Abstract
The spike glycoprotein on the virion surface docking onto the angiotensin‐converting enzyme (ACE) 2 dimer is an essential step in the process of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in human cells—involves downregulation of ACE2 expression with systemic renin‐angiotensin system (RAS) imbalance and promotion of multi‐organ damage. In general, the RAS induces vasoconstriction, hypertension, inflammation, fibrosis, and proliferation via the ACE/Ang II/Ang II type 1 receptor (AT1R) axis and induces the opposite effects via the ACE2/Ang (1‐7)/Mas axis. The RAS may be activated by chronic inflammation in hypertension, diabetes, obesity, and cancer. SARS‐CoV‐2 induces the ACE2 internalization and shedding, leading to the inactivation of the ACE2/Ang (1‐7)/Mas axis. Therefore, we hypothesize that two hits to the RAS drives COVID‐19 progression. In brief, the first hit originates from chronic inflammation activating the ACE/Ang II/AT1R axis, and the second originates from the COVID‐19 infection inactivating the ACE2/Ang (1‐7)/Mas axis. Moreover, the two hits to the RAS may be the primary reason for increased mortality in patients with COVID‐19 who have comorbidities and may serve as a therapeutic target for COVID‐19 treatment.
Keywords: coronavirus disease 2019, renin‐angiotensin system), two hits
1. INTRODUCTION
The first report related to the ongoing pandemic of coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome (SARS) coronavirus 2 (SARS‐CoV‐2), originated in Wuhan, China in early December 2019. Subsequently, COVID‐19 rapidly spread across China and then globally within 2 months after the first confirmed COVID‐19 case was reported outside of China, in Thailand, on 13 January 2020. The World Health Organization declared the outbreak to be a public health emergency of international concern on 30 January 2020, and then further elevated the level of emergency by recognizing it as a pandemic on 11 March 2020.1, 2 Thus far, more than 3.5 million confirmed COVID‐19 cases and more than 247 652 related deaths have been reported worldwide. 2 Yan et al. reported that the trimer of the spike glycoprotein on the virion surface docking onto the angiotensin‐converting enzyme (ACE) 2 dimer structure is an essential step in the attack by SARS‐CoV‐2 on human cells and leads to systemic organ injury.3, 4 This SARS‐CoV‐2‐ACE2 interaction has generated great interest in the development of renin‐angiotensin system (RAS)‐based therapeutic strategies for COVID‐19. 5
2. RAS
The RAS, a key hormone system, regulates blood pressure, fluid and electrolyte balance, and systemic vascular function particularly in maintaining plasma sodium concentration, arterial blood pressure, and extracellular volume. 6 In most organs, RAS activation can result in hypertension, inflammation, cell proliferation, and fibrosis.6, 7 Manipulating the RAS by stabilizing renin and angiotensin II (Ang II) levels and promoting ACE2 expression can prevent numerous chronic and acute diseases.7, 8, 9, 10 The RAS signal transduction system is regulated by two main axes: (a) ACE/Ang II/Ang II type 1 receptor (AT1R) axis, which promotes vasoconstriction, hypertension, inflammation, fibrosis, and proliferation, and (b) ACE2/Ang (1‐7)/Mas axis, which induces the opposite effects of ACE/Ang II/AT1R axis activation to inhibit any detrimental consequences (Figure 1).
FIGURE 1.
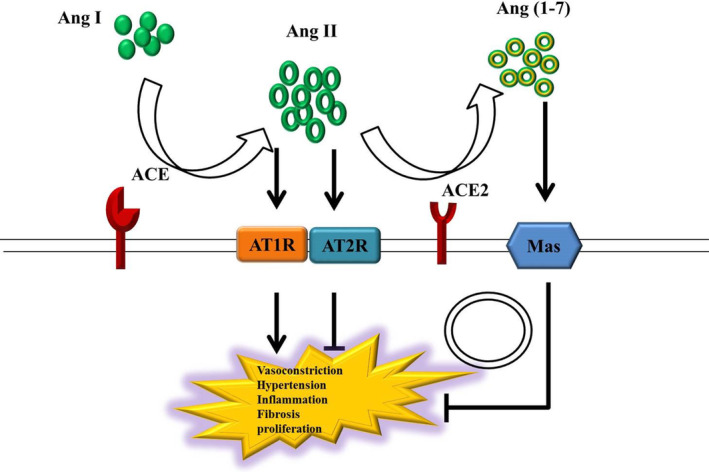
Steady‐state of the RAS under normal physiological conditions. The RAS signal transduction system is activated via the ACE/Ang II/AT1R axis; this promotes vasoconstriction, hypertension, inflammation, fibrosis, and proliferation. Studies on the biological functions of AT2R, a receptor of Ang II, are scant. Although AT2R activation induces the effects opposite to those of AT1R activation, AT2R expression is lower than AT1R expression in most adult tissues. Most functions of Ang II are performed via AT1R. The ACE2/Ang (1‐7)/Mas axis induces the opposite effects to suppress the harmful effects of the ACE/Ang II/AT1R axis induction
3. THE ROLE OF RAS IN COVID‐19
Several studies have indicated that circulating Ang II levels are significantly higher in individuals infected with SARS‐CoV‐2 than in healthy individuals; thus, SARS‐CoV‐2 infection must cause tissue ACE2 downregulation with systemic RAS imbalance and thus promote multi‐organ damage.1, 11, 12 SARS coronavirus (SARS‐CoV) interacts with ACE2 via trimers of the SARS spike protein, a loop of which extends into a hydrophobic pocket of ACE2. In addition, the difference between SARS‐CoV and SARS‐CoV‐2 is of only 380 amino acid substitutions, translating to differences only in five of the six important amino acids in the receptor‐binding domain (RBD) of the viral spike protein. Both SARS‐CoV‐2 and SARS‐CoV infect cells via viral spike protein‐host ACE2 receptor contact, however, the RBD has significantly higher binding affinity to ACE2 receptors in SARS‐CoV‐2 than in SARS‐CoV.1, 13 Furthermore, the entry of SARS‐CoV‐2 into host cells induces ACE2 internalization and shedding, which increases Ang II and reduces Ang (1‐7) levels, with a net increase in pro‐inflammatory function relative to the anti‐inflammatory function of RAS. 14
4. ROLE OF RAS IN COVID‐19 DEVELOPMENT IN PATIENTS WITH COMORBIDITIES
RAS activation induced by chronic inflammation stimulates pro‐inflammatory and anti‐inflammatory cytokine production in immune cells, which in turn upregulates the expression of RAS components and accelerates the formation of systemic and local Ang II. 15 Blood Ang II levels are elevated in patients with chronic inflammatory diseases, such as pulmonary arterial hypertension, diabetes, and obesity.16, 17, 18 Moreover, elevated plasma Ang II levels are correlated with shortened survival in patients with cancer cachexia. 19 In addition, senescent T cells are more sensitive to Ang II stimulation and have adverse effects on the target organ, in which senescent T‐cell‐derived IFN‐γ may play a key role. 20 These findings indicate that chronic inflammation and aging have complex but close relationship with activation of the ACE/Ang II/AT1R axis.
Patients with COVID‐19 develop acute inflammatory diseases, such as SARS‐CoV‐2‐induced acute respiratory distress syndrome. These patients, particularly those aged >65 years or with hypertension, diabetes, cardiovascular disease, chronic respiratory disease, low immune status, cancer, or obesity, have poor prognosis and may develop multiple organ failure.21, 22, 23, 24 Furthermore, increased levels of obesity‐related inflammatory cytokines possibly increase COVID‐19 morbidity. 25 Finally, patients with cancer might have an increased COVID‐19 risk because they generally have systemic immunosuppression due to the malignancy and the administered anticancer treatments. 26
A recent cohort study on 45 000 confirmed cases in China reported a considerably increased mortality rate in patients with COVID‐19 who had cardiovascular disease (10.5%), diabetes (7.3%), or hypertension (6.3%) compared with those who did not have these comorbidities (0.9%).27, 28 In addition, an analysis of the medical records of 355 patients who died due to COVID‐19 in Italy revealed that only 0.8% of these patients had no comorbidities. 29 Moreover, of all 355 patients, approximately half had at least three comorbidities, approximately one‐quarter had two comorbidities, and approximately one‐quarter had one comorbidity 29 ; moreover, approximately 73.8% had hypertension, approximately 33.9% had diabetes, and approximately 30.1% had heart disease.29, 30
Therefore, we hypothesize that the higher mortality rate in patients with COVID‐19 who have comorbidities may be attributable to the two hits to the RAS: The first hit originates from chronic inflammation, which activates the ACE/Ang II/AT1R axis of the RAS and the second hit originates from SARS‐CoV‐2 infection, which inhibits the ACE2/Ang (1–7)/Mas axis of the RAS. These two hits increase tissue damage and multiple organ failure risks in patients with SARS‐CoV‐2 infection (Figure 2).
FIGURE 2.
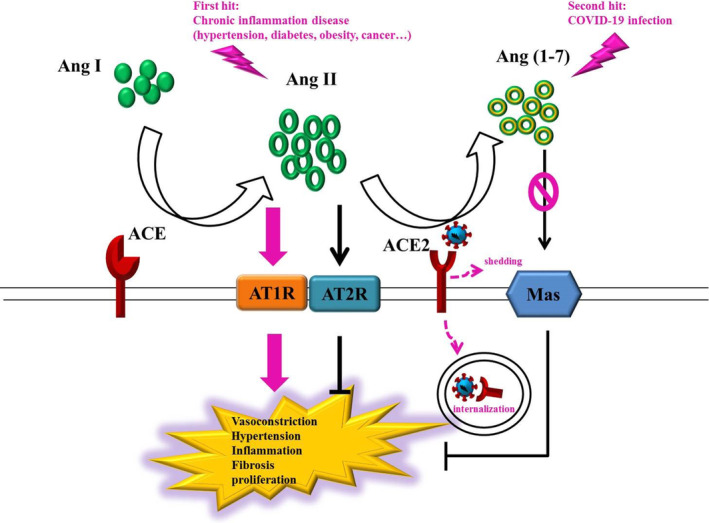
Two hits to the RAS by chronic inflammation and COVID‐19 infection. The ACE/Ang II/AT1R axis of the RAS is activated through chronic inflammation, including hypertension, diabetes, obesity, and cancer. Furthermore, SARS‐CoV‐2 induces ACE2 internalization and shedding, which lead to inactivation of the ACE2/Ang (1‐7)/Mas axis. Therefore, the two hits to the RAS may be the primary reason for the mortality rate being high among patients with COVID‐19 who have comorbidities. In brief, the first hit originates from chronic inflammation activating the ACE/Ang II/AT1R axis, and the second hit originates from the COVID‐19 infection inactivating the ACE2/Ang (1‐7)/Mas axis
5. INSIGHTS INTO COVID‐19 TREATMENT WITH RAS INHIBITORS
The lung is the primary target for SARS‐CoV‐2 infection, mainly because of its large surface area and high ACE2 expression in alveolar epithelial type II cells. In addition to being highly expressed in the lungs, ACE2 is highly expressed in tissues, such as the heart, kidney, endothelial, and intestinal tissues, which might explain the multiple organ dysfunction noted in patients with COVID‐19. 31 One study indicated low COVID‐19 severity in patients with hypertension receiving ACE inhibitors (ACEi) or angiotensin‐receptor blockers (ARB); moreover, these patients tended to have low blood interleukin 6 levels. 32 Compared with other antihypertensive drugs, ACEi or ARB use has also been reported to increase blood CD3 and CD8 T‐cell counts and reduce peak viral load. 32 Finally, ACE2 activation can reduce the severity of lipopolysaccharide‐induced acute lung injury via the activated serine/threonine protein kinase (AMPK)/mammalian target‐of‐rapamycin (mTOR) pathway. 33
6. CONCLUSION
Regulation of the RAS may serve as a therapeutic target for COVID‐19 treatment, and therefore, the underlying mechanism of its effects requires urgent clarification.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Tseng Y‐H, Yang R‐C, Lu T‐S. Two hits to the renin‐angiotensin system may play a key role in severe COVID‐19. Kaohsiung J Med Sci. 2020;36:389–392. 10.1002/kjm2.12237
REFERENCES
- 1. Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, et al. Angiotensin converting enzyme 2: SARS‐CoV‐2 receptor and regulator of the renin‐angiotensin system. Circ Res. 2020;126:1456–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) . Coronavirus disease (COVID‐19) Pandemic [Situation reports]. May 7, 2020. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 3. Malha L, Mueller FB, Pecker MS, Mann SJ, August P, Feig PU. COVID‐19 and the renin‐angiotensin system. Kidney Int Rep. 2020;5:563–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS‐CoV‐2 by full‐length human ACE2. Science. 2020;367(6485):1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gurwitz D. Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics. Drug Dev Res. 2020:1–4. 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel S, Rauf A, Khan H, Abu‐Izneid T. Renin‐angiotensin‐aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed Pharmacother. 2017;94:317–325. [DOI] [PubMed] [Google Scholar]
- 7. Song J, Hu B, Qu H, Wang L, Huang X, Li M, et al. Upregulation of angiotensin converting enzyme 2 by shear stress reduced inflammation and proliferation in vascular endothelial cells. Biochem Biophys Res Commun. 2020;525:812–818. [DOI] [PubMed] [Google Scholar]
- 8. Batlle D, Jose Soler M, Ye M. ACE2 and diabetes: ACE of ACEs? Diabetes. 2010;59(12):2994–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noguchi R, Kaji K, Namisaki T, Moriya K, Kitade M, Takeda K, et al. Serum angiotensin‐converting enzyme level for evaluating significant fibrosis in chronic hepatitis B. World J Gastroenterol. 2017;23(36):6705–6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qaradakhi T, Gadanec LK, McSweeney KR, Tacey A, Apostolopoulos V, Levinger I, et al. The potential actions of angiotensin‐converting enzyme II (ACE2) activator diminazene aceturate (DIZE) in various diseases. Clin Exp Pharmacol Physiol. 2020;47(5):751–758. [DOI] [PubMed] [Google Scholar]
- 11. Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang K, Gheblawi M, Oudit GY. Angiotensin converting enzyme 2: A double‐edged sword. Circulation. 2020. https://10.1161/CIRCULATIONAHA.120.047049. [DOI] [PubMed] [Google Scholar]
- 13. Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, et al. Genome composition and divergence of the novel coronavirus (2019‐nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sparks MA, South A, Welling P, Luther JM, Cohen J, Byrd JB, et al. Sound science before quick judgement regarding RAS blockade in COVID‐19. Clin J Am Soc Nephrol. 2020;15:714–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Satou R, Penrose H, Navar LG. Inflammation as a regulator of the renin‐angiotensin system and blood pressure. Curr Hypertens Rep. 2018;20(12):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sandoval J, Del Valle‐Mondragon L, Masso F, Zayas N, Pulido T, Teijeiro R, et al. Angiotensin converting enzyme 2 and angiotensin (1–7) axis in pulmonary arterial hypertension. Eur Respir J. 2020. 10.1183/13993003.02416-2019. [DOI] [PubMed] [Google Scholar]
- 17. South AM, Nixon PA, Chappell MC, Diz DI, Russell GB, Shaltout HA, et al. Obesity is associated with higher blood pressure and higher levels of angiotensin II but lower angiotensin‐(1–7) in adolescents born preterm. J Pediatr. 2019;205:55–60.e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hollenberg NK, Stevanovic R, Agarwal A, Lansang MC, Price DA, Laffel LM, et al. Plasma aldosterone concentration in the patient with diabetes mellitus. Kidney Int. 2004;65(4):1435–1439. [DOI] [PubMed] [Google Scholar]
- 19. Penafuerte CA, Gagnon B, Sirois J, Murphy J, MacDonald N, Tremblay ML. Identification of neutrophil‐derived proteases and angiotensin II as biomarkers of cancer cachexia. Br J Cancer. 2016;114(6):680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pan XX, Wu F, Chen XH, Chen DR, Chen HJ, Kong LR, et al. T cell senescence accelerates angiotensin II‐induced target organ damage. Cardiovasc Res. 2020. 10.1093/cvr/cvaa032. [DOI] [PubMed] [Google Scholar]
- 21. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eurosurveillance Editorial Team . Updated rapid risk assessment from ECDC on coronavirus disease 2019 (COVID‐19) pandemic: Increased transmission in the EU/EEA and the UK. Euro Surveill. 2020;25(12):2003121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dietz W, Santos‐Burgoa C. Obesity and its implications for COVID‐19 mortality. Obesity (Silver Spring). 2020. 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- 26. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS‐CoV‐2 infection: A nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151.32064853 [Google Scholar]
- 28. Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID‐19 with different severity: A multi‐center study of clinical features. Am J Respir Crit Care Med. 2020. 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020;323(18):1175–1176. [DOI] [PubMed] [Google Scholar]
- 30. Gentile S, Strollo F, Ceriello A. COVID‐19 infection in Italian people with diabetes: Lessons learned for our future (an experience to be used). Diabetes Res Clin Pract. 2020;162:108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, et al. Renin‐angiotensin system inhibitors improve the clinical outcomes of COVID‐19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang X, Zheng J, Yan Y, Ruan Z, Su Y, Wang J, et al. Angiotensin‐converting enzyme 2 regulates autophagy in acute lung injury through AMPK/mTOR signaling. Arch Biochem Biophys. 2019;672:108061. [DOI] [PubMed] [Google Scholar]


