings J, Smith ZR, eds. Letter to the Editor: Acute hypertriglyceridemia in patients with COVID‐19 receiving tocilizumab. J Med Virol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


