Abstract
The coronavirus pandemic has resulted in the need for rapid assessment of resource utilization within our hospital systems. Specifically, the overwhelming need for intensive care unit (ICU) beds within epicenters of the pandemic has created a need for consideration as to how acute coronary syndrome cases, and specifically ST‐elevation myocardial infarction (STEMI) patients, are managed postprocedure. While most patients in the United States continue to be managed in coronary care units after primary percutaneous coronary intervention, there is a robust literature regarding the ability to triage STEMI patients safely and efficiently with low‐risk features to non‐ICU beds. We review the various risk scores for STEMI triage and the data supporting their usage. In summary, these findings support an approach to low‐risk STEMI triage that does not come at the expense of quality patient care or outcomes, where up to two‐thirds of patients with STEMI may be able to be safely managed without ICU‐level care.
Keywords: acute coronary syndrome, pandemic, PCI, STEMI
1. INTRODUCTION
Almost overnight, the coronavirus disease 19 (COVID‐19) pandemic has resulted in extremely rapid and perhaps permanent changes to many aspects of society and, specifically, health care. Based on experiences in China and Italy, a key challenge relates to the overwhelming demand for intensive care unit (ICU) beds, which necessitates the need to consider management of patients with acute coronary syndromes, including ST‐elevation myocardial infarction (STEMI). In the United States, the overwhelming majority of STEMI patients are still initially managed in an ICU or cardiac care unit (CCU) setting, 1 despite strong evidence (and even guidelines) 2 , 3 that low‐risk STEMI patients can be identified for non‐ICU admission and early discharge without adverse impacts on short‐ or long‐term outcomes. 4 , 5 , 6 , 7 In a retrospective study of nearly 20,000 STEMI patients, 82% were treated in the ICU following percutaneous coronary intervention (PCI); however, only 16% suffered an event that required such a high level of care. 1 Institutions and cardiovascular service lines working on plans to address the COVID‐19 crisis should implement triage of low‐risk STEMI patients to non‐ICU settings, along with early hospital discharge protocols.
2. EXISTING RISK STRATIFICATION MODELS FOR STEMI
Multiple risk stratification methods for STEMI patients exist, the two most commonly utilized to identify low‐risk STEMI patients for early discharge are the CADILLAC and Zwolle Risk Scores. 4 , 5 The CADILLAC Risk Score, developed in 2,082 patients with acute MI, incorporates baseline left ventricular ejection fraction, renal insufficiency, Killip Class, final Thrombolysis In Myocardial Infarction (TIMI) flow, age > 65 years, anemia, and triple vessel disease and stratifies patients into low‐, intermediate‐, and high‐risk groups. When validated in 900 patients from the Stent‐PAMI Trial, the score performed well, predicting mortality at both 30 days (low, intermediate at high risk: 0.2 vs. 1.3 vs. 8.1%, p trend < .001) and 1 year (low, intermediate at high risk: 0.9 vs. 4.5 vs. 12.4%, p trend < .001). 4 The Zwolle Risk Score was developed using data on 1,800 STEMI patients undergoing primary PCI, with slightly different predictor variables (age, Killip class post‐PCI, TIMI flow post‐PCI, presence of triple vessel disease, anterior infarction, and ischemic time > 4 hr), with a score of 0–3 considered low risk, and a score ≥4 considered high risk, based on 30‐day mortality rates, with a strong discriminatory capacity (c statistic: 0.907) (Figure 1). In a validation cohort of 747 patients, 65% of STEMI patients were deemed low risk, with a mortality rate of 0.6% at 30 days. 5
FIGURE 1.
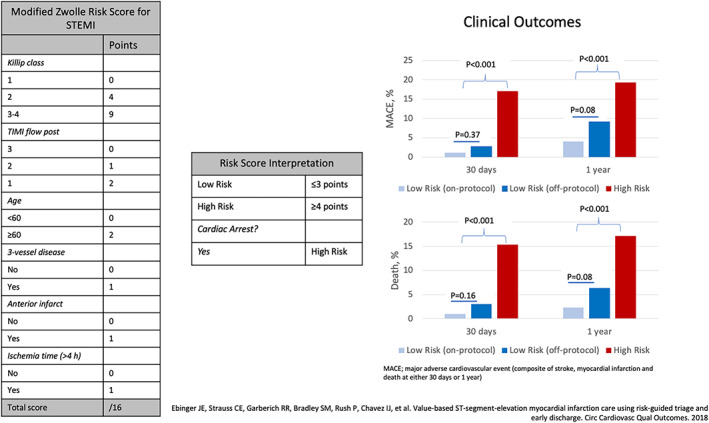
Left: Modified Zwolle Risk Score. Center: Classification of scoring into low‐ and high‐risk STEMI groups. Right: Outcomes of prospectively applied modified Zwolle Risk Score in a population of 549 STEMI patients. MACE, major adverse cardiovascular event (composed of stroke, myocardial infarction and death at either 30 days or 1 year); STEMI, ST‐elevation myocardial infarction. Source: Ebinger et al 7 [Color figure can be viewed at wileyonlinelibrary.com]
3. CONTEMPORARY ASSESSMENT OF RISK TRIAGE MODELS FOR STEMI
The feasibility, effectiveness, and safety of utilizing such protocols have also been demonstrated in contemporary STEMI systems. Recently, a small retrospective study in 228 patients over 2 years (42% of all STEMI patients) reported no adverse effects after 3 days and 0 deaths at 1 year using the Cadillac Risk Score. 6 We recently reported the results of a larger prospective trial using the Zwolle Risk Score for STEMI patient triage. 7 After initially validating the predictive ability of the score in 967 STEMI patients from our own retrospective data (death and major adverse cardiac events rates among low‐risk patients of 0.2 and 0.9% at 30 days, respectively), the Zwolle Risk Score was incorporated into the electronic health record (EHR), adding out‐of‐hospital cardiac arrest as an automatic high‐risk feature. The EHR risk calculator was then prospectively applied to 549 STEMI patients, 62% (n = 266) of whom were determined to be low risk, with 62% of those (n = 177) treated per protocol, triaged to telemetry rather than CCU admission and targeted for early (<48 hr) hospital discharge. The remainder included 109 low‐risk patients triaged to the CCU and 176 high‐risk STEMI patients. Reasons for low‐risk STEMI triaged to the CCU were multifactorial but appeared to be mostly related to procedural complications or the potential for bleeding (2.5% for “on protocol” vs. 8.9% “off protocol,” p = .018). At 30 days, low‐risk patients triaged to telemetry did extremely well, with shorter index hospital length of stay (LOS), markedly lower hospital costs, no inhospital mortality, and only one death at 30 days related to a stroke on admission. These findings persisted at 1‐year follow‐up, with lower long‐term mortality among the low‐risk, on‐protocol cohort (low‐risk on‐protocol, low‐risk off‐protocol, high risk: 2.3 vs. 6.4 vs. 17.1%, p < .001) (Figure 1). When used in conjunction with physician judgment, integration of the Zwolle Risk Score appropriately identifies STEMI patients who may be cared for in non‐ICU settings, freeing these resources for critically ill patients who require ICU‐level care.
A recent meta‐analysis of five randomized clinical trials demonstrated that an early discharge strategy (≤3 days) in selected low‐risk STEMI patients significantly reduced LOS without an adverse effect on mortality or readmission rates. 8 Based on these studies, society guidelines and recommendations have begun to follow suit. The 2018 European Society of Cardiology guidelines upgraded the 48–72 hr discharge recommendation for STEMI patients to level IIb to IIa, while the Society for Cardiovascular Angiography and Intervention 2018 consensus document on LOS also suggested 48–72 hr for stable STEMI patients after successful PCI. 2 , 3 These recommendations are consistent with the American College of Cardiology/American Heart Association guidelines, which stress the importance of considering economics and value of care to “supplement evidence of safety and efficacy with information about the resources needed to achieve health improvements.” 9
4. CONCLUSION
We believe that these findings support an approach to low‐risk STEMI triage that does not come at the expense of quality of patient care or outcomes. Furthermore, during the current pandemic, we suggest that programmatic efforts utilizing this approach offer an evidence‐based method of addressing the expected surge in the need for ICU beds. Finally, we propose that in this time of crisis, it is important for programs and their cardiology leaders to consider supporting a newly developed consortium of institutions to collect prospective data on the impact of the rapid enactment of a standardized protocol for STEMI risk stratification. We welcome sites and investigators to join us in this important endeavor.
Lopez JJ, Ebinger JE, Allen S, Yildiz M, Henry TD. Adapting STEMI care for the COVID‐19 pandemic: The case for low‐risk STEMI triage and early discharge. Catheter Cardiovasc Interv. 2021;97:847–849. 10.1002/ccd.28993
REFERENCES
- 1. Shavadia JS, Chen AY, Fanaroff AC, de Lemos JA, Kontos MC, Wang TY. Intensive care unit utilization in stable ST‐segment elevation myocardial infarction patients treated with rapid reperfusion: a report from Chest Pain‐MI Registry. JACC Cardiovasc Interv. 2019;12(8):709‐717. 10.1016/j.jcin.2019.01.230. [DOI] [PubMed] [Google Scholar]
- 2. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119‐177. 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 3. Seto AH, Shroff A, Abu‐Fadel M, et al. Length of stay following percutaneous coronary intervention: an expert consensus document update from the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2018;92(4):717‐731. 10.1002/ccd.27637. [DOI] [PubMed] [Google Scholar]
- 4. Halkin A, Singh M, Nikolsky E, et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. J Am Coll Cardiol. 2005;45:1397‐1405. [DOI] [PubMed] [Google Scholar]
- 5. De Luca G, Suryapranata H, van 't Hof AW, et al. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: implications for early discharge. Circulation. 2004;109(22):2737‐2743. [DOI] [PubMed] [Google Scholar]
- 6. Sharkawi MA, Filippaios A, Dani SS, et al. Identifying patients for safe early hospital discharge following ST‐elevation myocardial infarction. Catheter Cardiovasc Interv. 2017;89(7):1141‐1146. [DOI] [PubMed] [Google Scholar]
- 7. Ebinger JE, Strauss CE, Garberich RR, et al. Value‐based ST‐segment‐elevation myocardial infarction care using risk‐guided triage and early discharge. Circ Cardiovasc Qual Outcomes. 2018;11(4):e004553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gong W, Li A, Ai H, Shi H, Wang X, Nie S. Safety of early discharge after primary angioplasty in low‐risk patients with ST‐segment elevation myocardial infarction: a meta‐analysis of randomised controlled trials. Eur J Prev Cardiol. 2018;25(8):807‐815. 10.1177/2047487318763823. [DOI] [PubMed] [Google Scholar]
- 9. Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on performance measures and task force on practice guidelines. J Am Coll Cardiol. 2014;63(21):2304‐2322. 10.1016/j.jacc.2014.03.016. [DOI] [PubMed] [Google Scholar]


