Mariño‐Sánchez and colleagues have raised a number of issues regarding our recent article on olfactory problems associated with coronavirus disease 2019 (COVID‐19). 1 In our study we found that 59 of 60 COVID‐19 patients exhibited at least some degree of smell loss, as measured by the validated Persian version of the 40‐item University of Pennsylvania Smell Identification Test (UPSIT). However, only 25% were anosmic.
A major concern expressed by Mariño‐Sánchez et al is that “the UPSIT is commonly used in America. Nevertheless, this test may not be adequate for Iranian population.” Initially, they support this concern by citing the findings of a study by Kamrava et al 2 in which the American UPSIT was administered to Iranian subjects, with the result that a number of odorant items were found wanting. What they neglected to consider, despite the mention of this fact in our article, is that we did not use the American UPSIT in the study. As clearly indicated in the article, we modified and validated the 40‐item Persian UPSIT in an Iranian population to take into account cultural factors. Moreover, they failed to indicate that we employed age‐ and sex‐matched Iranian normal controls for comparisons. They also seem unaware of the fact that the UPSIT is available not only as an American (ie, North American) version, but has been culturally revised and translated into over 30 different languages for multiple cultures.
It is noteworthy that a number of UPSIT odorants we used and validated in Iran performed well for us, but this was not the case for Kamrava et al. Of the 40 items, 29 are common between the 2 tests, and 11 odorants are different. We found much higher identification rates for 16 of the odorants common to the 2 studies. For example, onion had a 100% identification rate in our Iranian healthy subjects (which is evident in Fig. 2 of our article1 for the matched controls), whereas Kamrava et al reported a 68% identification rate, which is surprising given the universality of onion. The same was true for wintergreen, which reached a 93% identification rate in our study, but only 43% in the Kamrava et al study. We speculate that the latter difference could be due to issues with translation (we employed the term “Vicks” in addition to wintergreen, per se, to facilitate identification).
It is important to note that, in the Kamrava et al study, there was no significant difference between the familiarity ratings of the 16 odorants that reached the 70% identification level and those 24 that did not reach this criterion (p = 0.13). Interestingly, wintergreen received the highest average familiarity score, even though the mean identification rate was low (43%). Leather had a very low familiarity score (only 5 of the 40 odorants rated lower), but it achieved a 75% identification rate. Kamrava et al noted that their data were based on “healthy adult volunteers,” but failed to mention the age of their subjects. If the patients were older, this could have contributed to the lower test scores. 3
As shown in Figure 1, we found that even the unaltered British version of the UPSIT achieved higher UPSIT scores for healthy Iranian subjects than those obtained by Kamrava et al. Although we did not know the ages of their study subjects, we plotted our research data for individuals between 20 and 60 years of age, when age‐related deficits would be expected to be minimal. Note the improvement by replacing odorants less familiar to Iranian subjects.
FIGURE 1.
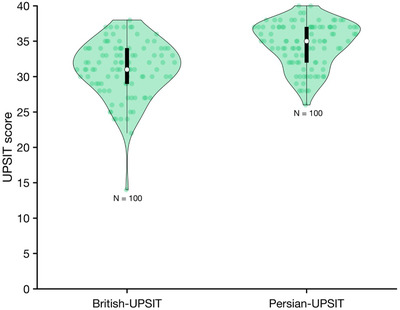
Comparison of the British and revised Persian UPSIT score in healthy subjects. Each circle represents 1 subject and the violin plots illustrate the probability density of the data at different values. The white circles indicate medians and the thick lines interquartile ranges. UPSIT = University of Pennsylvania Smell Identification Test.
To further support their criticism, Mariño‐Sánchez referred to a study by Taherkhani et al, who described the development of a 24‐odor modified version of the UPSIT. 4 They stated, “Curiously, the mean odor identification of healthy subjects [in the Taherkhani study] was 21.41 ± 1.37, very similar to the 20.98 mean UPSIT score obtained by Moein et al1 in COVID‐19 patients.” We found this statement to be peculiar because the Taherkhani test score is based on 24 items, reflecting an 89% correct response rate (21.41 of 24) and the Moein et al COVID‐19 test score is based on 40 items, resulting in a 52% correct response rate (20.98 of 40).
Mariño‐Sánchez et al referred to the high rate of smell loss we observed as “alarming.” It is noteworthy that a new study, for which the first author of this critique is coauthor, used a rather crude intensity measure and common household odors and found that 81% of those with confirmed COVID‐19 demonstrated smell dysfunction. 5 Moreover, based on the cutoff for normality, the following is stated in their study: “This is of course a conservative measure that will bias our estimates towards a potential lower prevalence given that there might be asymptomatic COVID‐19 patients or individuals with olfactory dysfunction prior to the COVID‐19 pandemic.”
Another point made by Mariño‐Sánchez et al is that, “… as the control group consisted of healthy subjects rather than severe acute respiratory syndrome‒coronavirus‐2 (SARS‐CoV‐2) polymerase chain reaction‒negative patients but with similar symptoms, the frequency of loss of smell could also be due to viral (common cold) or postviral infection.” As we noted in our study, the Iranian control group was tested outside the period of the first known COVID‐19 case in Iran. The controls were vetted for any ongoing infections or disturbances that would otherwise preclude their participation, and we clearly noted that “none of the controls had influenza or common cold symptoms at the time of testing.” If, as suggested, the control group also contained subjects with other virus‐related olfactory deficits, one would have expected lower control values than the ones that were found.
In light of their misapprehensions, the suggestion by Mariño‐Sánchez and colleagues that “Moein et al should reanalyze their data, using only odorants of UPSIT valid for Iranian population to give a more real clinical picture about the prevalence of smell dysfunction in COVID‐19,” is unwarranted.
How to Cite this Article:Moein ST, Hashemian SMR, Mansourafshar B, Khorram‐Tousi A, Tabarsi P, Doty RL. Reply to: Psychophysical olfactory testing in COVID‐19: is smell function really impaired in nearly all patients?. Int Forum Allergy Rhinol. 2020;10:953–954.
Potential conflicts of interest: R.L.D.: Acorda Therapeutics, Eisai Co, Merck Pharmaceuticals, the Michael J. Fox Foundation for Parkinson's Research, and Johnson & Johnson, consultant; Cambridge University Press, Johns Hopkins University Press, and John Wiley & Sons, royalties; R.L.D. is also president of, and a major shareholder in, Sensonics International (a manufacturer and distributor of smell and taste tests, including the test used in this study). A.K.‐T.: Cobel Darou in Iran, medical advisor. The remaining authors have no conflicts of interest to disclosure.
References
- 1. Moein ST, Hashemian SMR, Mansourafshar B, Khorram‐Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID‐19. Int Forum Allergy Rhinol. 2020;10:944‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kamrava SK, Farhadi M, Jalessi M, et al. University of Pennsylvania Smell Identification on Iranian population. Iran Red Crescent Med J. 2014;16:e7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226:1441‐1443. [DOI] [PubMed] [Google Scholar]
- 4. Taherkhani S, Moztarzadeh F, Seraj JM, et al. Iran Smell Identification Test (Iran‐SIT): a modified version of the University of Pennsylvania Smell Identification Test (UPSIT) for Iranian population. Chemosens Percept. 2015;8:183‐191. [Google Scholar]
- 5. Iravani B, Arshamian A, Ravia A, et al. Relationship between odor intensity estimates and COVID‐19 prevalence prediction in a Swedish population. Chem Senses. 2020; published online May 22. 10.1093/chemse/bjaa034 [DOI] [PMC free article] [PubMed] [Google Scholar]


