Summary
In late December 2019, a group of patients was observed with pneumonia‐like symptoms that were linked with a wet market in Wuhan, China. The patients were found to have a novel coronavirus genetically related to a bat coronavirus that was termed SARS‐CoV‐2. The virus gradually spread worldwide and was declared a pandemic by WHO. Scientists have started trials on potential preventive and treatment options. Currently, there is no specific approved treatment for SARS‐CoV‐2, and various clinical trials are underway to explore better treatments. Some previously approved antiviral and other drugs have shown some in vitro activity. Here we summarize the fight against this novel coronavirus with particular focus on the different treatment options and clinical trials exploring treatment as well as work done toward development of vaccines.
Keywords: bat coronavirus, chloroquine, remdesivir, SARS‐CoV‐2, vaccine
1. INTRODUCTION
Coronaviruses are a form of positive‐strand non‐segmented RNA viruses distributed among birds, mammals and humans that cause respiratory and neurological illnesses. 1 There are six different types that can cause disease among humans. Four of these (HKU1, 229E, NL63 and OC43) cause only the common cold in patients, 2 while two others cause severe acute respiratory syndrome (SARS‐Coronavirus) and Middle East respiratory syndrome coronavirus (MERS‐CoV). 3 SARS‐CoV caused severe respiratory illness in China during its outbreak in 2002‐2003, 4 while the MERS‐CoV outbreak started in the Middle East in 2012. 5 Due to the broad genetic variation and diversity of coronaviruses and higher chances of animal to human spread they are likely to emerge periodically in future. 6
During December 2019, an outbreak of pneumonia‐like symptoms occurred in patients that were linked to the seafood market in Wuhan China. 7 Investigation identified a new strain of coronavirus called SARS‐CoV‐2. 8
The detection of this novel coronavirus is key to confirm the cases and proceed to treatment. In an early method for detecting SARS‐CoV‐2, the samples from bronchoalveolar‐lavage fluids were collected, centrifuged to remove debris and inoculated onto human epithelial cells of airway origin. 9 About 20 000 sequences from each sample were obtained and genome matches showed more than 85% identity with SARS‐like betacornavirus. Results were also obtained from real‐time PCR, and isolated viruses were named SARS‐CoV‐2. 8 To further characterize SARS‐CoV‐2, the de‐novo sequence was obtained by using nanopore sequencing and Illumina methods. The airway epithelial cell cultures were suitable for visualization and identification of the novel coronavirus. 9
2. TREATMENT OPTIONS FOR CORONAVIRUS
Currently, no treatment is approved for SARS‐CoV‐2, but various already approved drugs are being tried in different clinical trials to check efficacy against this virus. The possible options include nucleoside analogs, interferon that can act as immune modulators and approved antimalarials having antiviral activities such as chloroquine and hydroxychloroquine.
2.1. Antiviral drugs
The nucleoside analogs such as ribavirin, favipiravir, galidesivir and remdesivir target RNA polymerase and block the synthesis of viral RNA. 10 Favipiravir was effectively used against influenza and has the potential to inhibit viral RNA synthesis and a new study also supports its activity against SARS‐CoV‐2. 11 Different clinical trials are underway where patients are being recruited to evaluate the efficacy of a combination of favipiravir and interferon‐α 12 and a combination of favipiravir and baloxavir marboxil is being evaluated. 13 Ribavirin is a guanine derivative antiviral drug approved for the treatment of HCV and RSV, but it causes anemia at higher doses that limit its use and its efficacy against coronavirus is uncertain. 14
Remdesivir is an adenine derivative antiviral drug. It has activity against a variety of viral strains such as SARS and MERS and a recent study also supports its activity against SARS‐CoV‐2. 11 A recent patient in US with SARS‐CoV‐2 has shown recovery with intravenous administration of remdesivir. 15 Recently, Phase‐III clinical trials have been started in USA to evaluate the efficacy of IV remdesivir as 200 mg OD or 100 mg OD for 9 days. 16 A recent RCT apparently reported no significant efficacy, but we await the published findings.
Some protease inhibitors like lopinavirand ritonavir have shown activity against SARS and MERS coronaviruses. 17 , 18 Clinical trials have been started to test the efficacy of lopinavir and ritonavir combination against SARS‐CoV‐2. These drugs are known to inhibit the chymotrypsin‐like protease of MERs and SARS coronaviruses. 19 A study in vitro using Vero E6 cells infected with SARS‐CoV‐2 assessed efficacy of different therapies by quantitative analysis using PCR (qRT‐PCR). Remdesivir and chloroquine showed promising results. Remdesivir has previously shown efficacy against MERS coronavirus and Ebola virus and is currently under clinical trial for treatment of Ebola. The results showed that EC90 value of remdesivir against SARS‐CoV‐2 was 1.76 μM. 15
Neuraminidase inhibitors are recognized antiviral agents for the treatment of influenza. The treatment with oral oseltamivir has been given to suspected patients in China. In past, oseltamivir has shown efficacy against MERS‐COV. 20
2.2. Chloroquine and hydroxychloroquine
Small molecular weight drugs such as chloroquine have shown inhibitory effects against SARS‐CoV‐2 (EC50 = 1.14 μM in Vero E6 cells) and is under evaluation in open‐label trials.. 15 Chloroquine (CQ) is a recognized anti‐malarial drug but also has antiviral activity. The antiviral activity of chloroquine was first noticed in the late 1960s. 21 Chloroquine and its analog hydroxychloroquine both have inhibitory effects against various viruses including SARS, 22 enterovirus 23 and Zika virus. 24 Chloroquine inhibits the virus by increasing endosomal pH and so reducing viral cell fusion and also interferes with cellular receptor glycosylation. 25 The EC90 value of chloroquine against SARS‐CoV‐2 is 6.90 μM that can be achieved with administration of 500 mg dose. 26 Remdesivir and chloroquine have shown activity in in vitro studies and can easily be tested in patients with SARS‐CoV‐2. 15
In another recent study, Gao et al found that chloroquine phosphate reduced the symptoms of pneumonia in SARS‐CoV‐2 patients and shortening the duration of disease. 27 The guidelines for the treatment of SARS‐CoV‐2 were revised six times since its issuance on 15 January 2020. The recent guidelines also include IFN‐α, remdesivir, ribavirin, ritonavir and chloroquine. The mode of administration of IFN‐α is through inhalation at a dose of 5 million units diluted with water for injection. The dose of ritonavir is 100 mg BD for adults. Ribavirin may be given in combination with IFN‐α or ritonavir at a dose of 500 mg BD or TDS. Chloroquine phosphate should be administered at a dose of 500 mg BD. Arbidol can be given three times a day at 200 mg. 28
Hydroxychloroquine (HCQ) is an approved disease‐modifying antirheumatic drug that also has immunomodulatory effects and prevents organ damage. 29 HCQ alters endosomal pH and interrupts the biding between RNA/DNA and toll‐like receptors that leads to suppression of TLR signaling. 30 , 31 , 32 Inside the cytoplasm, HCQ also interferes with the interaction between nucleic acid sensor cyclic GMP and cytosolic DNA. 33 These two mechanisms lead to increase production of IL‐1, TNF and type‐1 interferon. Such mechanism supports the idea that HCQ suppress the cytokine release storm (CRS) which is due to SARS‐CoV‐2 triggered overreaction of immune system. 34 In a recent study, HCQ was found to be more effective than chloroquine; a loading dose of 400 mg twice daily and maintenance dose of 200 mg twice a day is recommended for SARS‐CoV2 infection. 35
The mechanism involves in antiviral role of HCQ and CQ is inhibition of receptor binding and fusion of cell membrane. These two are crucial steps that are required for cell entry of SARS‐CoV‐2. Chloroquine interferes with the glycosylation of ACE‐2 (angiotensin‐converting enzyme receptors) receptors that are considered as cellular receptors for SARS‐CoV‐2 and block the fusion of SARS‐CoV‐2 with host cell. Thus, the binding of virus is blocked and infection is prevented. The HCQ and CQ after entry into the cell tend to concentrate in lysosomes and endosomes. The SARS‐CoV‐2 use endosome as a tool for cellular entry. HCQ and CQ increase the pH of endosomes and create a negative influence on the binding of SARS‐C0v‐2 with endosomes. 25 Lysosomal protease helps in viral fusion with cell membrane. Increase lysosomal pH prevents the action of protease and fusion and replication of virus is blocked 36 . The mechanism of action of chloroquine and hydroxychloroquine is represented as (Figure 1).
FIGURE 1.
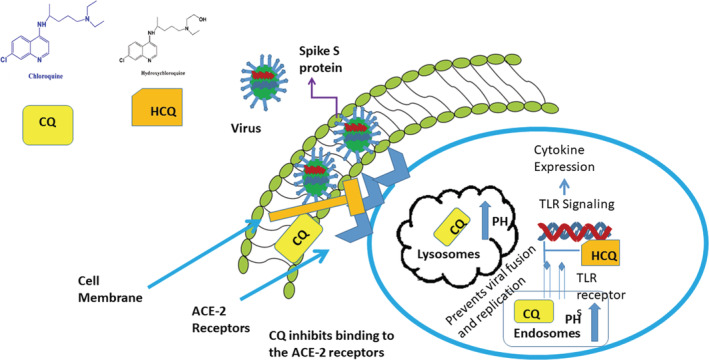
Mechanism of action of HCQ and CQ by blocking binding of virus with ACE‐2 receptors and increasing endosomal pH and preventing fusion with the cell
2.3. Chymotrypsin‐like inhibitors
Cinanserin is an antagonist of serotonin receptors. It can inhibit chymotrypsin‐like (3C‐like) protease and has shown promising results against SARS coronavirus. 37 The 3C‐protease was also investigated to be encoded in SARS‐CoV‐2. 38
Flavonoids such as chalcones, flavones and isoflavones produce antioxidant effects but they also have antiviral effects. 39 A study has found that flavonoids can inhibit the entry of hepatitis‐C virus. 40 Some flavonoids have activity against MERS coronavirus, presumably due to inhibition of 3C‐like protease. 41
2.4. Other treatment options
Pirfenidone is under clinical trial for the treatment of idiopathic pulmonary fibrosis resulting from SARS‐CoV‐2 infection. It also has an anti‐inflammatory effect and serves as an anti‐oxidant. The results obtained during SARS 2003 were promising and now beneficial effects could be obtained in patients with severe pneumonia from SARS‐CoV‐2. 42
Nitric oxide (NO) is a biological gas produced from arginine. NO after reaction with superoxide forms peroxynitrite which has cytotoxic bactericidal action. 43 Nitric oxide is also known to regulate airway function and reduce inflammation of airways. 44 The beneficial effect of NO in SARS patients was observed in some studies. 45 NO can also inhibit the synthesis of RNA and viral protein. 46 A study has found that organic nitric oxide could inhibit the replication cycle of SARS‐CoV‐2. 47
Αlpha‐lipoic acid (ALA) is used in hepatic disorders and polyneuropathies. 48 ALA is an antioxidant that protects cells against oxidative stress by increasing the glutathione level. 49 It was reported that individual oxidative stress plays a role in coronavirus infection and G6llPD deficiency was a crucial factor in SARS‐CoV‐2 patients. 50 So, ALA could be an option to decrease the oxidative stress in patients with SARS‐CoV‐2 infection.
Mucroporin‐M1 is a peptide derived from scorpion venom with broad spectrum antiviral activity against various infections like influenza H5N1 and SARS‐CoV. 51
The use of convalescent plasma for the treatment of SARS‐CoV‐2 is also under consideration with varying hope regarding efficacy. The pros of using plasma from recovered patients include easy availability and it can also be used as prophylactically in healthcare professionals who are at high risk of exposure to SARS‐CoV‐2 infection. The cons include that not all recovered patients have enough antibodies. Lack of availability of validated SARS‐CoV‐2 assay for detection of neutralizing antibodies may cause hindrance in identification of suitable donor. 52 A large scale randomized clinical trials are needed to establish the efficacy of convalescent plasma. 53
Some studies support the role of angiotensin‐converting enzyme (ACE) inhibitors. This is based on the hypothesis that ACE‐2 serves as receptor for SARS‐CoV‐2. 54 , 55 So, ACE inhibitor could potentially compete for site binding and reduce the mortality and morbidity associated with SARS‐CoV‐2. 56
The various ongoing clinical trials are summarized in Table 1.
TABLE 1.
Clinical trials for various treatment options for SARS‐CoV‐2
| Clinical Trial Registration Number | Trial information | Date | Type | References |
|---|---|---|---|---|
| ChiCTR2000030897 | The efficacy of Newgen beta‐gluten probiotic powder in patient with 2019‐nCOV. | 16 March 2020 | Interventional | 57 |
| ChiCTR2000030894 | The efficacy of combination of Favipiravir + Tocilizumab in patient with pneumonia (COVID‐19). | 16 March 2020 | Interventional | 58 |
| ChiCTR2000030892 | A prospective study on Pirfenidone in the treatment of Coronavirus Pneumonia (COVID‐19) Fibrosis. | 16 March 2020 | Interventional | 59 |
| ChiCTR2000030883 | The efficacy of traditional Chinese qingfei detoxification decoction (mixture) in coronavirus pneumonia (COVID‐19). | 16 March 2020 | Observational | 60 |
| ChiCTR2000030857 | A study for bronchoscopic alveolar lavage in patients undergoing trachea intubation with new coronavirus pneumonia (COVID‐19). | 16 March 2020 | Observational | 61 |
| ChiCTR2000030853 | The study of protective effect of dexmedetomidine in severe novel coronavirus pneumonia (COVID‐19) patients. | 16 March 2020 | Interventional | 62 |
| NCT04252885 | The combination of Lopinavir + Ritonavir and Arbidol against 2019‐nCOV. | 28 January 2020 | Interventional | 63 |
| NCT04273763 | The study of bromhexine hydrochloride along with standard treatment in patients with novel coronavirus pneumonia (COVID‐19). | 18 February 2020 | interventional | 64 |
| NCT04280588 | The use of Fingolimod in patient with COVID‐19. | 21 February 2020 | Interventional | 65 |
| NCT04288713 | The efficacy of Eculizumab (Soliris) in Covid‐19 Infected Patients (SOLID‐C19). | 28 February 2020 | Interventional | 66 |
3. POTENTIAL VACCINES
Vaccine provides immunity against a particular pathogen before exposure of that infectious agent. Several types of vaccines exist that can be nucleic acid based, live attenuated vaccine, subunit proteins or nanoparticle vaccines. 67 Different technologies are being utilized to develop potential vaccine for SARS‐CoV‐2 including DNA and mRNA‐based nanoparticles. Phase‐I trials of potential vaccines focus on safety and immunogenicity, for example, against MERS. 68 Two of the clinical trials on MERS vaccine are expected to be complete by the end of 2020 69 , 70 in Russia and one in Germany by December 2021. 71 The Inovo pharmaceutical company has tested its vaccine against MERS coronavirus funded by coalition for epidemic preparedness innovation (CEPI) using DNA‐based technology and named as INO‐4700. 72 The University of Oxford recombinant chimpanzee adenovirus has also begun Phase 1 randomized multicenter trials for intramuscular injection of vaccine ChAdOx1 against SARS‐CoV‐2. The 1100 participants have been divided into four groups and they will be observed for any serious adverse event for 6 months.
The ongoing clinical trials for development of vaccines have been summarized in Table 2 and completed vaccine trials for SARS and MERS viruses have been summarized in Table 3.
TABLE 2.
Ongoing clinical trials on vaccine development
| Company | Technology | Stage | Expected timeline | References |
|---|---|---|---|---|
| Inovo pharmaceuticals | INO‐4800 DNA based | Pre‐clinical studies funded by Coalition for Epidemic Preparedness Innovations (CEPI) | Human testing in few months | 73 |
| Vir Biotechnology | anti‐coronavirus monoclonal antibodies (mAbs), CRISPR‐ based screening | Pre‐clinical | No decided timeline | 74 |
| The university of oxford | Adenovirus vector and SARS‐CoV‐2 spike protein | Phase‐1 started on 23rd April in 1100 peoples | Upto 6 mo | 75 |
| University of Queensland | Molecular clamp technology | Pre‐clinical | 6 mo‐1 y | . 76 |
| Johnson & Johnson | Adenovirus based technology | Pre‐clinical | 1 y | |
| The University of Saskatchewan's | Not known | Pre‐clinical | 3‐4 mo | |
| GeoVax and BravoVax | Modified Vaccinia Ankara (MVA) platform technology | Pre‐clinical | Not known | |
| Chinese clinical trial center ChiCTR2000030906 | Clinical trial for recombinant novel coronavirus (2019‐CoV) vaccine based on adenoviral vector | Phase‐1 clinical trail | Not known | 80 |
TABLE 3.
Completed vaccine trials on SARS‐COV and MERS‐COV
| Company | Vaccine | Subjects | Results | Conclusion | References |
|---|---|---|---|---|---|
| Sinovac Biotech | inactivated SARS coronavirus (SARS‐CoV) vaccine | Thirty six subjects were enrolled. They received 16 units of vaccine two time or 32 units one time | On 56th day 100% of patients were seroconverted receiving 16 unit and 91% patients were seroconverted receiving 32 units one dose. | The inactivated vaccine against SARS‐COV is safe and well tolerated | 81 |
| NIH, Vaccine research center | single‐plasmid DNA vaccine encoding the Spike (S) glycoprotein | Ten healthy adults | Eight out of 10 subject develop antibody against SARS‐COV and detected by ELISA | The VRC SARS DN vaccine is safe and well tolerated | 82 |
| National Institute of Allergy and Infectious Diseases and Clinical Center, National Institutes of Health (NIH); the National Cancer Institute, NIH (contract number HHSN261200800001E); | Anti‐MERS coronavirus antibody | Double blind phase‐I single dose study including 43 participants | Out of 43 there were 38 eligible participant and 28 received the dose while 10 were placebo. They develop antibodies against MERS | Single dose at 50 mg/kg of SAB infusion appeared to be safe. | 83 |
| GeneOne Life Science and Inovio Pharmaceuticals. | GLS‐5300 DNA vaccine | Phase‐I open label, dose escalation study including 75 participants | Out of 75, 25 patients receive 0.67 mg, other 25 receive 2 mg and 3rd group receive 6 mg of GLS‐DNA vaccine 5300. | The immune response was dose‐independent and further trials needed to establish efficacy | 84 |
3.1. Development of neutralizing antibodies against coronaviruses
The fusion of coronavirus with a cell occurs after biding of S protein to a target receptor delivers viral nucleocapsid and initiates replication. The S protein causes syncytial formation at the receptor site. 85 A neutralizing antibody that can target the S protein on the surface of SARS‐CoV‐2 could also be an option to produce passive immunity in patients. 86 Recent research on the genome of SARS‐CoV‐2 (MN908947.3) allows scientists to express the S protein as immunogen on the surface and explore options such as yeast libraries for generating antibodies that neutralize the virus. 86 , 87
CONFLICT OF INTEREST
The authors have no conflict of interest in this manuscript.
Khan MM, Noor A, Madni* A, Shafiq M. Emergence of novel coronavirus and progress toward treatment and vaccine. Rev Med Virol. 2020;30:e2116. 10.1002/rmv.2116
Abbreviations: ACE, angiotensin‐converting enzyme; CQ, chloroquine; CRS, cytokine release storm; HCQ, hydroxychloroquine; MERS, Middle East respiratory syndrome; PCR, polymerase chain reaction; SARS, severe acute respiratory syndrome; SARS‐CoV‐2, severe acute respiratory syndrome Coronavirus‐2; TLR, Toll‐like receptors.
Contributor Information
Muhammad Muzamil Khan, Email: muzamilpharmacist@gmail.com.
Asadullah Madni*, Email: muzamilpharmacist@gmail.com.
REFERENCES
- 1. Weiss SR, Leibowitz JL. Coronavirus pathogenesis . Adv Virus Res. 2011;81:85‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cui J, Li F, Shi Z‐L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhong N, Zheng BJ, Li YM, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362(9393):1353‐1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814‐1820. [DOI] [PubMed] [Google Scholar]
- 6. Wong G, Liu W, Liu Y, Zhou B, Bi Y, Gao GF. MERS, SARS, and Ebola: the role of super‐spreaders in infectious disease. Cell Host Microbe. 2015;18(4):398‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wuhan Municipal Health Commission . report of clustering pneumonia of unknown etiology in Wuhan city; December 31, 2019.
- 8. Zhu N, Zhang D, Wang W, et al; China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jonsdottir HR, Dijkman R. Coronaviruses and the human airway: a universal system for virus‐host interaction studies. Virol J. 2016;13(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Clercq E. New nucleoside analogues for the treatment of hemorrhagic fever virus infections. Chem Asian J. 2019;14(22):3962‐3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019‐nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liao Xuejiao LY. Clinical study for safety and efficacy of favipiravir in the treatment of novel coronavirus pneumonia (COVID‐19); February 12, 2020.
- 13. Qiu Yunqing WL. A randomized controlled trial for the efficacy and safety of Baloxavir Marboxil, Favipiravir tablets in 2019‐nCoV pneumonia (novel coronavirus pneumonia, NCP) patients who are still positive on virus detection under the current antiviral therapy.
- 14. Zumla A, Chan JFW, Azhar EI, Hui DSC, Yuen KY. Coronaviruses—drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15(5):327‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30(3):269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bin Cao YW. Mild/Moderate 2019‐nCoV Remdesivir RCT.
- 17. Chu C, Cheng VC, Hung IF, et al; HKU/UCH SARS Study Group. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan JF‐W, Yao Y, Yeung ML, et al. Treatment with lopinavir/ritonavir or interferon‐β1b improves outcome of MERS‐CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212(12):1904‐1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Clercq E, Li G. Approved antiviral drugs over the past 50 years. Clin Microbiol Rev. 2016;29(3):695‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bleibtreu A, Jaureguiberry S, Houhou N, et al. Clinical management of respiratory syndrome in patients hospitalized for suspected Middle East respiratory syndrome coronavirus infection in the Paris area from 2013 to 2016. BMC Infect Dis. 2018;18(1):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shimizu Y, Yamamoto S, Homma M, Ishida N. Effect of chloroquine on the growth of animal viruses. Arch Gesamte Virusforsch. 1972;36(1–2):93‐104. [DOI] [PubMed] [Google Scholar]
- 22. Keyaerts E, Vijgen L, Maes P, Neyts J, Ranst MV. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323(1):264‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tan YW, Yam WK, Sun J, Chu JJH. An evaluation of chloroquine as a broad‐acting antiviral against hand, foot and mouth disease. Antiviral Res. 2018;149:143‐149. [DOI] [PubMed] [Google Scholar]
- 24. Li C, Zhu X, Ji X, et al. Chloroquine, a FDA‐approved drug, prevents Zika virus infection and its associated congenital microcephaly in mice. EBioMedicine. 2017;24:189‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mackenzie AH. Dose refinements in long‐term therapy of rheumatoid arthritis with antimalarials. Am J Med. 1983;75(1):40‐45. [DOI] [PubMed] [Google Scholar]
- 27. Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. BioSci Trends. 2020;14(1):72‐73. [DOI] [PubMed] [Google Scholar]
- 28. Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID‐19). Drug Discov Ther. 2020;14(1):58‐60. [DOI] [PubMed] [Google Scholar]
- 29. Ruiz‐Irastorza G, Ramos‐Casals M, Brito‐Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(01):20‐28. [DOI] [PubMed] [Google Scholar]
- 30. Kužnik A, Benčina M, Švajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol. 2011;186(8):4794‐4804. [DOI] [PubMed] [Google Scholar]
- 31. Ewald SE, Lee BL, Lau L, et al. The ectodomain of Toll‐like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456(7222):658‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vollmer J, Tluk S, Schmitz C, et al. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll‐like receptors 7 and 8 . J Exp Med. 2005;202(11):1575‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. An J, Woodward JJ, Sasaki T, Minie M, Elkon KB. Cutting edge: antimalarial drugs inhibit IFN‐β production through blockade of cyclic GMP‐AMP synthase–DNA interaction. J Immunol. 2015;194(9):4089‐4093. [DOI] [PubMed] [Google Scholar]
- 34. Dijkmans B, Verweij C. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor‐alpha, interleukin 6, and interferon‐gamma production by peripheral blood mononuclear cells. J Rheumatol. 1997;24(1):55‐60. [PubMed] [Google Scholar]
- 35. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al‐Bari MAA. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases . Pharmacol Res Perspect. 2017;5(1):e00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen L, Gui C, Luo X, et al. Cinanserin is an inhibitor of the 3C‐like proteinase of severe acute respiratory syndrome coronavirus and strongly reduces virus replication in vitro. J Virol. 2005;79(11):7095‐7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Panche A, Diwan A, Chandra S. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shimizu JF, Lima CS, Pereira CM, et al. Flavonoids from Pterogyne nitens inhibit hepatitis C virus entry. Sci Rep. 2017;7(1):16127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jo S, Kim S, Shin DH, Kim MS. Inhibition of SARS‐CoV 3CL protease by flavonoids. J Enzyme Inhib Med Chem. 2020;35(1):145‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang H. A Study to Evaluate the Efficacy and Safety of Pirfenidone With Novel Coronavirus Infection.
- 43. Robbins RA, Grisham MB. Nitric oxide. Int J Biochem Cell Biol. 1997;29(6):857‐860. [DOI] [PubMed] [Google Scholar]
- 44. Barnes PJ. Nitric oxide and airway disease. Ann Med. 1995;27(3):389‐393. [DOI] [PubMed] [Google Scholar]
- 45. Rossaint R, Gerlach H, Schmidt‐Ruhnke H, et al. Efficacy of inhaled nitric oxide in patients with severe ARDS. Chest. 1995;107(4):1107‐1115. [DOI] [PubMed] [Google Scholar]
- 46. Hui D. An overview on severe acute respiratory syndrome (SARS) . Monaldi Arch Chest Dis. 2005;63(3):149‐57. [DOI] [PubMed] [Google Scholar]
- 47. Åkerström S, Mousavi‐Jazi M, Klingström J, Leijon M, Lundkvist Å, Mirazimi A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J Virol. 2005;79(3):1966‐1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sachse G, Willms B. Efficacy of thioctic acid in the therapy of peripheral diabetic neuropathy . Horm Metab Res Suppl. 1980;9:105‐107. [PubMed] [Google Scholar]
- 49. Tibullo D, Li Volti G, Giallongo C, et al. Biochemical and clinical relevance of alpha lipoic acid: antioxidant and anti‐inflammatory activity, molecular pathways and therapeutic potential. Inflamm Res. 2017;66(11):947‐959. [DOI] [PubMed] [Google Scholar]
- 50. Wu Y‐H, Tseng CP, Cheng ML, Ho HY, Shih SR, Chiu DTY. Glucose‐6‐phosphate dehydrogenase deficiency enhances human coronavirus 229E infection. J Infect Dis. 2008;197(6):812‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li Q, Zhao Z, Zhou D, et al. Virucidal activity of a scorpion venom peptide variant mucroporin‐M1 against measles, SARS‐CoV and influenza H5N1 viruses. Peptides. 2011;32(7):1518‐1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sullivan HC, Roback JD. Convalescent plasma: therapeutic hope or hopeless strategy in the SARS‐CoV‐2 pandemic. Transfus Med Rev. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang B, Liu S, Tan T, et al. Treatment with convalescent plasma for critically ill patients with SARS‐CoV‐2 infection. Chest. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade‐long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gurwitz D. Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics. Drug Dev Res. 2020;81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Honghua Ye TC. Evaluation of the effect of taking Newgen beta‐gluten probiotic composite powder to nutrition intervention of patients with novel coronavirus pneumonia (COVID‐19).
- 58. Guiqiang Wang HZ. Favipiravir Combined with Tocilizumab in the Treatment of Novel Coronavirus Pneumonia (COVID‐19) ‐ A Multicenter, Randomized, Controlled Trial.
- 59. Luo Q. Efficacy and Safety of Pirfenidone in the Treatment of Severe Post‐Novel Coronavirus Pneumonia (COVID‐19) Fibrosis: a prospective exploratory experimental medical study.
- 60. Si‐Jin Y, Rao‐Qiong W. Clinical research and preparation development of qingfei detoxification decoction (mixture) for prevention and treatment of novel coronavirus pneumonia (COVID‐19).
- 61. Yan SLJ. Clinical study for bronchoscopic alveolar lavage in the treatment of critically trachea intubation patients with new coronavirus pneumonia (COVID‐19).
- 62. Jianli WJW. Evaluation of the protective effect of dexmedetomidine on patients with severe novel coronavirus pneumonia (COVID‐19).
- 63. Coronoavirus clinical trial. 2020.
- 64. Evaluating the Efficacy and Safety of Bromhexine Hydrochloride Tablets Combined With Standard Treatment/ Standard Treatment in Patients With Suspected and Mild Novel Coronavirus Pneumonia (COVID‐19).
- 65. Wang N. Fingolimod in COVID‐19.
- 66. Pitts T. Eculizumab (Soliris) in Covid‐19 Infected Patients (SOLID‐C19).
- 67. Ralph R, Lew J, Zeng T, et al. 2019‐nCoV (Wuhan virus), a novel coronavirus: human‐to‐human transmission, travel‐related cases, and vaccine readiness. J Infect Dev Ctries. 2020;14(01):3‐17. [DOI] [PubMed] [Google Scholar]
- 68. Pang J, Wang MX, Ang IYH, et al. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019‐nCoV): a systematic review. J Clin Med. 2020;9(3):623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.NCT. Study of safety and immunogenicity of BVRS‐GamVac; 2019.
- 70.NCT. Study of safety and immunogenicity of BVRS‐GamVac‐Combi; 2019.
- 71.NCT. Randomized, double‐blind, placebo‐controlled, phase ib study to assess the safety and immunogenicity of MVA‐MERS‐S_DF‐1. 2019.
- 72. INOVO vaccine against MERS coronavirus.
- 73. Matone B. Inovio Selected by CEPI to Develop Vaccine Against New Coronavirus.
- 74. An obscure biotech stock skyrockets 38% after saying it's testing a coronavirus antibody (VIR).
- 75.University of Oxford. A Study of a Candidate COVID‐19 Vaccine (COV001). 2020.
- 76. Young PP. Race to develop coronavirus vaccine.
- 77. Stoffels DP. J&J scientific officer ‘pretty confident’ they can create coronavirus vaccine as outbreak widens.
- 78.Saskatchewan lab joins global effort to develop coronavirus vaccine.
- 79.GeoVax and BravoVax (Wuhan, China) to Collaborate on Development of Coronavirus Vaccine.
- 80. Zhu Fengcai HL. A Phase I Clinical Trial for Recombinant Novel Coronavirus (2019‐COV) Vaccine (Adenoviral Vector). http://www.chictr.org.cn/showprojen.aspx?proj=51154. [Google Scholar]
- 81. Lin J, Zhang JS, Su N, et al. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir Ther. 2007;12(7):1107‐1113. [PubMed] [Google Scholar]
- 82. Martin JE, Louder MK, Holman LA, et al; VRC 301 Study Team. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a phase I clinical trial. Vaccine. 2008;26(50):6338‐6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Beigel JH, Voell J, Kumar P, et al. Safety and tolerability of a novel, polyclonal human anti‐MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double‐blind, single‐dose‐escalation study. Lancet Infect Dis. 2018;18(4):410‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Modjarrad K, Roberts CC, Mills KT, et al. Safety and immunogenicity of an anti‐Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open‐label, single‐arm, dose‐escalation trial. Lancet Infect Dis. 2019;19(9):1013‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tortorici MA, Veesler D. Structural insights into coronavirus entry. Adv Virus Res. 2019;105:93‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Casadevall A, Pirofski LA. The Ebola epidemic crystallizes the potential of passive antibody therapy for infectious diseases. PLoS Pathog. 2015;11(4):e1004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Keck ZY, Wang Y, Lau P, SKH F. Isolation of HCV neutralizing antibodies by yeast display. Methods Mol Biol. 2019;1911:395‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]


