Abstract
Aim
To summarize what we know so far about coronavirus disease (COVID‐19) in children.
Method
We searched PubMed, Scientific Electronic Library Online, and Latin American and Caribbean Center on Health Sciences Information from 1 January 2020 to 4 May 2020. We selected randomized trials, observational studies, case series or case reports, and research letters of children ages birth to 18 years with laboratory‐confirmed COVID‐19. We conducted random‐effects meta‐analyses to calculate the weighted mean prevalence and 95% confidence interval (CI) or the weighted average means and 95% CI.
Result
Forty‐six articles reporting 551 cases of COVID‐19 in children (aged 1 day‐17.5 years) were included. Eighty‐seven percent (95% CI: 77%‐95%) of patients had household exposure to COVID‐19. The most common symptoms and signs were fever (53%, 95% CI: 45%‐61%), cough (39%, 95% CI: 30%‐47%), and sore throat/pharyngeal erythema (14%, 95% CI: 4%‐28%); however, 18% (95% CI: 11%‐27%) of cases were asymptomatic. The most common radiographic and computed tomography (CT) findings were patchy consolidations (33%, 95% CI: 23%‐43%) and ground glass opacities (28%, 95% CI: 18%‐39%), but 36% (95% CI: 28%‐45%) of patients had normal CT images. Antiviral agents were given to 74% of patients (95% CI: 52%‐92%). Six patients, all with major underlying medical conditions, needed invasive mechanical ventilation, and one of them died.
Conclusion
Previously healthy children with COVID‐19 have mild symptoms. The diagnosis is generally suspected from history of household exposure to COVID‐19 case. Children with COVID‐19 and major underlying condition are more likely to have severe/critical disease and poor prognosis, even death.
Keywords: COVID‐19, CT, meta‐analysis, severe acute respiratory syndrome coronavirus 2, signs, symptoms
Abbreviations
- ALT
alanine aminotransferase
- CDC
center for disease control
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- CT
computed tomography
- GGO
ground glass opacity
- LILACS
Latin American and Caribbean Center on Health Sciences Information
- LOS
length of hospital stay
- NPT
nasopharyngeal and/or throat
- PCT
procalcitonin
- RT‐PCR
reverse transcription‐polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SciELO
Scientific Electronic Library Online
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) is an emerging infectious disease, caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). COVID‐19 was first reported in December 2019 in Wuhan, the capital of Hubei province, China. The World Health Organization declared COVID‐19 a public health emergency of international concern on 30 January 2020, and recognized it as a pandemic on 11 March. As of 4 May, three and half million cases of COVID‐19 have been reported in over 187 countries and regions, resulting in approximately 260 000 deaths. 1
COVID‐19 appears to be less common in children than adults. Early data from the Chinese Center for Disease Control (CDC) showed that 2.1% of 44.672 patients with laboratory‐confirmed COVID‐19 as of 11 February 2020, were children up to 10 years old. 2 As of 2 April, among the 149 760 laboratory‐confirmed cases in the Unites States, 1.7 percent were children aged <18 years, which make up 22% of the U.S. population. 3 In Europe, children and adolescents made up a small proportion of the 266 393 cases reported to the European Surveillance System‐European CDC (1.1%: <10 years, 2.5%: 10‐19 years). 4
Since the first papers published on 24 January 2020, 2 , 5 , 6 there has been a growing number of publications related to COVID‐19. However, the number of studies in children is still limited, and most of them were case series or case reports with a small number of patients. 7 , 8 , 9 A systematic and quantitative synthesis of data from such studies is needed to provide a more comprehensive and accurate overall picture of COVID‐19 in children.
Three systematic reviews have been published to date to address COVID‐19 in children. One review included 45 scientific papers or letters, 10 showing milder symptoms and better prognosis in children with COVID‐19. However, the clinical picture of COVID‐19 was narratively described, based on the data of 15 reports, mainly from two papers. Another review with 34 studies described clinical, radiological, and laboratorial characteristics of children with COVID‐19. 11 The authors used simple arithmetic means to estimate overall prevalence of clinical findings, and several duplicate publications were included in the review. The latest review 12 included 18 studies (16 primary studies and 2 secondary studies), and a qualitative synthesis of data was performed.
We conducted this systematic review and meta‐analysis of currently available studies to summarize what we know so far about the epidemiological, clinical, radiological, and laboratory features, as well as therapeutic and prognostic aspects, of COVID‐19 in children.
2. METHODS
We followed the preferred reporting items for systematic reviews and meta‐analyses guidelines to conduct and report this review. The review protocol was registered on PROSPERO, an International Prospective Register of Systematic Reviews (CRD42020178178). According to the Brazilian National Commission of Ethics in Research and the National Health Council, ethical approval is not required for literature review research (council resolution no. 510/2016).
2.1. Data sources and search strategy
We searched PubMed from 1 January 2020 to 4 May 2020, using the following search strategy: “Novel coronavirus” OR “Novel coronavirus 2019” OR “2019 nCoV” OR “COVID‐19” OR “SARS‐CoV‐2.” We also searched the Scientific Electronic Library Online (SciELO), the Latin American and Caribbean Health Sciences Literature (LILACS), and Google Scholar. We checked reference lists of retrieved articles for additional studies. There was no restriction in language.
2.2. Study selection
To be included in this review, studies needed to meet the following criteria: (a) Study design: randomized trials, observational studies (cross‐sectional, cohort and case‐control), case series or case reports, and research letters; (b) Participants: children up to 18 years of age with laboratory‐confirmed COVID‐19; (c) Variables: epidemiological and demographic characteristics, clinical, radiological and laboratory findings, treatments, and prognosis. We excluded editorials, comments, and review articles. We also excluded studies reporting nationwide aggregated data and those reporting the same patients' data to avoid overlapping and duplicate publications.
Two authors (SMVF, PTG) independently assessed the titles and abstracts of all citations identified by the searches. We obtained the full articles when they met the inclusion criteria or there were insufficient data in the title and abstract for assessment of eligibility. The definitive inclusion of studies was made after reviewing the full‐text articles. Any disagreement between two reviewers was resolved by discussion with the third reviewer (ZL).
2.3. Data extraction
One author (SMVF) extracted the data from selected articles using a standardized form. These were checked by another author (ZL). We extracted the following data: (a) Study identification: first author, year and date of publication, study setting, and country or region; (b) Study design; (c) Participants: age, gender, sample size, date of recruitment, inclusion and exclusion criteria; (d) Variables: epidemiologic data, signs and symptoms, chest radiographic and CT findings, treatment, and prognosis.
2.4. Assessment of risk of bias in included studies and risk of publication bias
Two authors (PTG, ZL) independently assessed the risk of bias and study quality of each included observational study or case series, using the National Institutes of Health Study Quality Assessment Tools. 13
2.5. Data synthesis and statistical analysis
For dichotomous variables, we calculated the weighted mean prevalence and 95% CI whenever there were three or more studies with at least 50 patients. We aggregated the data of a single case report into a series of cases for meta‐analysis because an individual case report has no denominator for any variables. We conducted sensitivity analysis excluding aggregated single cases from the analysis to assess the influence of such data management on the results of meta‐analysis. For continuous variables, we calculated the weighted average means and 95% CI whenever there were three or more studies with at least 50 patients. We used random‐effects model for meta‐analyses.
We planned to perform subgroup analyses according to illness severity (mild/moderate vs severe/critical) and study countries (China vs others). However, the limited number of severe/critical cases and cases outside China did not allow us to conduct such subgroup analyses.
We assessed heterogeneity between studies using I 2 statistic which measures the percentage of observed total variation across studies that is due to real heterogeneity rather than chance. The heterogeneity was considered substantial if I 2 > 50%. We assessed publication bias using funnel plot with Egger's test for each variable whenever there were 10 or more studies. All meta‐analyses were performed in Stata version 11.0 (Stata‐Corp, College Station, TX).
3. RESULTS
The search strategy identified 8475 records (8058 from PubMed, 223 from LILACS, and 194 from SciELO). After screening the titles and abstracts, we retrieved 64 potentially relevant full text articles for further evaluation. Twenty‐four articles were excluded, of which eight were duplicate publications of the patients' data from Wuhan Children's Hospital in the city of Wuhan, 14 , 15 the epicenter of SARS‐CoV‐2 outbreak (Figure 1). We also excluded another two Chinese nationwide reports of COVID‐19 in children. 16 , 17 We identified eight additional articles on Google Scholar. Thus, 46 articles 7 , 8 , 9 , 14 , 15 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 reporting 551 laboratory‐confirmed cases of COVID‐19 in children were included in the review (Figure 1). Thirty‐five articles (429 cases) were from China, 7 , 8 , 9 , 14 , 15 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 and the remaining papers from Iran (one cases), 48 , 49 Italy (109 case), 50 , 51 , 52 Korea (one case), 53 Malaysia (four cases), 54 Singapore (one case), 55 Spain (one case), 56 United States (one case), 57 and Vietnam (one case). 58 Among 35 articles from China, six reported patients (204 cases) from Wuhan city, 14 , 15 , 28 , 34 , 38 , 44 epicenter of the outbreak. We included two studies from Wuhan Children's Hospital, 14 , 15 but the larger one with 171 patients 14 had contributed the data to all but one category of variables (laboratory findings) for which we used the data from a subset of 82 patients. 15 Thirty‐nine articles were case series or case reports, and six were research letters. 14 , 22 , 28 , 36 , 51 , 56 We considered one observational cohort study 31 as a case series because this paper described clinical and epidemiological features of 36 cases, in which patients' data were retrospectively collected from electronic medical records. We rated the quality of 26 case series as poor (n = 2), fair (14), and good (n = 10; Table S1). The characteristics of included studies and the main epidemiological and clinical findings of COVID‐19 are summarized in Table 1. The laboratory and radiological findings of COVID‐19 are given in Table S2.
Figure 1.
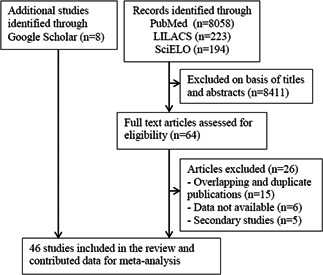
Flow diagram of study selection. LILACS, Latin American and Caribbean Center on Health Sciences Information; SciELO, Scientific Electronic Library Online
Table 1.
Study characteristics and main epidemiological and clinical findings of COVID‐19 in children
| Study ID (country) | Study design and participants | Exposure to COVID‐19 case and IP | Symptoms and signs | Main treatments | Prognosis (hospital discharge (LOS) (death)) |
|---|---|---|---|---|---|
| Cai et al, 7 China |
Case series, multicenter (n = 10) Age: median of 78 mo (range, 3‐131 mo); male/female: 4/6 |
Household (n = 7) Community (n = 3) IP: median of 7 d (range, 2‐10 d) |
Fever (n = 8, 37.7 ‐39.2°C), cough (n = 6), stuffy nose (n = 3), rhinorrhea (n = 2), sneezing (n = 2), sore throat (n = 4), dyspnea (n = 0), diarrhea (n = 0) | Symptomatic treatment (n = 10), antibiotics (n = 5), oxygen therapy (n = 0) | All discharged LOS: NA |
| Canarutto et al, 50 Italy |
Case report Age: 32 d; male |
NA | Fever (38.2°C), cough, rhinitis | Supportive care | Discharged LOS: 5 d |
| Chan et al, 9 China |
Case report Age: 10 y; male |
Household | Asymptomatic | Supportive care | Remained in hospital on day 15 |
| Chen et al, 18 China |
Case report Age: 7 y; male |
Household | Fever (37.2°C) | Symptomatic treatment | Discharged LOS: NA |
| Cui et al, 19 China |
Case report Age: 55 d; female |
Household | Rhinorrhea, dry cough, tachycardia | Inhaled interferon α‐1b, amoxicillin, oxygen therapy | Discharge criteria were met on day 10, but remained in hospital due to persistent fecal viral shedding |
| Denina et al, 52 Italy |
Case series (n = 8) Age: mean of 4.2 y (range, 0.2‐10 y); male/female:5/3 |
Household (n = 8) | Fever (n = 6), dry cough (n = 5), dyspnea/tachypnea (n = 3), pharyngeal congestion/sore throat (n = 3), vomiting/diarrhea (n = 3) | Oxygen therapy (n = 2), mechanical ventilation (n = 0) | NA |
| Díaz et al, 56 Spain |
Case report (n = 1) Age: 1 d; female |
Household | Asymptomatic | Supportive care | Remained asymptomatic on day 13 |
|
Du et al, 20 China |
Case series, multicenter (n = 14) Age: median of 6.2 y (range, 0‐16 y); male/female: 6/8 |
Household (n = 14) | Fever (n = 5, ≥38.5°C, n = 1), cough (n = 3), fatigue (n = 1), dyspnea (n = 0), sore throat (n = 1), headache (n = 1), vomiting (n = 0), diarrhea (n = 0) | NA | NA |
| Feng et al, 8 China |
Case series (n = 15) Age: median of 7 y (range: 4‐14 y); male/female: 5/10 |
Household (n = 12) Community (n = 3) |
Fever (n = 5), nasal congestion (n = 1), cough (n = 1) | NA | Discharged as of the reporting date (n = 5), with a mean LOS of 12 d |
| Han et al, 21 China |
Case series (n = 7) Age: median of 1.3 y (range, 0.2‐13 y); male/female: 4/3 |
Household (n = 7) IP: median of 5 d (range, 3‐12 d) |
Fever (n = 5, >37.3°C), cough (n = 5), myalgia or fatigue (n = 0), diarrhea and/or vomiting (n = 4), shortness of breath (n = 3), pharyngalgia (n = 1) | Oxygen therapy (n = 2), antiviral (n = 0), antibiotic (n = 0), glucocorticoids (n = 1) | All discharged LOS: NA |
| He et al, 22 China |
Case report Age: 11 y; male |
Household | High fever | Interferon α‐2b | NA |
| Huang et al, 23 China |
Case report Age: 16 y; female |
Household IP: 3 or 7 d |
Fever (37.2 – 38.5°C), rhinorrhea, sneezing, headache, diarrhea | Moxifloxacin, interferon α‐1b, ribavirin, oseltamivir | Discharged LOS: NA |
| Ji et al, 24 China |
Case series (n = 2) Age: 15, 9 y; male/female: 2/0 |
Household (n = 2) | Fever (n = 1), pharyngeal congestion (n = 1), diarrhea (n = 1), normal breath sounds (n = 2) | Symptomatic treatment (n = 2), probiotic (n = 1) | NA |
| Jones et al, 57 EUA |
Case report Age: 6 mo; female |
NA | Fever (38.8°C), fussiness, refusal to eat, tachypnea, mild subcostal retractions, Kawasaki disease | Symptomatic treatment, acetylsalicylic acid used for Kawasaki disease | Discharged LOS: NA |
| Kam et al, 55 Singapore |
Case report Age: 6 mo; Male |
Household | Fever (38.5°C) | NA | Remained well in hospital as of the reporting date |
| Kamali et al, 49 Iran |
Case report Age: 15 d; male |
Household | Fever (38.2°C), lethargy, mottling, tachycardia, tachypnea, mild subcostal retraction | Oxygen therapy, vancomycin +amikacin, oseltamivir | Discharged LOS: 6 d |
| Le et al, 58 Vietnam |
Case report Age: 3 mo; female |
Household | Low grade fever, rhinorrhea, nasal congestion, fussy | Azithromycin | Discharged LOS: 15 d |
| Li et al, 25 China |
Case series (n = 5) Age: median of 3 y (range: 10 mo‐6 y); male/female: 4/1 |
Household (n = 4) Community (n = 1) |
Asymptomatic (n = 4), fever (n = 1), cough (n = 1), sputum (n = 1), sore throat (n = 1), runny nose (n = 1) |
Antiviral (n = 2), antibiotics (n = 2), immunoglobulin (n = 5), interferon (n = 2), montelukast (n = 3) |
Discharged as of the reporting date (n = 3) LOS: 12, 13 and 14 d |
| Li et al, 26 China |
Case series (n = 22), Age: mean of 8 y male/female: 12/10 |
NA | Asymptomatic (n = 2), fever (n = 14), cough (n = 13) | NA | NA |
| Lin et al, 27 China |
Case report Age: 7 y; female |
Household IP: 5 d |
Cough, rhinorrhea, fatigue, nausea, vomiting, diarrhea, abdominal discomfort | Interferon α‐1b, oseltamivir | Remained in quarantine ward as of the reporting date |
| Liu et al, 28 China |
Case series (n = 6) Age: median of 3 y (range: 1‐7 y); male/female: 2/4 |
Unknown (n = 6) | Fever (n = 6), cough (n = 6), swollen tonsils (n = 6), pharyngeal congestion (n = 5), vomiting (n = 4), wheeze (n = 2), tachypnea (n = 1), rhinorrhea (n = 1), chill (n = 1) |
Ribavirin (n = 2), oseltamivir (n = 6), glucocorticoids (n = 4), oxygen therapy (n = 1), immunoglobulin (n = 1), ICU care (n = 1) |
All discharged LOS: mean of 7.5 d (range, 5 to 13 d) |
| Lou et al, 29 China |
Case series (n = 3), Age: 6, 6, and 8 mo; male/female: 1/2 |
Household (n = 3) | Fever (n = 3), cough (n = 1), nasal congestion (n = 2), rhinitis (n = 2), fatigue (n = 2), diarrhea (n = 2), headache (n = 2) | Interferon‐ α2b (n = 2) | All discharged LOS: mean of 10 d |
| Lu et al, 14 China |
Case series (n = 171) Age: median of 6.7 y (range: 1 d to 15 y); male/female: 104/67 |
Household (n = 154) Community (n = 2) Unknown (n = 15) |
Asymptomatic (n = 27), fever (n = 71), cough (n = 83), pharyngeal erythema (n = 79, diarrhea (n = 15), fatigue (n = 13), rhinorrhea (n = 13), vomiting (n = 11), nasal congestion (n = 9), tachypnea (n = 49), tachycardia (n = 72) | ICU care and invasive mechanical ventilation (n = 3) |
Discharged as of the reporting date (n = 149) LOS: NA Death (n = 1) |
| Pan et al, 30 China |
Case report Age: 3 y; male |
Community | Asymptomatic | NA | NA |
| Park et al, 53 Korea |
Case report Age: 10 y; female |
Household | Mild fever, sputum | Supportive care | Discharged LOS: 15 d |
| Parri et al, 51 Italy, |
Case series, multicenter (n = 100) Age: median of 3.3 y (range, 0‐17.5 y); male/female: 57/43 |
Household (n = 45) Others (n = 48) Unknown (n = 7) |
Fever (n = 54, >39°C, n = 11), cough (n = 44), shortness of breath (n = 11), rhinorrhea (n = 22), drowsiness (n = 11), vomiting (n = 11), diarrhea (n = 9), headache (n = 4), sore throat (n = 4), asymptomatic (n = 21) | 9 patients received respiratory support (4 low‐flow oxygen, 3 high‐flow oxygen, 1 noninvasive ventilation, 1 invasive MV) | 33 discharged from emergency departments |
| Qiu et al, 31 China |
Case series, multicenter (n = 36) Age: mean of 8.3 y (range: 1‐16 y); male/female: 23/13 |
Household (n = 32) Community (n = 4) |
Fever (n = 13), dry cough (n = 7), dyspnea or tachypnea (n = 1), pharyngeal congestion (n = 1, sore throat (n = 2), vomiting or diarrhea (n = 2), headache (n = 3) | Oxygen therapy (n = 6), interferon alfa (n = 36), lopinavir‐ritonavir (n = 14) | All discharged LOS: mean of 14 d (range, 10‐20 d) |
| Quan et al, 32 China |
Case report Age: 4 y; female |
Household | Asymptomatic | Interferon α‐1b | Discharged LOS: 10 d |
| Rahimzadeh et al, 48 Iran |
Case series (n = 3) Age: 2, 5, and 5 y; male/female: 3/0 |
Household (n = 3) | Fever (n = 3), cough (n = 3), tachypnea (n = 3), chills (n = 3), myalgia (n = 3), weakness (n = 3), tachycardia (n = 2), crackle in both lungs (n = 3) | Oxygen therapy (n = 3), antibiotics (n = 3), oseltamivir (n = 3) | NA |
| See et al, 54 Malaysia |
Case series (n = 4) Age: 20 mo, 4, 9, and 11 y; male/female: 3/1 |
Household (n = 2) | Asymptomatic (n = 1), fever (n = 1), cough (n = 2), runny nose (n = 1), diarrhea (n = 1) | Symptomatic treatment (n = 4), antibiotic (n = 1) | Discharged LOS: NA |
| Shen et al, 33 China |
Case series (n = 9) Age: median of 8 y (range: 1‐12 y); male/female: 3/6 |
Household (n = 9) IP: median of 7.5 d (range, 1‐16 d) |
Asymptomatic (n = 2), fever (4/9, 37.5‐39.1°C), diarrhea (n = 2), sore throat(n = 1), cough (n = 1) | Oxygen therapy (n = 9), lopiravir/ritonavir (n‐9), azithromycin (n = 5), glucocorticoids (n = 1), immunoglobulin (n = 1) |
Discharged as of the reporting date (n = 6) LOS: 11, 12, 15, 16, 16, and 22 d |
| Shi et al, 34 China |
Case report Age: 4 mo; male |
Household | Cough, wheeze, dyspnea | Interferon, antibiotic, methylprednisolone, immunoglobulin | Discharged LOS: NA |
| Song et al, 47 China |
Case series (n = 16) Age: median of 8.5 y (range: 11.5 mo‐14 y); Male/Female: 10/6 |
Household (n = 12) | Fever (n = 5), cough (n = 6), dyspnea (n = 0), vomiting (n = 0), diarrhea (n = 0), fatigue (n = 0), asymptomatic (n = 6) | Lopinavir‐ritonavir (n = 4), oseltamivir (n = 11), antibiotics (n = 9) | All discharged LOS: median of 14 d (range, 8‐26 d) |
| Su et al, 35 China |
Case series (n = 9) Age: median of 3.6 y (range: 11 mo‐9 y); male/female: 3/6 |
Household (n = 9) | Fever (n = 2), cough (n = 1), asymptomatic (n = 6) | Interferon (n = 9), ribavirin (n = 1) | All discharged LOS: 2‐3 wk |
| Tang et al, 36 China |
Case report Age: 10 y; Male |
Household | Asymptomatic | Interferon α‐2b, abidol hydrochloride | Remained in hospital on day 14 |
| Wang et al, 38 China, |
Case report Age: 1 d; male |
Household | Asymptomatic | Symptomatic treatment, penicillin G | Discharged LOS: 18 d |
| Xing et al, 39 China |
Case series (n = 3) Age: 1.5, 5, and 6 y; male/female: 2/1 |
Household (n = 3) | Fever (n = 3), cough (n = 1), runny nose (n = 1), diarrhea (n = 1) | Symptomatic treatment | All discharged LOS: 19 d |
| Wang et al, 37 China |
Case series (n = 31) Age: mean of 7.1 y (range: 6 mo‐17 y); male/female: 15/16 |
Household (n = 21) Community (n = 1) Imported (n = 9) |
Asymptomatic (n = 4), fever (n = 20, >39°C, n = 1), cough (n = 14), diarrhea (n = 3), fatigue (n = 3), sore throat (n = 2), headache or dizziness (n = 3), runny nose (n = 2), vomiting (n = 2) | Interferon alone (n = 10), oseltamivir alone (n = 1), combined use of two or more antiviral drugs (n = 18), antibiotics (n = 6), immunoglobulin (n = 2) | Discharged as of the reporting date (n = 24) |
| Xu et al, 40 China |
Case series (n = 10) Age: (range: 2 mo‐15 y); male/female: 6/4 |
Household (n = 7) Community (n = 3) |
Asymptomatic (n = 1), fever (n = 7), cough (n = 5), sore throat (n = 4), rhinorrhea (n = 2), diarrhea (n = 3) | NA |
Discharged as of the reporting date (n = 4) LOS: NA |
| Zhang et al, 41 China |
Case report Age: 3 mo; female |
Household | Fever, cough | Azithromycin + ceftazidime, peramivir | Discharged LOS: 15 d |
| Zhang et al, 42 China |
Case report Age: 1 y 3 mo; female |
Household | Fever, cough, pharyngeal congestion | Interferon α2b, immunoglobulin | Discharged LOS: 9 d |
| Zhang et al, 43 China |
Case series (n = 3) Age: (range: 6‐9 y); male/female: 3/0 |
Household (n = 3) | Fever (n = 2), cough (n = 1), sore throat (n = 1), nasal congestion/runny nose (n = 2), fatigue (n = 0/), vomiting/diarrhea (n = 0) | Antiviral (n = 2), antibiotic (n = 1) | NA |
| Zheng et al, 44 China |
Case series (n = 25) Age: median of 3 y (range: 3 mo‐14 y); male/female: 14/11 |
Household (n = 16) Community (n = 5) Unknown (n = 4) |
Fever (n = 13), cough (n = 11), nasal congestion (n = 2), dyspnea (n = 2), abdominal pain (n = 2), vomiting (n = 2), diarrhea (n = 3) | Antiviral (n = 12), antibiotics (n = 13), glucocorticoids (n = 2), immunoglobulin (n = 2), kidney replacement therapy (n = 1), invasive MV (n = 2) | Discharged as of the reporting date (n = 1) |
| Zhou et al, 45 China |
Case series (n = 9) Age: mean of 1.3 y (range: 7 mo‐3 y); male/female: 4/5 |
Household (n = 9) |
Asymptomatic (n = 5), fever (n = 4) cough (n = 2), runny nose (n = 1) |
Interferon (n = 9), ritonavir (n = 9) | Remained in hospital as of the reporting date (n = 9) |
| Zhu et al, 46 China |
Case series, multicenter (n = 10) Age: (range: 1‐17 y); male/female: 5/5 |
Household (n = 7) | Asymptomatic (n = 4), fever (n = 4), cough (n = 3), sore throat (n = 0), headache (n = 0), shortness of breath (n = 0), vomiting (n = 0), diarrhea (n = 0) | Oxygen therapy (n = 1), antiviral (n = 5), antibiotic (n = 1), glucocorticoids (n = 0), immunoglobulin (n = 0), ICU care (n = 0) |
Discharged as of the reporting date (n = 7) LOS: NA |
Abbreviations: COVID‐19, coronavirus disease‐2019; ICU, intensive care unit; IP, incubation period; LOS, length of stay; MV, mechanical ventilation NA, not available.
3.1. Demographic and epidemiological characteristics
Of 551 children with laboratory‐confirmed COVID‐19, 311 were males (57%, 95% CI: 53%‐62%). The patients' age ranged from 1 day to 17.5 years old, and 216 (48%, 95% CI: 37%‐58%) were children under 5 years of age. At least seven cases were neonates aged up to 28 days. All but three studies 26 , 50 , 57 provided data on exposure to COVID‐19 case. Household exposure was most common, with a pooled mean prevalence of 87% (95% CI: 77%‐95%) (Table 2). Thirty‐four patients (1%, 95% CI: 0%‐4%) had unknown exposure information. Three small case series with a total of 26 patients reported the incubation period, with a median (range) of 7 days (2‐10 days), 7 5 days (3‐12 days), 21 and 7.5 days (1‐16 days), 33 respectively.
Table 2.
Meta‐analysis of epidemiological, clinical, radiological, and laboratory findings of COVID‐19 in children
| Variables | No. of studies a | Events/patients (n) | Pooled mean prevalence (95% CI) | I 2 (%) |
|---|---|---|---|---|
| History of exposure to COVID‐19 patient | ||||
| Household exposure | 42 | 402/516 | 87% (77‐95%) | 79 |
| Other types of exposure | 42 | 79/516 | 9% (2‐20%) | 83 |
| Unknown | 42 | 35/516 | 1% (0‐4%) | 35 |
| Clinical findings | ||||
| Asymptomatic | 45 | 117/551 | 18% (11‐27%) | 69 |
| Fever | 45 | 269/551 | 53% (45‐61%) | 52 |
| >39.0°C | 11 | 38/409 | 7% (4‐10%) | 0 |
| Cough | 45 | 231/551 | 39% (30‐47%) | 59 |
| Sore throat/pharyngeal erythema | 41 | 121/502 | 14% (4‐28%) | 87 |
| Nasal symptoms (rhinorrhea, stuffy nose, sneezing) | 41 | 68/509 | 7% (3‐14%) | 55 |
| Tachypnea/dyspnea | 41 | 82/503 | 8% (2‐15%) | 73 |
| Diarrhea | 41 | 53/502 | 8% (3‐14%) | 54 |
| Vomiting | 38 | 31/485 | 2% (0‐5%) | 25 |
| Fatigue/weakness | 10 | 31/390 | 5% (0‐13%) | 69 |
| Headache | 10 | 14/391 | 3% (0‐12%) | 59 |
| Radiological findings | ||||
| Normal | 44 | 162/434 | 36% (28‐45%) | 46 |
| Ground‐glass opacities | 45 | 151/470 | 28% (18‐39%) | 73 |
| Patchy consolidations | 44 | 150/434 | 33% (23‐43%) | 64 |
| Laboratory findings | ||||
| Leukocytosis | 22 | 47/243 | 15% (9‐21%) | 8 |
| Leukopenia | 15 | 48/314 | 14% (6‐26%) | 75 |
| Lymphocytosis | 8 | 67/157 | 35% (14‐59%) | 81 |
| Lymphcytopenia | 24 | 61/323 | 13% (7‐20%) | 48 |
| Increased CRP | 27 | 50/265 | 17% (7‐29%) | 67 |
| Increased PCT | 22 | 56/254 | 10% (1‐22%) | 79 |
| Increased ALT | 24 | 35/290 | 9% (3‐15%) | 44 |
| Increased AST | 23 | 58/280 | 18% (9‐28%) | 64 |
| Increased creatinine | 9 | 48/184 | 4% (0‐25%) | 91 |
| Increased urea | 5 | 22/139 | 5% (0‐19%) | 73 |
| Increased LDH | 11 | 59/196 | 29% (16‐43%) | 60 |
| Increased creatine kinase | 21 | 68/206 | 21% (8‐37%) | 75 |
| Increased D‐dimer | 19 | 37/269 | 12% (4‐22%) | 45 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; COVID‐19, coronavirus disease‐2019; CRP, C‐reactive protein; CT, computed tomography; LDH, lactate dehydrogenase; PCT, procalcitonin.
3.2. Clinical and radiological findings
All 551 children with laboratory‐confirmed COVID‐19 were hospitalized or treated in the emergency department (n = 100), of which 18% (95% CI: 11%‐27%) were asymptomatic. The most common symptoms and signs were fever (53%, 95% CI: 45%‐61%), cough (39%, 95% CI: 30%‐47%), and sore throat or pharyngeal erythema (14%, 95% CI: 4%‐28%). The less common symptoms and signs included tachypnea/dyspnea, nasal symptoms, diarrhea, vomiting, fatigue/weakness, and headache (Table 2). The sensitivity analysis excluding series of 20 single cases yielded almost identical results.
Nine patients had severe/critical COVID‐19, of which three were from one series of 171 cases, 14 two from one series of 25 cases, 44 two from one series of 100 cases, 51 one from a series of six cases, 28 and one from a single case report. 19 All but two patients had underlying medical conditions, such as hydronephrosis (n = 1), leukemia receiving maintenance chemotherapy (n = 1), intussusception (n = 1), encephalopathy (n = 1), and congenital heart diseases (n = 3).
Chest images were obtained in all but one patient, 52 and most of them (86%) were chest CT scans. The most common findings were patchy consolidations (33%, 95% CI: 23%‐43%) and ground glass opacities associated or not with consolidations (28%, 95% CI: 18%‐39%), but 36% (95% CI: 28%‐45%) of patients had normal CT images (Table 2). The sensitivity analysis excluding series of 20 single cases yielded almost identical results. Among 161 patients with available data on lesion distribution of CT images, 68% (95% CI: 47%‐87%) of abnormalities were unilateral.
3.3. Laboratory findings
SARS‐CoV‐2 was detected in nasopharyngeal and/or throat (NPT) specimens by reverse transcription‐polymerase chain reaction (RT‐PCR) testing in all 551 patients. Nine case series with a total of 92 patients reported duration of viral RNA shedding in NPT specimens. 7 , 33 , 35 , 37 , 39 , 40 , 43 , 47 , 54 The pooled average mean duration was 11.2 days (95% CI: 9.6‐12.8 days, I 2 = 62). The range of shedding duration reported by nine case series was 6 to 22 days (n = 10), 7 9 to 20 days (n = 6), 33 7 to 16 days (n = 9), 35 7 to 23 days (n = 31), 37 10 to 15 days (n = 3), 39 3 to 16 days (n = 10), 40 7 to 14 days (n = 3), 43 2 to 24 days (n = 16), 47 and 9 to 18 days (n = 4), 54 respectively.
In four series of cases 7 , 35 , 39 , 40 and one single case, 9 RT‐PCR testing for SARS‐CoV‐2 was performed in both NPT and fecal/rectal specimens. Among the 29 tested patients, 22 had positive fecal/rectal RT‐PCR tests. The duration of viral RNA shedding in fecal/rectal specimens was much longer than that in NPT specimens in all 22 patients. All but one patient had a duration of viral RNA shedding longer than 2 weeks in fecal/rectal specimens. In at least five patients, fecal/rectal RT‐PCR tests remained positive for more than 30 days.
A total of 141 patients with COVID‐19 from six case series had tests for other respiratory pathogens. In a subset (n = 82) of a series of 171 cases, 15 23 patients had co‐infections (three respiratory syncytial virus RSV, one influenza virus, one adenovirus, 17 Mycoplasma pneumoniae, one bacteria). In another series of 25 cases, 44 six patients had co‐infections (two influenza virus, three mycoplasma pneumoniae, and one bacteria). Six patients from a series of nine cases had testing, 45 and RSV was identified in one patient. No co‐infection was found in three series of cases with a total of 28 patients. 7 , 11 , 40 The pooled prevalence of co‐infections of SARS‐CoV‐2 with other respiratory pathogens was 10% (95% CI: 1%‐24%, I 2 = 65).
A battery of laboratory tests was performed in children with COVID‐19. The more common laboratory abnormalities included lymphocytosis (35%, 95% CI: 14%‐59%), increased lactate dehydrogenase (29%, 95% CI: 16%‐43%), increased creatine kinase (21%, 95% CI: 8%‐37%), and increased aspartate aminotransferase (18%, 95% CI: 9%‐28%) (Table 2).
Four case series reported oxygen saturation. In one series of 36 cases, all had normal oxygen saturation. 31 One percent of patients in a series of 100 cases 51 and 2.3% of patients in another series of 171 cases 14 had oxygen saturation <92% during the period of hospitalization. In a series of three cases from Iran, 48 all had oxygen saturation below 92%. The pooled mean prevalence of oxygen saturation <92% was 4% (95% CI: 0%‐17%, I 2 = 84).
3.4. Treatment
Forty‐two studies 7 , 9 , 14 , 15 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 53 , 54 , 55 , 56 , 57 , 58 with 381 cases of COVID‐19 provided available data on treatments. All patients received symptomatic treatment. Antiviral agents were given in 227 patients (74%, 95% CI: 52%‐92%). Inhaled interferon α (IFN‐α) was the most commonly used antiviral drug. Other reported antivirals were ribavirin, oseltamivir, lopinavir, ritonavir, and litonavir. Antibiotics were used in 138 patients (40%, 95% CI: 19%‐63%). Other treatments included intravenous immunoglobulin therapy (n = 15, 3%, 95% CI: 0%‐10%), oxygen therapy (n = 30, 4%, 95% CI: 1%‐10%), and systematic corticosteroids (n = 10, 1%, 95% CI: 0%‐3%). Six patients with severe/critical COVID‐19 needed invasive mechanical ventilation.
3.5. Prognosis
Of 451 hospitalized children with COVID‐19, 83% (95% CI: 67%‐95%) were discharged as of the reporting date, with at least one negative RT‐PCR testing for SARS‐CoV‐2 in nasopharyngeal/throat specimens. The pooled average mean length of hospital stay was 12.5 days (95% CI: 11.1‐14.0 days) among 182 discharged patients with available data. Of nine patients with severe/critical COVID, six needed intensive care with mechanical ventilation, 14 , 44 , 51 and all had major underlying medical conditions. One 10‐month‐old child with intussusception had multiple organ failure, and died 4 weeks after admission. 14
4. DISCUSSION
This systematic review included 46 studies reporting 551 pediatric patients with laboratory‐confirmed COVID‐19 between early January and late March 2020, of which 429 cases (78%) were from China, 11 cases (2%) from other Asian countries, and 110 cases (20%) from Europe (Italy and Spain). The patients' age ranged from 1 day to 17.5 years old, and 57% (95% CI: 53%‐62%) were males. Eighty‐seven percent (95% CI: 77%‐95%) of patients had household exposure to COVID‐19 cases. Some similar demographic and epidemiological characteristics were reported in a preliminary analysis of 2,572 pediatric cases of COVID‐19 in the USA. 3 The median age was 11 years, ranging from 0 to 17 years, and 57% of patients were males. Among 184 (7.2%) pediatric cases with known exposure information, 91% had exposure to a patient with COVID‐19 in the household or community.
Mild‐to‐moderate fever and cough were the most common symptoms among 551 children with COVID‐19 (53%, 95% CI: 45%‐61% and 39%, 95% CI: 30%‐47%, respectively), but much less frequently than that reported by a systematic review in adult patients (92.8%, 95% CI: 89.4%‐96.2% and 57.6%, 95% CI: 40.8%‐74.4%, respectively). 59 Eighteen percent (95% CI: 11%‐27%) of 551 children with laboratory‐confirmed COVID‐19 were asymptomatic, while the prevalence of asymptomatic cases was 12.9% among 731 pediatric patients with laboratory‐confirmed COVID‐19 reported to the Chinese CDC from 16 January to 8 February 2020. 16 Among 2572 children with COVID‐19 in the USA, only 291 (11%) had available data on signs and symptoms. 3 Fifty‐six percent of pediatric patients had fever, 54% had cough, and 13% had shortness of breath, compared with 71%, 80%, and 43%, respectively, among patients aged 18 to 64 years. The prevalence of asymptomatic cases was not estimated in this U.S. cohort of pediatric patients with COVID‐19 because of incomplete symptom information.
Biochemical laboratory tests were often used to measure inflammatory markers and to detect possible hepatic, renal, and cardiac damage in patients with COVID‐19. However, only a small proportion of pediatric cases included in this review had abnormal results. Lymphocytopenia is common at the early stage of COVID‐19 in adult patients, and it can predict the disease severity and prognosis. 60 However, most of pediatric patients included in the review had normal white blood cell count without lymphocyte depletion, which may suggest less immune dysfunction in children after the SARS‐CoV‐2 infection.
Chest CT scans were routinely used in patients with COVID‐19. Ground glass opacity (GGO) and consolidation were the most common CT findings in adult patients with COVID‐19, ranging from 54% to 100% for GGO and 31% to 71% for consolidation, and with a predominantly bilateral and peripheral distribution. 61 , 62 , 63 One systematic review of 10 studies involving 2657 adult patients showed a pooled sensitivity of 93% (95% CI: 85%‐97%) of chest CT on detection of COVID‐19 confirmed by RT‐PCR testing. 64 Low specificity was reported by one included study (25%, 95% CI: 22%‐30%). Ground glass opacities on chest CT were observed in a subgroup of 15 asymptomatic adult patients with COVID‐19, substantiating previous anecdotal reports that asymptomatic patients could have CT abnormalities before symptom onset. All these results suggest that chest CT is a sensitivity modality for detecting COVID‐19 pneumonia in adults, even in asymptomatic individuals, and may be considered as a screening tool, together with RT‐PCR. This systematic review showed that GGO and consolidation were also the most common CT abnormalities in children with COVID‐19. However, the prevalence of such CT findings in pediatric cases was much lower than that in adult patients, and the lesions were usually unilateral and less extensive. Moreover, 36% (95% CI: 28%‐45%) of children with laboratory‐confirmed COVID‐19 had normal CT images. Thus, the role of chest CT scan, and even single or serial chest X‐rays, in the diagnosis and assessment of pediatric patients with COVID‐19 needs to be defined further.
There is currently no approved treatment for COVID‐19 in adults as well as in children. However, among 381 pediatric cases in this review with available data on treatments, 74% (95% CI, 52%‐92%) were treated with at least one antiviral drug, and inhaled IFN‐α was the most commonly used antiviral agent. IFN‐α exerts its antiviral effect mainly by inducing the expression of antiviral proteins and activating cellular immunity. Several multi‐center studies from China have shown that inhaled IFN‐α can reduce viral load, alleviate symptoms, and shorten disease duration in children with viral infections, including bronchiolitis and viral pneumonia. 65 , 66 , 67 The Chinese Experts Consensus Statement on Diagnosis, Treatment, and Prevention of COVID‐19 in Children has listed nebulized IFN‐α as a choice of treatment. 68 This may explain high prevalence of children with COVID‐19 treated with inhaled IFN‐α in this review. There is at least one ongoing randomized trial to assess the efficacy and safety of inhaled IFN‐α in children with COVID‐19. 69 Until new evidence is available, it seems prudent to not recommend use of inhaled IFN‐α in these patients.
Other antiviral agents used in the patients included in this review were lopinavir and ritonavir. However, one latest randomized trial with 199 adult patients with COVID‐19 failed to show significant benefit of treatment with lopinavir‐ritonavir on time to clinical improvement, mortality, intensive care unit (ICU) length of stay, and length of hospital stay, compared with standard care. 70 Moreover, lopinavir‐ritonavir was stopped early in 13.8% of patients because of adverse events.
Children with COVID‐19 seem to have less severe disease and better prognosis than adults. Of 551 pediatric cases with laboratory‐confirmed COVID‐19 included in this review, only nine had severe/critical disease. Six patients needed invasive mechanical ventilation, and one of them died. All of these six patients had major underlying medical condition. Among a nationwide case series of 2135 pediatric patients with COVID‐19 (728 laboratory‐confirmed cases) reported to the Chinese CDC, 16 55.4% were classified as asymptomatic or mild, 38.8% were classified as moderate, and 5.8% were classified as severe/critical. The proportion of severe and critical cases was 10.6%, 7.3%, 4.2%, 4.1%, and 3.0% for the age group of <1, 1 to 5, 6‐10, 11 to 15, and ≥16 years, respectively. One 14‐year‐old boy died, but it is not clear whether this adolescent had underlying condition.
In the U.S. cohort of pediatric COVID‐19 cases, 3 children aged <1 year accounted for the highest percentage (15%‐62%) of hospitalization among 745 (29% of total cohort) cases with known hospitalization status. Among 345 cases with information on underlying conditions, 23% had at least one health problem, such as chronic lung disease (including asthma), cardiovascular disease, and immunosuppressive conditions. Seventy‐seven percent of hospitalized patients, including six patients admitted to an ICU, have one or more underlying condition. Three deaths were reported in the U.S. cohort of 2572 pediatric cases; however, review of these cases is ongoing to confirm COVID‐19 as the likely cause of death. These results may suggest that patient's age and underlying medical condition are possible host factors associated with susceptibility to COVID‐19, disease severity, and prognosis in pediatric patients.
The results of this systematic review have implications for clinical practice and research. First, previously healthy children with COVID‐19 usually have mild symptoms and good prognosis. The diagnosis is generally suspected from history of household exposure to COVID‐19 case. For these patients, the management should focus on symptomatic and supportive care. In mild cases, unnecessary laboratory and imaging evaluation and unproven treatment should be avoided. Second, more attention should be given to children with COVID‐19 and major underlying medical conditions. These patients are more likely to have severe or critical disease and poor prognosis. Third, currently available evidence regarding COVID‐19 in children is mainly descriptive and anecdotal, and many questions remain unanswered. What are the risk factors for COVID‐19 in children? Why do children seem to be less affected by COVID‐19? What is the role of radiological imaging in the diagnosis and assessment of children with COVID‐19, and is there any advantage of CT scan over plain X‐ray? What are the effective treatments for children with COVID‐19? Could the WHO algorithm for the management of acute respiratory infections in children be applicable to patients with mild‐ to moderate COVID‐19, especially in low‐middle‐income countries? What are the prognosis factors for children with COVID‐19? What is the clinical implication of prolonged fecal shedding of SARS‐CoV‐2 RNA in children with COVID‐19? Further prospective multicenter studies are needed to answer these questions.
This review has several limitations. The majority of included studies were conducted in China and other Asian countries, and thus caution should be taken when applying the findings of this review to Western populations. The retrospective data collection might have caused lack of accuracy and missing data for some clinical and laboratory variables. All the studies included in the review were case series or case reports which provide low‐ to very low‐ level evidence. However, this is the best available evidence to date for a previously unknown disease. We used a comprehensive search strategy to identify larger number of relevant studies, and both visual inspection of the funnel plots and Egger's test did show substantial publication bias (Figures S1,2). We recognize, however, that some Chinese studies published in local medical journals might not be identified and included in the review.
In conclusion, children of all ages can get COVID‐19, although they appear to be affected less commonly than adults. Mild‐to‐moderate fever and cough are the most common symptoms, but much less frequently than that reported in adults, and 18% of patients may be asymptomatic. Ground glass opacity and consolidation are the most common CT abnormalities in children with COVID‐19. The prevalence of such CT findings in pediatric cases is lower than that in adult patients, and 36% of children with laboratory‐confirmed COVID‐19 may have normal CT images. Previously healthy children with COVID‐19 usually have mild symptoms and good prognosis. However, children with COVID‐19 and major underlying medical condition are more likely to have severe or critical disease, and poor prognosis, even death. To date, there is no approved treatment for COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
LZ conceptualized and designed the study, participated in trial selection, quality assessment, data collection, data analysis and interpretation, drafted the protocol and the review article, and approved the final manuscript as submitted. TGP and MVFS provided input for study conception and design, participated in trial selection, quality assessment and data collection, critically revised the manuscript, and approved the final manuscript as submitted. PC provided input for study conception and design, critically revised the manuscript, and approved the final manuscript as submitted.
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Zhang L, Peres TG, Silva MVF, Camargos P. What we know so far about Coronavirus Disease 2019 in children: A meta‐analysis of 551 laboratory‐confirmed cases. Pediatric Pulmonology. 2020;55:2115–2127. 10.1002/ppul.24869
REFERENCES
- 1. Dong E, Du H, Gardner. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20:533‐534. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 3. CDC COVID‐19 Response Team . Coronavirus disease 2019 in children—United States, 12 February to 2 April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422‐426. 10.15585/mmwr.mm6914e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. European Centre for Disease Prevention and Control Rapid risk assessment: coronavirus disease 2019 (COVID‐19) pandemic: increased transmission in the EU/EEA and the UK—eighth update. https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-coronavirus-disease-2019-covid-19-pandemic-eighth-update. Accessed 10 April 2020.
- 5. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cai J, Xu J, Lin D, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020. 10.1093/cid/ciaa198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feng K, Yun YX, Wang XF, et al. Analysis of CT features of 15 children with 2019 novel coronavirus infection. Zhonghua Er Ke Za Zhi. 2020;58(0):E007. 10.3760/cma.j.issn.0578-1310.2020.0007 [DOI] [PubMed] [Google Scholar]
- 9. Chan JFW, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395:514‐523. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ludvigsson JF. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088‐1095. 10.1111/apa.15270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Souza TH, Nadal JA, Nogueira RJN, Pereira RM, Brandao MB. Clinical manifestations of children with COVID‐19: a systematic review. MedRxiv. 10.1101/2020.04.01.20049833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020. 10.1001/jamapediatrics.2020.1467 [DOI] [PubMed] [Google Scholar]
- 13. National Institutes of Health . Study quality assessment tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 6 April 2020.
- 14. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020. 10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu H, Cai Q, Dai X, Liu X, Sun H. The clinical and epidemiological features and hints of 82 confirmed COVID‐19 pediatric cases aged 0‐16 in Wuhan, China. medRxiv 10.1101/2020.03.15.20036319 [DOI] [Google Scholar]
- 16. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020:e20200702. 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 17. Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020;323:1313. 10.1001/jama.2020.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen D, Li Y, Deng X, et al. Four cases from a family cluster were diagnosed as COVID‐19 after 14‐day of quarantine period. J Med Virol. 2020. 10.1002/jmv.25849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cui Y, Tian M, Huang D, et al. A 55‐day‐old female infant infected with 2019 novel coronavirus disease: presenting with pneumonia, liver injury, and heart damage. J Infect Dis. 2020;221:1775‐1781. 10.1093/infdis/jiaa113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Du W, Yu J, Wang H, et al. Clinical characteristics of COVID‐19 in children compared with adults in Shandong province, China. Infection. 2020. 10.1007/s15010-020-01427-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han YN, Feng ZW, Sun LN, et al. A comparative‐descriptive analysis of clinical characteristics in 2019‐coronavirus‐infected children and adults. J Med Virol. 2020:jmv.25835. 10.1002/jmv.25835 [DOI] [PubMed] [Google Scholar]
- 22. He G, Sun W, Wu J, Cai J. Serial computed tomography manifestations in a child with coronavirus disease (COVID‐19). Indian Pediatr. 2020;57:467‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang L, Zhang X, Zhang X, et al. Rapid asymptomatic transmission of COVID‐19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16‐23 years outside Wuhan and characteristics of young patients with COVID‐19: a prospective contact‐tracing study. J Infect. 2020;80:e1‐e13. 10.1016/j.jinf.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ji LN, Chao S, Wang YJ, et al. Clinical features of pediatric patients with COVID‐19: a report of two family cluster cases. World J Pediatr. 2020. 10.1007/s12519-020-00356-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li W, Cui H, Li K, Fang Y, Li S. Chest computed tomography in children with COVID‐19 respiratory infection. Pediatr Radiol. 2020;50:796‐799. 10.1007/s00247-020-04656-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li B, Shen J, Li L, Yu C. Radiographic and clinical features of children with 2019 novel coronavirus (COVID‐19) pneumonia. Indian Pediatr. 2020;57:423‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin J, Duan J, Tan T, Fu Z, Dai J. The isolation period should be longer: lesson from a child infected with SARS‐CoV‐2 in Chongqing, China. Pediatr Pulmonol. 2020;55. 10.1002/ppul.24763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu W, Zhang Q, Chen J, et al. Detection of Covid‐19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020;382:1370‐1371. 10.1056/NEJMc2003717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lou XX, Shi CX, Zhou CC, Tian YS. Three children who recovered from novel coronavirus 2019 pneumonia. J Paediatr Child Health. 2020;56:650‐651. 10.1111/jpc.14871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pan X, Chen D, Xia Y, et al. Asymptomatic cases in a family cluster with SARS‐CoV‐2 infection. Lancet Infect Dis. 2020;20(4):410‐411. 10.1016/S1473-3099(20)30114-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID‐19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689‐696. 10.1016/S1473-3099(20)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Quan SD, Li X, Ding T, Sun S, Sun G. A case report of child with SARS‐CoV‐2 infection in Liaoning province. Zhongguo Xiaoer Jijiu Yixue. 2020:27. 10.3760/cma.j.issn.1673-4912.2020.0005 [DOI] [Google Scholar]
- 33. Shen Q, Guo W, Guo T, et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr Pulmonol. 2020;55:1424‐1429. 10.1002/ppul.24762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shi B, Xia Z, Xiao S, Huang C, Zhou X, Xu H. Severe pneumonia due to sars‐cov‐2 and respiratory syncytial virus infection: a case report. Clin Pediatr. 2020:9922820920016. 10.1177/0009922820920016 [DOI] [PubMed] [Google Scholar]
- 35. Su L, Ma X, Yu H. The different clinical characteristics of corona virus disease cases between children and their families in China—the character of children with COVID‐19. Emerg Microbes Infect. 2020;9(1):707‐713. 10.1080/22221751.2020.1744483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tang A, Tong ZD, Wang HL, et al. Detection of novel coronavirus by rt‐pcr in stool specimen from asymptomatic child, China. Emerg Infect Dis. 2020;26(6):1337‐1339. 10.3201/eid2606.200301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang D, Ju XL, Xie F, et al. [Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua Er Ke Za Zhi. 2020;58(4):E011‐E274. 10.3760/cma.j.cn112140-20200225-00138 [DOI] [PubMed] [Google Scholar]
- 38. Wang J, Wang D, Chen GC, Tao XW, Zeng LK. SARS‐CoV‐2 infection with gastrointestinal symptoms as the first manifestation in a neonate. Chin. J Contemp Pediatr. 2020;22(3):211‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xing YH, Ni W, Wu Q, et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect. 2020. 10.1016/j.jmii.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS‐CoV‐2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26(4):502‐505. 10.1038/s41591-020-0817-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang YH, Lin DJ, Xiao MF, et al. 2019‐novel coronavirus infection in a three‐month‐old baby. Zhonghua Er Ke Za Zhi. 2020;58(0):E006. 10.3760/cma.j.issn.0578-1310.2020.0006 [DOI] [PubMed] [Google Scholar]
- 42. Zhang GX, Zhang AM, Huang L, Cheng LYm Liu ZX, Peng XL, Wang HW. Twin girls infected with SARS‐CoV‐2. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22(3):221‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang T, Cui X, Zhao X, et al. Detectable SARS‐CoV‐2 viral RNA in feces of three children during recovery period of COVID‐19 pneumonia. J Med Virol. 2020. 10.1002/jmv.25795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zheng F, Liao C, Fan Q, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. 2020;40(2):1‐6. 10.1007/s11596-020-2172-6 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou Y, Yang GD, Feng K, et al. Clinical features and chest CT findings of coronavirus disease 2019 in infants and young children. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22(3):215‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhu L, Wang J, Huang R, et al. Clinical characteristics of a case series of children with coronavirus disease 2019. Pediatr Pulmonol. 2020;55:1430‐1432. 10.1002/ppul.24767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Song W, Li J, Zou N, Guan W, Pan J, Xu W. Clinical features of pediatric patients with coronavirus disease (COVID‐19). J Clin Virol. 2020;127:104377. 10.1016/j.jcv.2020.104377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rahimzadeh G, Ekrami Noghabi M, Kadkhodaei Elyaderani F, et al. COVID‐19 infection in Iranian children: a case series of 9 patients. J Pediatr Rev. 2020;8(2):139‐144. [Google Scholar]
- 49. Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infect Dis. 2020;52:1‐3. 10.1080/23744235.2020.1747634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Canarutto D, Priolo A, Russo G, Pitea M, Vigone MC, Barera G. COVID‐19 infection in a paucisymptomatic infant: raising the index of suspicion in epidemic settings. Pediatr Pulmonol. 2020;55, 10.1002/ppul.24754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Parri N, Lenge M, Buonsenso D. Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group. Children with Covid‐19 in pediatric emergency departments in Italy. N Engl J Med. 2020:NEJMc2007617. 10.1056/NEJMc2007617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Denina M, Scolfaro C, Silvestro E, et al. Lung ultrasound in children with COVID‐19. Pediatrics. 2020:e20201157. 10.1542/peds.2020-1157 [DOI] [PubMed] [Google Scholar]
- 53. Park JY, Han MS, Park KU, Kim JY, Choi EH. First pediatric case of coronavirus disease 2019 in Korea. J Korean Med Sci. 2020;35(11):e124. 10.3346/jkms.2020.35.e124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. See KC, Liew SM, Ng DCE, et al. COVID‐19: Four paediatric cases in Malaysia. Int J Infect Dis. 2020;94:125‐127. 10.1016/j.ijid.2020.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kam K, Yung CF, Cui L, et al. A well infant with coronavirus disease 2019 with high viral load. Clin Infect Dis. 2020. 10.1093/cid/ciaa201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Díaz CA, Maestro ML, Pumarega MTM, Antón BF, Alonso CP. Primer caso de infección neonatal por Covid‐19 en España. An Pediatr (Barc), 2020:2816. 10.1016/j.anpedi.2020.03.002 [DOI] [Google Scholar]
- 57. Jones VG, Mills M, Suarez D, et al. COVID‐19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020:2020‐0123. 10.1542/hpeds.2020-0123 [DOI] [PubMed] [Google Scholar]
- 58. Le HT, Nguyen LV, Tran DM, et al. The first infant case of COVID‐19 acquired from a secondary transmission in Vietnam. Lancet Child Adolesc Health. 2020;4:405‐406. 10.1016/S2352-4642(20)30091-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutiérrez‐Ocampo E, et al. Clinical, laboratory and imaging features of COVID‐19: a systematic review and meta‐analysis. Travel Med Infect Dis. 2020;34:101623. 10.1016/j.tmaid.2020.101623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. 10.1038/s41392-020-0148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases. Radiology. 2020:200642. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li Y, Xia LM. Coronavirus disease 2019 (COVID‐19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020;214:1‐7. 10.2214/AJR.20.22954 [DOI] [PubMed] [Google Scholar]
- 64. Xu B, Xing Y, Peng J, et al. for detecting covid‐19: a systematic review and meta‐analysis of diagnostic accuracy. Research Squre. 2020. 10.21203/rs.3.rs-20481/v1.\69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen PL, Zhang TX, Hu YH, Zhou CF, Wang SD. A multicenter randomized controlled clinical study of recombinant human interferon‐typeα1b in the treatment of viral pneumonia in children. Lin Chuang Er Ke Za Zhi. 2005;23:244‐245. [Google Scholar]
- 66. Shang YX, Huang Y, Liu EM, Chen Q, Cao L, Lu M. A multicenter study on the treatment of acute bronchiolitis by nebulized human recombinant human interferon‐alpha 1b. Zhongguo Shi Yong Er Ke Za Zhi. 2014;29:840‐804. [Google Scholar]
- 67. Chen L, Shi M, Deng Q, et al. A multi‐center randomized prospective study on the treatment of infant bronchiolitis with interferon α1b nebulization. PLoS One. 2020;15(2):e0228391. 10.1371/journal.pone.0228391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shen K, Yang Y, Wang T, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts' consensus statement. World J Pediatr. 2020. 10.1007/s12519-020-00343-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tu YF, Chien CS, Yarmishyn AA, et al. A review of SARS‐CoV‐2 and the ongoing clinical trials. Int J Mol Sci. 2020;21(7):2657. 10.3390/ijms21072657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cao B, Wang Y, Wen D, et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe COVID‐19. N Engl J Med. 2020;382:1787‐1799. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Supporting information
Supporting information


