Abstract
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a global health emergency, in which its effective treatment and prevention remain obscured. Hyperpyrexia is an elevation of body temperature above 106.7°F (41.5°C) due to an abnormally increased hypothalamic‐thermoregulatory set. The pathophysiology, impact, and outcomes of hyperpyrexia in patients with COVID‐19 have not yet been studied. Herein, we present clinical features and outcomes of six patients with COVID‐19 who had developed hyperpyrexia during hospitalization. All patients expired shortly after the onset of hyperpyrexia. Hyperpyrexia seems to adversely impact the outcomes and mortality in patients with COVID‐19. The underlying mechanisms of developing hyperpyrexia in COVID‐19 are mysterious. We propose it may be caused by SARS‐CoV‐2‐related brain injury, exuberant immune response, and thrombus formation. More research is needed to verify our results. Understanding the association between hyperpyrexia and SARS‐CoV‐2 will help to elucidate the COVID‐19 pathogenesis, which is mandatory for developing effective treatment strategies.
Keywords: COVID, fever, hyperpyrexia, SARS‐CoV‐2
Highlights
Hyperpyrexia is a negative prognostic factor for COVID‐19.
The pathogenesis of hyperpyrexia in COVID‐19 is undetermined but it may be caused by SARS‐CoV‐2‐related brain injury, exuberant host immune response, and thrombus formation.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has shaken the global health systems, with more than 5 million people, have infected and of which almost 400 000 died. The clinical courses of COVID‐19 vary from asymptomatic to multiorgan failure and death. 2 Despite the immense effort on COVID‐19 research, the mechanisms underlying the progression to severe disease remain mysterious. Several clinical factors, including certain comorbidities, hypoxia, elevated inflammatory markers, and acute kidney injury, are identified as the ominous clinical predictors of SARS‐CoV‐2 infection. 2 , 3 , 4
Hyperpyrexia is defined as an elevation of body temperature (BT) above 106.7°F (41.5°C) to achieve an abnormally increased hypothalamic‐thermoregulatory threshold, as opposed to hyperthermia, which is an elevation of core BT exceeding the normal hypothalamic‐thermoregulatory limit. 5 , 6 The most common cause of hyperpyrexia is brain dysfunction, while infection and sepsis are thought to be infrequent causes. 6 , 7 The effect of hyperpyrexia on patients who have infectious diseases is contradictory. Hyperpyrexia may adversely impact survival in patients with a bacterial infection, but may not increase mortality among patients with viral illnesses. 7 , 8 To date, the impact of hyperpyrexia on the clinical course and prognoses of patients with COVID‐19 has not yet been reported. Herein, we are presenting the clinical features and outcomes of confirmed patients with COVID‐19 who had developed hyperpyrexia and were admitted to our COVID‐19 unit from 1 to 10 April 2020.
2. CASE PRESENTATION
Six patients were identified; four were females and two were males; and patients' median age was 60.5 years (range, 54‐62 years). Five patients had multiple comorbidities proven to negatively impact COVID‐19 prognoses. Fever, dyspnea, and altered mental status were among the most prevalent clinical manifestations. Gastrointestinal (GI) symptoms were reported in one patient. The median duration of illness before hospitalization was 2 days (range, 1‐7 days). COVID‐19 was diagnoses by a positive SARS‐CoV‐2 reverse transcription‐polymerase chain reaction test from nasopharyngeal swab specimens. All patients presented with hypoxia (oxygen saturation on room air range, 60%‐89%) and had abnormal chest X‐ray compatible with viral pneumonia (Figure 1). Two patients did not have lymphocytopenia initially but later developed during the hospital course. Ferritin was measured in five patients, and all revealed the remarkably high result. C‐reactive protein (CRP) was initially elevated in four of five patients, and D‐dimer (DD) was high in five of five patients.
Figure 1.
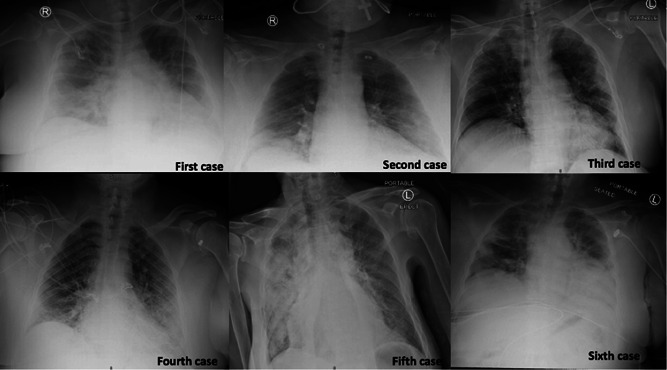
Chest X‐ray of each patient: Case 1—Bilateral perihilar predominant infiltrates. Case 2—Bibasilar alveolar infiltrates. Case 3—Bibasilar alveolar infiltrates, predominately on the left‐sided. Case 4—Multifocal patchy pneumonic infiltration in the bilateral peripheral lung field. Case 5—Bilateral multifocal patchy pneumonic infiltration predominantly on the right. Case 6—Patchy ground‐glass infiltrates throughout the left lung and at the right lung base
All patients received a 5‐day course of hydroxychloroquine (400 mg twice daily on the first day, and 200 mg twice daily for the following 4 days) for the COVID‐19 treatment per hospital protocol, and intravenous ceftriaxone and azithromycin to cover possible bacterial pneumonia. However, blood culture revealed negative results in all. None received steroids, immunosuppressive agents, or convalescent plasma.
All patients were intubated due to acute hypoxemic respiratory failure within 3 days of hospitalization, of which four were intubated during the first admission day. The positive end‐expiratory pressure (PEEP) and the fraction of inspired oxygen (FiO2) requirement ranged from 8 to 20 cm H2O, and 40% to 100%, respectively. Five patients had developed acute kidney injury. The median onset of hyperpyrexia was 8 days from admission (range, 6‐12) with the measured axillary BT between 107.2 and 109.7°F. External cooling with ice pads was applied as well as oral acetaminophen administration to control the BT. Patients 4 and 5 showed improvement of lung mechanics demonstrated by a decreased requirement of PEEP and FiO2 over the hospitalization courses However, both patients failed spontaneous breathing trials due to impair of consciousness, despite not receiving any sedation. All patients ultimately expired. The duration from the first occurrence of hyperpyrexia to death ranged between 3 and 45 hours (mean 17 hours and median 9.5 hours). Table 1 summarizes the demographic, clinical features, and outcomes of the included patients. Figure 2 demonstrates the dynamic changes of measured BT (°F) of each patient.
Table 1.
Demographic, clinical features, and outcomes of patients
| Patient | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Age, sex | 62, Female | 54, Male | 61, Male | 60, Male | 56, Male | 62, Female |
| Comorbidities | DM, HTN, HLD, and COPD | DM, HTN, HLD, and CAD | DM, HTN, and CKD | DM | Cerebral palsy | DM, HTN, CKD, and stroke |
| BMI | 37.37 | 47.95 | 39.98 | 39.17 | 25.70 | 38.55 |
| Presenting symptoms | Dyspnea, dry cough, fever, abdominal pain, and nausea | Dyspnea, fever, and dry cough | Altering mental status | Dyspnea, fever, cough, and altering the mental status | Altering mental status and fever | Altering mental status |
| Duration of symptoms | 2 d | 4 d | 2 d | 7 d | N/A | 1 d |
| SpO2 RA | 84% | 85% | 87% | 60% | 89% | 89% |
| Initial labs | WBC 7.2 | WBC 9.23 | WBC 11.43 | WBC 15.86 | WBC 7.87 | WBC 7.93 |
| L 21% | L 12% | L 9.5% | L 19.8% | L 4.8% | L 11.9% | |
| Cr 0.56 | Cr 1.4 (BL 1.1) | Cr 2.33 | Cr 0.98 | Cr 0.84 | Cr 2.3 (BL 1.5) | |
| CRP 8 mg/dL | CRP 9.24 mg/dL | CRP 25.64 mg/dL | CRP 15.27 mg/dL | CRP 0.55 mg/dL | FER 1308 DD 302 ng/mL | |
| FER 791 mg/dL | FER 869 mg/dL | FER 1106 mg/dL | FER 977 mg/dL | IL‐6 10 pg/mL | ||
| DD 506 ng/mL | DD 1025 ng/mL | DD 640 ng/mL | DD 1929 ng/mL | |||
| IL‐6 79 pg/mL | ||||||
| Intubation | D 1 | D 1 | D 2 | D 1 | D 1 | D 3 |
| Last labs | WBC 13.67 | WBC 13.6 | WBC 11.85 | WBC 19.65 | WBC 9.58 | WBC 10.6 |
| L 13.2% | L 8.9% | L 10% | L 7.3% | L 7.3% | L 3.9% | |
| Cr 0.56 | Cr 7.03 | Cr 6.55 | Cr 12.94 | Cr 3.15 | Cr 13.19 | |
| CRP 14 mg/dL | CRP 21.89 mg/dL | CRP 34.42 mg/dL | CRP 6.77 mg/dL | DD 2236 ng/mL | ||
| PEEP/FiO2 | 20/100 | 18/100 | 18/80 | 8/40 | 8/50 | 18/100 |
| TTD, h | 7 | 3 | 4 | 20 | 12 | 45 |
| Peak BT | 109.7°F (D 6) | 109.7°F (D 10) | 107.6°F (D 6) | 107.2°F (D 8) | 109.7°F (D 12) | 107.2°F (D 8) |
| Outcome | Death (D 6) | Death (D 10) | Death (D 6) | Death (D 9) | Death (D 13) | Death (D 10) |
Abbreviations: BL, baseline value; BMI, body mass index; BT, body temperature; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; Cr, Creatinine; CRP, C‐reactive protein; D, day of event; DD, D‐dimer; DM, diabetes mellitus; FER, ferritin; FiO2, fraction of inspired oxygen; HLD, hyperlipidemia; HTN, hypertension; IL‐6, interleukin‐6; L, lymphocytes; NA, not applicable; PEEP, positive end‐expiratory pressure; SpO2 RA, oxygen saturation on room air; TTD, time from first onset of hyperpyrexia to death; WBC, white blood cell.
Figure 2.
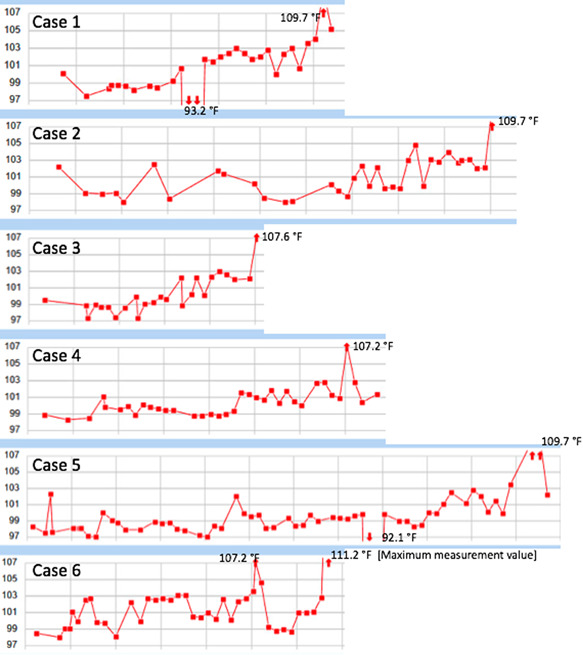
The dynamic changes of measured body temperature (°F) of each patient
3. DISCUSSION
Extremely high‐measured core BT is a devastating condition requiring urgent management and prompt recognition of the causes. High fever leads to direct cellular damages and worsens inflammatory responses so the cooling methods should be implemented immediately. 9 In addition, the causes of severely elevated BT need to be determined as many etiologies can lead to death even the fever symptom is under control. 6 It is important to distinguish whether the high BT is a result of hyperpyrexia or hyperthermia as the etiologies underlying these two conditions are different. The pathology of hyperpyrexia is centrally targeted, resulting in a reset of hypothalamic regulation to a higher level, whereas hyperthermia is usually caused by peripheral etiologies leading to excessive heat production or defective heat loss. 6
Our patients were deemed to have severely elevated BT from hyperpyrexia because of the high ranges of BT fluctuation and the elevation of inflammatory markers, especially CRP. 10 Besides, our patients did not have muscle rigidity nor developed rhabdomyolysis, which are the characteristic features in patients with hyperthermia. 6
To date, no published literature describes the pathophysiology of hyperpyrexia in COVID‐19. We propose three possible underlying mechanisms based on our current knowledge: (a) direct brain injury from SARS‐CoV‐2, (b) persistent immune dysfunction and dysregulation of cytokines, and (c) vascular thrombosis.
SARS‐CoV‐2 binds angiotensin‐converting enzyme 2 (ACE2) receptors to gain cellular entry into human organs. 11 ACE2 receptors are highly expressed in airway epithelia, lung parenchyma, and GI epithelia explaining why dyspnea, cough, and GI complaints are predominately symptoms in patients with COVID‐19. 12 Recent evidence suggested that neuron, astrocytes, and oligodendrocytes also have ACE2 receptors expression in a specific spatial localization pattern. 13 The direct invasion of SARS‐CoV‐2 to the nervous systems may explain COVID‐19‐related neurological complications, including stroke, encephalitis, and encephalopathy.
Brain injury from the SARS‐CoV‐2 invasion may lead to hyperpyrexia by two distinct mechanisms: direct injury to the neurons in hypothalamic‐thermoregulatory pathways and injury to other parts of the brain leading to the local production of proinflammatory and pyrogenic cytokines. Absence of normal circadian rhythm and the presence of hypothermia are pathognomonic signs of hyperpyrexia caused by brain injury. 14 According to our case series, the lack of normal daily temperature variation in patients 4 and 5, and the presence of hypothermia in patients 1 and 5 support the hypothesis that direct brain injury from SARS‐CoV‐2 leads to hyperpyrexia.
SARS‐CoV‐2 may cause injury to the brain‐stem respiratory center explaining why patients with COVID‐19 often report lesser perception of dyspnea than the actual degree of hypoxia and the extent of lung pathology. 15 Patients 4 and 5 showed a discrepancy between the improvement of COVID‐19 pneumonia, demonstrated by a decrease in oxygen requirement and better pulmonary mechanics, compared to the degree of respiratory function compromise, illustrated by an absence of spontaneous breathing despite sedation vacation. This further supports the possibility of brain injury from SARS‐CoV‐2.
Moreover, our case series revealed a remarkably high incidence of altering mental status (66.7%) as a presenting complaint, which indicated that many had encephalopathy before the admission. The early onset of encephalopathy (median 2 days [range, 1‐7 days]) raises the possibility that this encephalopathy was caused by the direct neuroinvasion of SARS‐CoV‐2 rather than its indirect effect from toxic‐metabolic encephalopathy.
SARS‐CoV‐2 leads to a unique pattern of immune dysfunction characterized by a defective antigen presentation and lymphoid cells, but preserved monocytes function to secrete tumor necrosis factor‐α (TNF‐α) and interleukin‐6 (IL‐6). 16 Patients with severe COVID‐19 have substantially higher serum levels of proinflammatory cytokines (TNF‐α, IL‐1, and IL‐6) and chemokines (IL‐8) than individuals with mild disease. 17 IL‐6 can also be used as a marker to predict SARS‐CoV‐2 disease deterioration. 18 IL‐1, IL‐6, and IL‐8 are known endogenous pyrogens. 19 Studies compared to the differential cytokines expression in malaria patients with and without hyperpyrexia discovered that IL‐6 was significantly higher in the hyperpyrexia group. 20 Thus, high IL‐6 may associate with hyperpyrexia in patients with COVID‐19.
Patients with COVID‐19 have an increased risk of arterial and venous thrombosis. 21 SARS‐CoV‐2 can directly destroy vascular endothelial cells and induce uncontrolled inflammatory responses leading to systemic thrombosis and generalized coagulopathy. Hematoma formation is well recognized but uncommon cause of hyperpyrexia. 6 Although this is the least likely possibility in our cases, it should be considered especially for patient 6, who had an eightfold increase in DD and a sudden increase in oxygen requirement during hospitalization. Furthermore, patient 6 is the only patient who had survived more than 24 hours after the onset of hyperpyrexia, which may suggest a different etiology underlying her extremely high BT.
Our case series revealed 100% mortality and suggested that hyperpyrexia is a poor prognostic outcome and may predict mortality in patients with COVID‐19. However, this assumption urgently needs more evidence as our case series is limited to only six patients. Another interesting point is that the patients' age in our case series is restricted to their 50 to 60 seconds. If this unique age group involvement exists in larger studies, it will be worthwhile to investigate the reasons.
Our case series also highlights the need to determine underlying mechanisms of hyperpyrexia in patients with COVID‐19 as each cause requires different management. Antiviral drugs, neuroprotective agents, and medications modifying neurotransmitters will be beneficial if the direct invasion of SARS‐CoV‐2 to the nervous systems is an underlying etiology. Immunosuppressive agents, particularly drugs targeting the causative cytokines, will be of greatest benefit if the immune dysregulation is identified. In contrast, thrombolytic treatment is mandatory if the patients have thromboembolic complications.
4. CONCLUSION
Our case series showed 100% mortality in patients with COVID‐19 who had developed hyperpyrexia. Hyperpyrexia may indicate poor clinical outcomes and worsen mortality in patients with COVID‐19. The underlying mechanisms of hyperpyrexia in COVID‐19 are unknown but may be a result of SARS‐CoV‐2‐related brain injury, exuberant immune response, and thrombus formation.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Suwanwongse K, Shabarek N. Hyperpyrexia in patients with COVID‐19. J Med Virol. 2020;92:2857–2862. 10.1002/jmv.26154
REFERENCES
- 1. WHO Coronavirus disease 2019 (COVID‐19) situation report—132. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200531-covid-19-sitrep-132.pdf?sfvrsn=d9c2eaef_2. Accessed 31 May 2020.
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China. JAMA Intern Med. 2020. https://10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in‐hospital death of patients with COVID‐19. Kidney Int. 2020;97(5):829‐838. 10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loscalzo J, Fauci A, Braunwald E, Dennis KL, Hauser SL, Longo DL. Chapter 17: fever versus hyperthermia. Harrison's Principles of Internal Medicine. New York, NY: McGraw‐Hill Medical; 2008. [Google Scholar]
- 6. Hussein O, Torbey M. Hyperpyrexia as the presenting symptom of intracranial hypotension. Neurocrit Care. 2017;28(3):395‐399. 10.1007/s12028-017-0481-9 [DOI] [PubMed] [Google Scholar]
- 7. Mackowiak PA, Wasserman SS, Levine MM. An analysis of the quantitative relationship between oral temperature and severity of illness in experimental shigellosis. J Infect Dis. 1992;166(5):1181–1184. 10.1093/infdis/166.5.1181 [DOI] [PubMed] [Google Scholar]
- 8. Sioson PB, Brown RB. Hyperpyrexia among patients in a large community hospital: causes, features, and outcomes. South Med J. 1993;86(7):773‐776. 10.1097/00007611-199307000-00011 [DOI] [PubMed] [Google Scholar]
- 9. Walter EJ, Hanna‐Jumma S, Carraretto M, Forni L. The pathophysiological basis and consequences of fever. Crit Care. 2016;20:200‐2016. 10.1186/s13054-016-1375-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dahan E, Dichtwald S, Amar E, Sorkine P, Weinbroum AA. Low plasma C‐reactive protein level as an early diagnostic tool for heatstroke vs central nervous system‐associated infection in the ED. Am J Emerg Med. 2013;31(8):1176‐1180. 10.1016/j.ajem.2013.04.030 [DOI] [PubMed] [Google Scholar]
- 11. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen R, Wang K, Yu J, Chen Z, Wen C, Xu Z. The spatial and cell‐type distribution of SARS‐CoV‐2 receptor ACE2 in human and mouse brain. https://www.biorxiv.org/content/10.1101/2020.04.07.030650v1. Accessed 31 May 2020. [DOI] [PMC free article] [PubMed]
- 14. Dinarello CA. Thermoregulation and the pathogenesis of fever. Infect Dis Clin North Am. 1996;10(2):433‐449. 10.1016/s0891-5520(05)70306-8 [DOI] [PubMed] [Google Scholar]
- 15. Gandhi S, Srivastava AK, Ray U, Tripathi PP. Is the collapse of the respiratory center in the brain responsible for respiratory breakdown in COVID‐19 patients? ACS Chem Neurosci. 2020;11(10):1379‐1381. 10.1021/acschemneuro.0c00217 [DOI] [PubMed] [Google Scholar]
- 16. Giamarellos‐Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID‐19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992‐1000. 10.1016/j.chom.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID‐19 patients reveals IL‐6 and IL‐10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123‐1130. 10.1080/22221751.2020.1770129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leon LR. Invited review: cytokine regulation of fever: studies using gene knockout mice. J Appl Physiol. 1985;92(6):2648‐2655. 10.1152/japplphysiol.01005.2001 [DOI] [PubMed] [Google Scholar]
- 20. Seoh JY, Khan M, Park SH, et al. Serum cytokine profiles in patients with Plasmodium vivax malaria: a comparison between those who presented with and without hyperpyrexia. Am J Trop Med Hyg. 2003;68(1):102‐106. [PubMed] [Google Scholar]
- 21. Spiezia L, Boscolo A, Poletto F, et al. COVID‐19‐related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120:998‐1000. 10.1055/s-0040-1710018 [DOI] [PMC free article] [PubMed] [Google Scholar]


