To the Editor,
SARS‐CoV‐2, responsible for COVID‐19, is a new virus that can infect different cellular lines, including endothelia and epithelial cells of the gastrointestinal tract mucosa. At this level, the virus can cause different types of direct damage, even in paucisymptomatic, young patients. Virus can be detected directly by immunohistochemical techniques.
Coronaviruses are a family of positive single‐stranded RNA viruses known since 1960s 1 , 2 , 3 that can infect mainly the respiratory system, the liver and the bowel in various animal species, including humans. Emerging infections, such as Middle‐East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS), are caused by coronaviruses. The most recent pathogen in this family is SARS‐CoV‐2, responsible for COVID‐19. 4
SARS‐CoV‐2 can infect different cellular lines expressing angiotensin‐converting enzyme 2 (ACE‐2), including ciliated respiratory cells in nasal sinuses and bronchial tree, alveolar pneumocytes, kidney tubular cells, small bowel, and colon mucosa. 5 , 6 , 7 , 8 Cellular entry of the virus depends on the binding of the Spike protein present on the virus capsid to ACE2 protein, and on the priming of the spike by the cellular serine protease TMPRSS2. The binding is crucial not only for virus internalization, but also for COVID‐19 pathogenesis, as blockage and downregulation of the receptors result in impaired cardiovascular function that may lead to Acute Respiratory Distress Syndrome (ARDS), which is the main clinical manifestation of the disease. 6 , 7 , 8
Along the development of COViD‐19 pandemics, gastro‐intestinal symptoms such as diarrhea and abdominal pain have been reported in SARS‐CoV‐2‐positive patients and are now recognized as a part of COViD‐19 clinical spectrum. 8 SARS‐CoV‐2 was recently found within endothelial cells of various organs, including the small bowel and the central nervous system, with Transmission Electron Microscopy (TEM) techniques. 9 , 10
We describe the histopathological findings in a 40 years old SARS‐CoV‐2‐positive woman, presenting with diarrhea and abdominal pain, who underwent endoscopic biopsy sampling of the large bowel, in which we searched for the virus with immunohistochemical reaction on formalin‐fixe paraffin‐embedded tissue with antibodies directed against the SARS‐CoV‐2 nucleocapsid. The woman, with mild respiratory symptoms including cough and fever (>37,5°C) was quarantined after positive SARS‐CoV‐2 nasal swab and was later referred to the emergency unit for diarrhea and abdominal pain with anemia (Hb 7,8 g/dL). Upon admission, lung CT‐scan was consistent with mild interstitial pneumonia. To investigate the causes of fecal occult blood test positivity a colonoscopy was performed. The exam highlighted two small ulcerative lesions on the ileocecal valve in an otherwise normal colon mucosa. Both lesions were sampled and sent for histopathological examination. The patient was treated with a unit of concentrated red blood cells plus iron supplementation and was discharged after normalization of Hb levels.
Biopsy samples consisted of mucosal and submucosal tissue, with extensive lymphoplasmacellular inflammatory infiltrate. Immunophenotyping showed a substantial share of T‐lymphocytes (mainly CD3+/CD4+, with a lesser proportion of CD3+/CD8+) (Figure 1A,B), prominent multifocal vasculitis (Figure 1C,D), and bizarre modifications of the endothelium of small‐ and middle‐sized vessels (Figure 1E,F), sometimes showing obliterating arteriolitis (Figure 1G,H). Interestingly, no fibrinic microthrombi were found in these vessels. The mucosa showed ischemic damage. Immunohistochemical stains with an antibody directed against the nucleocapsid protein of SARS‐CoV‐2 (Rabbit monoclonal anti‐nucleocapsid protein; Sino Biological Inc, Chesterbrook, PA) revealed the presence of virus particles in the cytoplasm of the endothelial cells with hobnail and bizarre features (Figure 1H). The immunohistochemical reaction was completely negative in non‐endothelial cells (Figure 1I) and in control samples. These modifications may represent a peculiar cytopathic effect of the virus in this cellular line.
Figure 1.
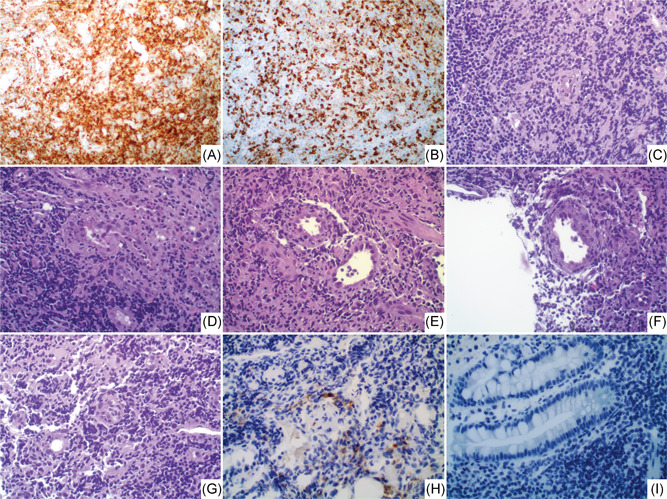
CD3 immunohistochemistry demonstrates the prevalent T‐cell share of the inflammatory infiltrate: CD4+ T‐cell share (A) is more consistent than CD8+ (B). C and D, Vasculitis of small diameter vessels, with bizarre nuclei and hobnail modifications (E and F), and aspects of obliteration (H). SARS‐CoV‐2 immunohistochemistry (IHC) demonstrates the direct presence of the virus within these endothelial cells. (I) No staining for SARS‐CoV‐2 is detectable in other cells. [A, B, H, I: IHC, ×40, C–G: HE, ×40]
The clinical setting of the patient and the recent reports of endothelial cells infection by SARS‐CoV‐2 suggest that patients with COVID‐19 may suffer GI tract damage due to hyperinflammatory response, 5 , 7 hypercoagulability state, and endothelial dysfunction. Our findings suggest that this later feature might be directly caused by endothelial cell infection, resulting in endothelial cytopathic modifications, vascular obliteration and, even if not observed in our case, thrombosis of small‐ and middle‐sized vessels. Similar findings were already described with TEM techniques on endothelia of the small bowel 9 and central nervous system. 10 We found a similar distribution of the virus in cytoplasm, also arranging in small clusters of particles. In the case of central nervous system infection, a viral hematogenous route using inflammatory cells as Trojan horse was proposed but, since we cannot demonstrate directly the presence of the virus in lymphocytes, 10 this possibility seems now less probable to us: the direct infection of blood‐brain barrier vessels may represent a more likely gate for the virus. Hobnail modifications of the endothelial cells, with bizarre nuclear shapes, may be an important hallmark to identify direct cytopathic effect of SARS‐CoV‐2 in this cellular line. These modifications and the presence of the virus with immunohistochemistry can be recognized directly by the pathologist, saving time and money to confirm a potential suspect of COVID‐19, where reverse‐transcription polymerase chain reaction on a swab was not already performed.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- 1. Tsang KW, Ho PL, Ooi GC, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1977‐1985. 10.1056/NEJMoa030666 [DOI] [PubMed] [Google Scholar]
- 2. Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967‐1976. 10.1056/NEJMoa030747 [DOI] [PubMed] [Google Scholar]
- 3. Chiu SS, Chan KH, Chu KW, et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005;40(12):1721‐1729. 10.1086/430301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses . The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5:536‐544. 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS‐CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622‐630. 10.1002/path.1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631‐637. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. To KF, Lo AW. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS‐CoV) and its putative receptor, angiotensin‐converting enzyme 2 (ACE2). J Pathol. 2004;203:740‐743. 10.1002/path.1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song Y, Liu P, Shi XL, et al. SARS‐CoV‐2 induced diarrhoea as onset symptom in patient with COVID‐19. Gut. 2020;69:1143‐1144. 10.1136/gutjnl-2020-320891 [DOI] [PubMed] [Google Scholar]
- 9. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paniz‐Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). J Med Virol. 2020;92(7):699‐702. 10.1002/jmv.25915 [DOI] [PMC free article] [PubMed] [Google Scholar]


