Abstract
Coronavirus disease 2019 (COVID‐19), a respiratory tract infection caused by a novel human coronavirus, the severe acute respiratory syndrome coronavirus 2, leads to a wide spectrum of clinical manifestations ranging from asymptomatic cases to patients with mild and severe symptoms, with or without pneumonia. Given the huge influence caused by the overwhelming COVID‐19 pandemic affecting over three million people worldwide, a wide spectrum of drugs is considered for the treatment in the concept of repurposing and off‐label use. There is no knowledge about the diagnosis and clinical management of the drug hypersensitivity reactions that can potentially occur during the disease. This review brings together all the published information about the diagnosis and management of drug hypersensitivity reactions due to current and candidate off‐label drugs and highlights relevant recommendations. Furthermore, it gathers all the dermatologic manifestations reported during the disease for guiding the clinicians to establish a better differential diagnosis of drug hypersensitivity reactions in the course of the disease.
Keywords: COVID‐19, desensitization, drug hypersensitivity reactions, SARS‐CoV‐2
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is a respiratory tract infection caused by a novel member of human coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 It causes a wide spectrum of clinical manifestations ranging from asymptomatic cases to patients with mild, uncomplicated illness and severe cases, with or without pneumonia. 2 Hospitalization and oxygen support, and admission to an intensive care unit are required in 14% and 5% of the patients, respectively. 1 Gastrointestinal symptoms and positive viral nucleic acid testing on rectal swabs are considered as indicators of infection in digestive system and fecal‐oral transmission of COVID‐19. 3 Moreover, skin symptoms, including exanthems, may appear during the evolution of the disease leading to differential diagnosis with drug hypersensitivity reactions (DHRs). 4
In critically ill patients, COVID‐19 can be complicated by acute respiratory distress syndrome (ARDS), septic shock, and multi‐organ dysfunction syndrome. 1 In such patients, in response to viral infection, the excessive activation and expansion of T lymphocytes and macrophages lead to an overproduction of cytokines, which causes a cytokine storm and a hyperinflammatory state. 5 , 6 Acute hyperinflammation may activate coagulation cascade and inhibit fibrinolytic reaction, thus promoting thrombosis. Coagulopathy and thrombocytopenia are serious complications which increase the risk of hemorrhage and thrombosis and progress to disseminated intravascular coagulation (DIC). 7
The periodically updated World Health Organisation interim guidance allows reliable comparison of investigational therapeutic interventions as part of randomized controlled trials, provides recommendations for the management, and forms the basis of many institutional or national protocols. 1 Unfortunately, none of the drugs used for COVID‐19 have been proven to be truly effective yet; besides, no specific antiviral drugs have been approved for COVID‐19 by health authorities. 8 , 9 At the moment, there is no specific treatment for COVID‐19, and standard practice of care focuses on treating the clinical symptoms with supportive care. 1
In this review, diagnosis and management of DHRs, which are expected to be caused by current or candidate repurposed and off‐label drugs used for COVID‐19 treatment mostly based on prior knowledge, are discussed. 8 , 10 , 11 Drugs in this review are classified into four groups according to their potential roles in different phases of the disease as antiviral drugs, antiviral and/or immunomodulatory drugs used in viral pneumonia; anti‐cytokine and anti‐inflammatory drugs considered during macrophage activation syndrome (MAS) and cytokine storm; anti‐inflammatory drugs in ARDS; and anti‐aggregant and anti‐coagulant drugs in coagulopathy (Figure 1). Information of DHRs due to the use of additional drugs for various purposes can be found in the relevant European Academy of Allergy and Clinical Immunology (EAACI) resources. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20
Figure 1.
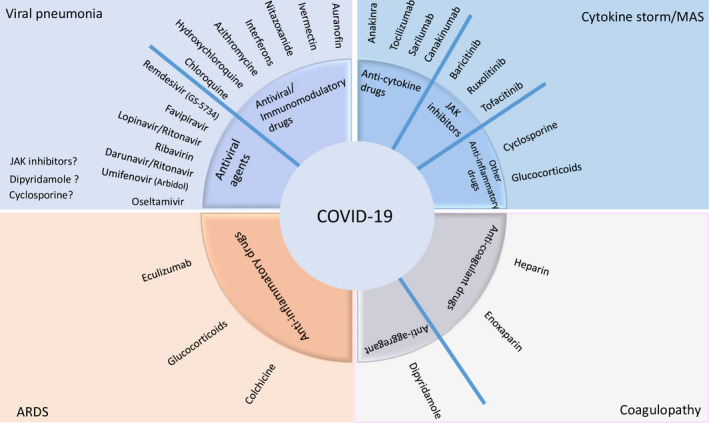
Currently investigated drugs in COVID‐19 grouped according to their clinical use
Since emerging recent findings are dynamically changing the clinical interventions, it is expected that the list of drugs determined according to current knowledge may change with upcoming recommendations in future.
2. SKIN MANIFESTATIONS INDUCED BY COVID‐19
There have been increasing reports of dermatologic manifestations associated with COVID‐19 (Table 1). It is knowledge, although in progress, rapidly evolving as evidenced by most publications being ahead of print and available only in an electronic version or reported in networks.
Table 1.
Skin manifestations reported associated with COVID‐19
| Manifestation | Clinical description | Relative frequency* | Similarity to skin rashes of other infections | References |
|---|---|---|---|---|
| 1. Skin manifestations similar to those in other viral infections | ||||
| Acute urticaria | Sudden appearance of wheals with a fleeting nature. Continual appearance and disappearance of new lesions is characteristic. | 19% | Unspecific for COVID‐19; infections are common elicitors for acute urticaria | (4, 22, 23, 31) |
| Maculopapular exanthem (“erythematous rash”) | Acute erupting, widespread distribution of multiple small, round to oval erythematous macules and/or papules with different degrees of confluence. Mostly trunk, low pruritus. | 47% | Unspecific for COVID‐19; infections are common elicitors for maculopapular exanthem | (4, 21, 22, 24, 31, 32) |
| Varicella‐like exanthem (“chickenpox‐like rash”) | Monomorphic papulovesicular skin eruption. Erythematous papules and vesicles bilaterally and symmetrically mostly on the trunk. | 9% | May be more specific for COVID‐19, vesicles are quite uncommon for virus exanthems and more specific for varicella | (4, 22, 25, 26) |
| Symmetrical intertriginous exanthem | Flexural erythematous maculopapular exanthem on axillary lesions and trunk +/‐antecubital fossa. | Individual case reports | Untypical for infectious exanthems | (30) |
| 2. Skin manifestations associated with vascular pathologies | ||||
| Purpuric exanthem (“purpuric rash”) | Skin rash with petechiae. | Individual case reports | Untypical for infectious exanthems, except, for example Parvovirus B19 | (22, 33) |
| Erythema ab igne (“livedo reticularis”) | Transient macular erythema in a broad reticular pattern on thigh unilaterally. | 6% together with cutaneous acro‐ischemia | Untypical for infectious exanthems | (34) |
| Chilblain‐like lesions | Acute‐onset, violaceous, infiltrated, and painful plaques on the toes and lateral feet. Vesicles and erosions may be present. | 19% | Untypical for infectious exanthems | (22, 35, 36, 37) |
| Cutaneous acro‐ischemia | Finger and toe cyanosis, purpura, hematoma, skin bulla, and dry gangrene. | 6% together with Erythema ab igne (“livedo reticularis”) | Typical for severely ill patients with sepsis | (38, 39) |
Relative frequency in percent of this skin manifestations associated with COVID‐19 infections according to Ref. (26). In cases, where no numbers are given, only individual case reports do exist.
According to pathogenetic mechanisms, skin manifestations reported so far can be divided into (1) skin manifestations similar to those in other viral infections and (2) skin manifestations related to thrombovascular events and vascular pathologies.
2.1. Skin manifestations similar to those in other viral infections
During the COVID‐19 outbreak in China, it was not a focus to document skin manifestations. Consequently, skin rash has only been reported in 2 out of 1.099 infected patients (0.2%). 21 In contrast, a study by dermatologists from Italy reported skin manifestations in 18/88 patients (20.4%) with COVID‐19. 4 Cutaneous manifestations seen were either erythematous rash (n = 14), widespread urticaria (n = 3), or chickenpox‐like vesicular rash (n = 1). In Spain, among 375 patients with suspected or confirmed COVID‐19, maculopapular eruptions (MPEs), sometimes similar to pityriasis rosea, were observed in 47% of the cases, urticarial lesions in 19%, and vesicular eruptions of the trunk in 9%. 22 Another case of urticaria was presented in France (Figure 2A) 23 and patients with morbilliform exanthem in the USA(Figure 2B). 24 Varicella‐like lesions predominantly on the trunk were described in 22 patients with proven COVID‐19 infection in Italy. 25 Predominance of vesicles was reported in 54.5% and generally mild itching in nine (40.9%) patients. The vesiculopapular exanthem appears to develop early in the course of the disease (Figure 2C). 22 , 26 An outbreak of severe Kawasaki‐like disease has been reported at epicenters of COVID‐19 infection also associated with a polymorphic rash in 30%‐50% of affected children. 27 , 28 In one case, picture of a urticaria‐like rash in a 6‐month‐old child with Kawasaki‐like disease associated with COVID‐19 infection is shown (Figure S1). 29 Two patients with bilateral flexural exanthems resembling systemic drug‐related intertriginous exanthems (SDRIFE), one with axillary purpuric lesions associated with thrombocytopenia, have been published (Figure 2D). 30 A prospective study from France reported a prevalence of 5/103 (4.9%) and confirmed association of pruritic erythematous rash (n = 2) and urticaria (n = 2) with COVID‐19 infections 31 ; they additionally observed one oral herpes simplex virus type 1 reactivation. The histopathological picture of exanthematic skin lesions generally resembles that of viral exanthems. However, in individual patients, early microthrombi and an interface dermatitis with necrotic keratinocytes surrounded by lymphocytes have been reported. 32
Figure 2.
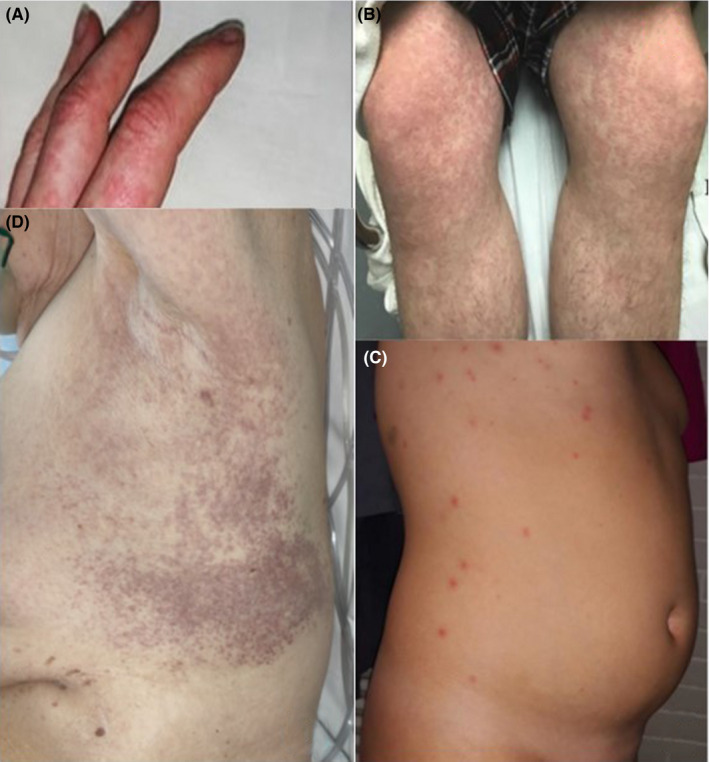
Skin manifestations similar to those in other viral infections. A, Urticaria, 23 B, Morbilliform maculopapular exanthem, 24 C, Vesiculopapular (chickenpox‐like) exanthem, 26 D, Intertriginous purpuric rash 30
2.2. Skin manifestations associated with thrombovascular events and vascular pathologies
COVID‐19 exanthems have also been reported with petechiae and low platelet count resembling dengue. 33 In two patients, unilateral lesions on the thigh resembling livedo reticularis or erythema ab igne have been described with microthromboses discussed as possible etiology (Figure 3A). 34
Figure 3.
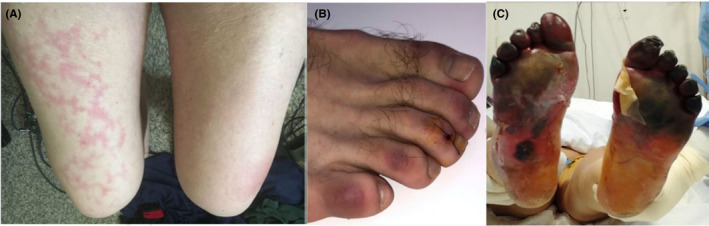
Skin manifestations associated with thrombovascular events and vascular pathologies. A, Transient unilateral livedo reticularis (erythema ab igne), 34 B, COVID‐19‐induced chilblains, 35 C, Acro‐ischemia with cyanosis, skin bulla, and dry gangrene in critically ill patient 38
Chilblain‐like skin lesions have been frequently reported to be associated with COVID‐19 22 , 35 , 36 , 37 (Figure 3B). 35 They appear in up to 19% of patients, typically in mildly affected ones, and late in the evolution of the disease. 22 , 37 Vesicles, pustules, and erosions on these violaceous plaques may occur. 37 In Spain, they were observed in 19% of 375 cases. 22
Seven patients had cutaneous acro‐ischemia including finger and toe cyanosis, skin bulla, and dry gangrene associated with COVID‐19 infection‐induced hypercoagulation including definitive DIC in four patients. Five of these patients finally died (Figure 3C). 38 A catastrophic microvascular injury syndrome mediated by activation of complement pathways and an associated procoagulant state were described in severe COVID‐19 with purpuric skin rash in 3/5 patients. 39
In conclusion, the prevalence of cutaneous manifestations in COVID‐19‐infected patients has been reported between 0.2%, 4.9%, and 20.9%. 4 , 21 , 30 Most skin manifestations resemble cutaneous involvement commonly occurring during viral infections, that is, erythematous rash and acute urticaria. Drug exanthems have to be considered as differential diagnosis. 15 Vesicular varicella‐like exanthems may be more specific for COVID‐19. Flexural distribution, and petechiae as well as erythema ab igne‐like lesions have been described. Violaceous, infiltrated painful plaques resembling chilblains have been frequently reported and discussed as typical manifestations. Necrotic lesions occurred in older and in severely ill patients with increased mortality. 22 Cutaneous acro‐ischemic microthromboses and small blood vessel occlusion have to be further explored for their causality and specificity for COVID‐19 manifestations.
3. ANTIVIRAL AGENTS USED FOR VIRAL PNEUMONIA
3.1. Clinical use in COVID‐19
Most antiviral agents used for COVID‐19 act either by inhibiting RNA‐dependent RNA polymerase [remdesivir (GS‐5734)] or proteases [lopinavir/ritonavir (LPV/r), favipiravir (FPV), ribavirin, and darunavir]. 40 , 41 , 42 , 43 Additionally, umifenovir plays a role in viral entry by inhibiting the hemagglutinin‐mediated membrane fusion, and oseltamivir is a neuraminidase inhibitor which blocks the release of viral particles from the host cells in influenza infection. 44 Remdesivir and FPV are considered to be the most effective agents and are mostly used in combination with other COVID‐19 medications like hydroxychloroquine. 40 , 41 , 42 , 43 Oseltamivir is recommended for concomitant influenza infection. 45 Darunavir or LPV/r can be concomitantly administered with chloroquine or hydroxychloroquine. 43
3.2. Hypersensitivity reactions
Drug hypersensitivity reactions to ribavirin, darunavir, LPV/r, remdesivir, and oseltamivir are rarely reported, whereas no DHRs to favipiravir and umifenovir are known at present 46 , 47 , 48 , 49 , 50 , 51 (Table 2).
Table 2.
Hypersensitivity reactions due to drugs with antiviral properties investigated for the treatment of COVID‐19 in clinical trials or in vitro studies
| Drug groups | Drugs | Purpose of use in COVID‐19 | Hypersensitivity reactions | In vivo tests in IHRs | In vivo tests in NIHRs | In vitro tests for IHRs | In vitro tests for NIHRs | Desensitization |
|---|---|---|---|---|---|---|---|---|
| Antiviral drugs | Favipiravir | Viral pneumonia | None | |||||
| Lopinavir/Ritonavir | AGEP 61 , MPE 60 | |||||||
| Darunavir/Ritonavir |
MPE 66 |
DNIHR 66 , 67 | ||||||
| Umifenovir (Arbidol) | None | |||||||
| Ribavirin |
Urticaria 58 MPE 56 |
DPT 56 | LTT 57 | DNIHR 58 , 59 | ||||
| Remdesivir (GS‐5734) | MPE 51 | |||||||
| Oseltamivir |
Anaphylaxis 68 |
SPT 68 | LTT 50 | |||||
| Immunomodulatory drugs | Azithromycine |
MPE 73 , 74 , ACD 75 , 76 , FDE 77 Anaphylaxis 72 Urticaria 71 Leukocytoclastic vasculitis 82 Hypersensitivity myocarditis 83 |
DPT 87 |
PT 75 DPT 87 |
DIHR 88 |
|||
| Hydroxychloroquine / Chloroquine |
Erythema multiforme 96 Bullous erythema 97 Photoallergic dermatitis 101 ACD 102 |
SPT 109 |
DPT 90 |
|||||
| Auranofin | None | |||||||
| Interferons |
Local reaction 116 |
DPT 125 |
PT 119 |
DIHR 123 |
||||
| Nitazoxanide | None | |||||||
| Ivermectin | FDE 129 , DRESS 130 , SJS 131 , TEN 132 |
Abbreviations: ACD, Acute contact dermatitis; AGEP, Acute generalized exanthematous pustulosis; BAT, Basophil activation test; DIHR, Desensitization for immediate hypersensitivity reactions; DNIHR, Desensitization for nonimmediate hypersensitivity reactions;DPT, Drug provocation test; DRESS, Drug‐related eosinophilia systemic symptoms; FDE, Fixed drug eruption; IDT, Intradermal test; IHR, Immediate hypersensitivity reaction; LTT, Lymphocyte transformation test; MPE, Maculopapular eruption; NIHR, Nonimmediate hypersensitivity reaction; PT, Patch test; SJS, Stevens Johnson syndrome; SPT, Skin prick test; ST, Skin test; TEN, Toxic epidermal necrolysis.
3.2.1. Ribavirin
Ribavirin is used in combination with pegylated‐interferon α2a (peg‐IFN‐α2a) for treating chronic hepatitis C, and both have been associated with several cutaneous DHRs. 52 Ribavirin alone causes dermatitis, alopecia, and photoallergic eczematous reactions, 53 , 54 and the risk of DHR increases with combination therapy: rash [response rate (RR), 1.74; 95% confidence interval (CI), 1.17‐2.6], dermatitis (RR, 1.67; 95% CI, 1.21‐2.30), and pruritus (RR, 1.62; 95% CI, 1.29‐2.02). 55 A meta‐analysis revealed that, on combination therapy, mild to moderate cutaneous reactions appear in 13.3% of patients, localized cutaneous reactions in2.6%, generalized reactions‐pruritus, skin xerosis, and eczematous changes in 10.3%, alopecia in 4.1%, and exacerbation of lichen planus in less than 1% 46 (Table 2).
The etiological diagnosis is difficult in case of combination therapy. A drug provocation test (DPT) confirmed the diagnosis of ribavirin hypersensitivity in a patient having MPE due to combined use of peg‐IFN‐α2a and ribavirin. 56 In another case, an erythema multiforme‐type drug eruption occurred with peg‐IFN‐α2a, ribavirin, and/or fluvastatin sodium therapy and a positive lymphocyte transformation test (LTT) confirmed the diagnosis of ribavirin hypersensitivity. 57 Successful desensitization protocols were reported 58 , 59 (Table 2).
3.2.2. Lopinavir/ritonavir (LPV/r)
Lopinavir/ritonavir, either alone or in combination, has been rarely reported to be associated with DHRs. In human immunodeficiency virus (HIV)‐infected patients who received LPV/r combination, MPE rate was reported as 2%‐4%. 60 Acute generalized exanthematous pustulosis (AGEP) was described in two cases receiving LPV/r 61 (Table 2).
In a multicentre randomized study that evaluated the long‐term efficacy and safety of the combination of efavirenz or LPV/r plus abacavir/lamivudine, 2/63 patients in the LPV/r group discontinued the study because of a DHR. 62
In a recent cohort of 199 severe COVID‐19‐infected patients who received LPV/r combination, only two (1%) experienced self‐limited skin eruptions. 47 A recent study evaluating 217 patients from China revealed that most of the adverse drug reactions (ADRs) were associated with LPV/r and umifenovir with 63.8% and 18.1%, respectively, and history of a drug allergy was higher in these patients (8.5%) comparing with the ones without ADRs (2.2% vs, P < .044). 63
3.2.3. Darunavir
Darunavir can induce a variety of delayed skin eruptions from mild MPE in most cases, to severe bullous cutaneous reactions in HIV‐infected patients. 48 , 64 A phase III randomized clinical trial performed in 604 patients treated with darunavir/r or LPV/r showed that the percentage of patients experiencing rash was higher in those receiving darunavir/r compared with others (16% vs 7%). Two patients receiving darunavir/r required treatment cessation due to a severe rash. 48 Darunavir contains a sulfonamide moiety and should be used with caution in patients with a known sulfonamide allergy. 65 Desensitization was reported to be successful in patients with nonimmediate hypersensitivity reactions (NIHRs) to darunavir 66 , 67 (Table 2).
3.2.4. Oseltamivir
Oseltamivir, used in influenza, causes rare hypersensitivity reactions although close monitoring of patients is important as two cases with Stevens‐Johnson syndrome (SJS)/Toxic epidermal necrolysis (TEN) have been reported, 49 , 50 with only one being confirmed by LTT. 50 Another case report revealed anaphylaxis due to oseltamivir confirmed by a skin prick test (SPT) 68 (Table 2).
3.2.5. Remdesivir
A recent multicentre study showed that only one (1.6%) out of 61 patients with COVID‐19, experienced MPE during remdesivir treatment and therefore discontinued it prematurely 51 (Table 2).
4. ANTIVIRAL AND/OR IMMUNOMODULATORY DRUGS USED FOR VIRAL PNEUMONIA
4.1. Azithromycin
4.1.1. Clinical use in COVID‐19
Azithromycin interferes with virus internalization process in influenza infection 69 and has shown clinical effects in COVID‐19‐infected patients, although its mechanism against SARS‐CoV‐2 remains unclear. 70
4.1.2. Hypersensitivity reactions
Regarding immediate hypersensitivity reactions (IHRs), urticaria is the most frequent manifestation 71 ; furthermore, anaphylaxis can occur. 72 Concerning NIHRs, MPE is described to occur independently 73 or only in the presence of a concurrent infection. 74 Azithromycin has been implicated in contact dermatitis in occupational 75 and nonoccupational settings. 76 Cases of fixed drug eruption (FDE), 77 AGEP, 78 and Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), 79 SJS, 80 , 81 leukocytoclastic vasculitis, 82 and hypersensitivity myocarditis 83 were reported(Table 2).
Diagnosis is complex as skin testing is not validated, presenting discrepancies in nonirritating dilutions for SPT and intradermal test (IDT). 84 , 85 For NIHRs, positive responses to patch tests (PTs)were described. 75 In addition, no validated in vitro tests are available. 86 Oral DPT remains as the gold standard for diagnosis. 87 A successful desensitization protocol was reported in a case of mast cell activation syndrome 88 (Table 2).
4.2. Hydroxychloroquine/Chloroquine
4.2.1. Clinical use in COVID‐19
Hydroxychloroquine/chloroquine have in vitro antiviral effects against SARS‐Cov‐2 by preventing virus/cell fusion, and immunomodulatory effects by inhibiting production of inflammatory cytokines. 89
4.2.2. Hypersensitivity reactions
Dermatologic ADRs are difficult to be distinguished as a side effect of or an allergic reaction to these drugs or a flare of the underlying dermatological disease. 90 , 91 The most common manifestation is mild pruritic MPEs within initial 4 weeks of treatment. 90 High association with AGEP [OR: 39 (8‐191)] was described. 92 Cases of DRESS, 93 , 94 pustular DRESS, 95 erythema multiforme, 96 bullous erythema, 97 SJS/TEN, 98 , 99 , 100 photoallergic dermatitis, 101 and occupational contact dermatitis 102 have been reported(Table 2).
PTs are reported to be useful for the diagnosis of NIHRs, 96 , 98 , 103 confirming a T cell–mediated mechanism. However, in a series of 14 patients with ADRs due to chloroquine/hydroxychloroquine, skin tests (STs) were negative in all cases. 90 DPT is useful in nonsevere cutaneous ADRs in order to differentiate allergic reactions from dermatological adverse effects since only 30% of the patients reporting cutaneous ADRs reveal a positive DPT. 90 Successful desensitization protocols of hydroxychloroquine in MPE were reported. 104 , 105 , 106 , 107 Recently, a 5‐hour desensitization protocol for nonimmediate urticaria was successfully administered 108 (Table 2).
Two cases of IHR were reported 109 , 110 and one was confirmed by SPTs 109 ; however, there are no available data for in vitro diagnosis. A hydroxychloroquine desensitization procedure that enables the turning of positive SPTs into negative was published. 109 In a case of anaphylaxis, a 7 day‐desensitization procedure was successfully performed with premedication 110 (Table 2).
4.3. Auranofin
4.3.1. Clinical use in COVID‐19
Auranofin is an anti‐inflammatory compound that can possibly inhibit the replication of SARS‐CoV‐2 in cell culture and reduce the expression of cytokines caused by SARS‐CoV‐2 and the associated lung damage. 111
4.3.2. Hypersensitivity reactions
There are no reported hypersensitivity reactions due to auranofin.
4.4. Interferons
4.4.1. Clinical use in COVID‐19
Type I IFNs (IFN‐α and IFN‐β) can inhibit the replication of both SARS and Middle East respiratory syndrome coronavirus (MERS‐CoV) and are recommended in combined therapies with other antiviral agents. 112 , 113
4.4.2. Hypersensitivity reactions
Cutaneous eruptions induced by IFNs are common, with an incidence of 13%‐23%. 114 , 115 Localized reactions at injection sites are most frequent at 48 weeks. 116 Diffuse skin symptoms including urticaria, generalized eczema, papules are common and mostly treated with symptomatic treatment. 114 , 117 , 118 Among 26 patients with nonimmediate reactions to IFNs, 12 cases reported generalized eczema, 10 MPE, 3 generalized urticaria, and 1 lichenoid eruption. 119 Cases of FDEs, 120 and subacute cutaneous lupus 121 were described (Table 2).
There are few case reports of immediate urticaria 122 , 123 and anaphylaxis. 124 , 125 For IHRs to IFN‐β, positive STs were reported. 122 , 124 For NIHRs, PTs have a low value and are not recommended, whereas delayed reading IDTs are useful. 119 , 126 A positive DPT was reported in a patient experiencing anaphylaxis due to peg‐INF‐α2a with negative STs. 125 Successful desensitization protocols both for IHRs 123 and NIHRs 119 , 127 due to different IFNs were reported (Table 2).
4.5. Ivermectin
4.5.1. Clinical use in COVID‐19
Ivermectin is an antiparasitic drug also shown to have an in vitro activity against SARS‐CoV‐2 by inhibition of viral replication. 128
4.5.2. Hypersensitivity reactions
Rare case reports of multiple FDEs, 129 confirmed DRESS by skin biopsy and blood eosinophilia, 130 confirmed SJS 131 and TEN 132 by skin biopsy were published (Table 2). No data about STs, in vitro tests, or DPT are available. In addition, no cases of desensitization were reported.
4.6. Nitazoxanide
4.6.1. Clinical use in COVID‐19
Nitazoxanide is an antiparasitic agent which also has antiviral activities. Combined with hydroxychloroquine or azithromycin, a synergistic effect has been suggested as hydroxychloroquine and azithromycin inhibit viral entry and fusion, while nitazoxanide upregulates innate immune response to prevent ongoing viral replication in COVID‐19. 133
4.6.2. Hypersensitivity reactions
No DHRs to nitazoxanide are reported.
5. ANTI‐CYTOKINE/ANTI‐INFLAMMATORY DRUGS USED FOR MAS/CYTOKINE STORM/ARDS
5.1. Tocilizumab
5.1.1. Clinical use in COVID‐19
Tocilizumab, an anti‐IL‐6 receptor humanized monoclonal antibody, is under investigation for treatment of COVID‐19 and has shown promising results in cytokine storm. 6
5.1.2. Hypersensitivity reactions
The rate of all ADRs to tocilizumab is reported to be around 8%, among them 0.1%‐0.7% are DHRs. 134 DHRs to tocilizumab are both NIHRs 135 , 136 and IHRs 137 , 138 , 139 , 140 (Table 3). In an adult cohort, the incidence of IHRs was reported as 5.5% 139 whereas in a pediatric cohort it was 13.6%. 137
Table 3.
Hypersensitivity reactions due to other drugs investigated for the treatment of COVID‐19–related complications in clinical trials or in vitro studies
| Drug groups | Drugs |
Purpose of use in COVID‐19 |
Hypersensitivity reactions | In vivo tests in IHRs | In vivo tests in NIHRs | In vitro tests for IHRs | Desensitization |
|---|---|---|---|---|---|---|---|
| Anti‐cytokine or anti‐inflammatory drugs | Tocilizumab | Cytokine storm/MAS |
Papular skin lesions 136 Nonimmediate urticaria 141 |
IDT 140 |
DIHR 134 DNIHR 141 |
||
| Sarilumab | Pruritic rash 155 | ||||||
| Anakinra |
U/Angioedema 149 Erythematous plaques 152 |
DNIHR 152 |
|||||
| Canakinumab | U 137 | ||||||
| JAK inhibitors Baricitinib | Palmoplantar pustulosis 160 | ||||||
| JAK inhibitors Ruxolitinib | morbiliform rash, exfoliative dermatitis 159 | ||||||
| JAK inhibitors Tofacitinib |
U 161 , palmoplantar pustulosis 162 |
||||||
| Cyclosporine | Anaphylaxis 164 , 165 , 166 , 167 | BAT 166 | |||||
| Anti‐inflammatory drugs | Glucocorticoids |
Cytokine storm/MAS ARDS |
IHR 178 , 179 , 180 , 181 , 182 , 183 ACD 179 |
||||
| Colchicine |
Anaphylaxis 151 |
DPT 168 PT 170 |
DNIHR 169 | ||||
| Eculizumab |
Anaphylaxis 174 |
SPT 174 , IDT 174 | DIHR 174 | ||||
| Anti‐coagulant or anti‐aggregant drugs |
Heparin Enoxaparin |
Coagulopathy |
ISR 192 |
SPT 17 |
PT 17 IDT 17 |
BAT 204 , 206 , 207 | DIHR 209 , 210 |
| Dipyridamole |
Eczema 215 |
PT 215 |
Abbreviations: ACD, Acute contact dermatitis; AGEP, Acute generalized exanthematous pustulosis; BAT, Basophil activation n test; DIHR, Desensitization for immediate hypersensitivity reactions; DNIHR, Desensitization for nonimmediate hypersensitivity reactions; DPT, Drug provocation test; DRESS, Drug related eosinophilia systemic symptoms; FDE, Fixed drug eruption; HIT, Heparin induced thrombocytopenia; IDT, Intradermal test; IHR, Immediate hypersensitivity reaction; ISR, Injection site reaction; GDE, Generalized delayed exanthema; LTT, Lymphocyte transformation test; MPE, Maculopapular eruption; NIHR, Nonimmediate hypersensitivity reaction; PT, Patch test; SJS, Stevens Johnson syndrome; SPT, Skin prick test; ST, Skin test; TEN, Toxic epidermal necrolysis; U, Urticaria.
Regarding NIHRs, cases of nonimmediate urticaria, 141 DRESS, 142 , 143 SJS, 144 and AGEP 145 were reported. Younger age, shorter stature, lighter weight, and increased disease activity in the early period of tocilizumab administration have been identified as risk factors for DHRs. 146
Although not standardized, DPTs, SPTs, and IDTs were used for diagnosis of IHRs in case reports. 137 , 139 Only one study revealed that STs have a low negative predictive value in NIHR. 140 Desensitization to tocilizumab in NIHRs was effectively applied in a weekly scheme with premedication in one case. 141 Rapid drug desensitization is successfully and routinely used for IHRs 19 , 134 (Table 3).
5.2. Anakinra
5.2.1. Clinical use in COVID‐19
Anakinra, a recombinant IL‐1 receptor antagonist, is under investigation for the treatment of cytokine storm seen during COVID‐19. 5
5.2.2. Hypersensitivity reactions
Anakinra causes ADRs in 75% of patients. Many of them are related to injection site reactions within the first weeks of application and can present either as an IHR or NIHR. 147 , 148 Systemic IHRs such as urticaria, angioedema, anaphylaxis, 149 , 150 , 151 and NIHRs 152 as infiltrating erythematous skin plaques were rarely reported as single cases. IHR after a first dose of anakinra was reported in a case possibly due to components that are able to induce a direct mast cell degranulation 151 , 153 (Table 3).
For evaluating IHRs to anakinra, SPTs and IDTs were performed with the undiluted drug. 150 , 151 For both IHRs 149 , 151 and NIHRs, 152 successful desensitization protocols were reported (Table 3).
5.3. Sarilumab
5.3.1. Clinical use in COVID‐19
Sarilumab, another IL‐6 receptor antagonist, is under investigation in a phase II/III clinical trial in patients with severe COVID‐19 infection. 154
5.3.2. Hypersensitivity reactions
It is generally a well‐tolerated drug; however, it can cause local reactions on injection site. In an open‐label study, in 3% of the patients it caused a pruritic generalized rash which did not affect the treatment 155 (Table 3).
5.4. Canakinumab
5.4.1. Clinical use in COVID‐19
Canakinumab, a high‐affinity human anti‐IL‐1β monoclonal antibody, is considered as a candidate in treatment of severe COVID‐19. 156
5.4.2. Hypersensitivity reactions
This anti‐IL‐1 agent is normally well tolerated and indicated as an alternative in cases with an anaphylactic reaction to anakinra. 138 However, there is a recently reported case who developed immediate diffuse urticaria after the tenth canakinumab administration and was prevented from further reactions with cetirizine premedication 137 (Table 3).
5.5. Janus kinase (JAK) inhibitors (Baricitinib, Ruxolitinib, Tofacitinib)
5.5.1. Clinical use in COVID‐19
Janus kinase inhibitors are under investigation for their potential role in regulating the overactive signaling in the JAK‐STAT pathway seen during cytokine storm in critically ill COVID‐19‐infected patients. Baricitinib with its potential to inhibit clathrin‐mediated endocytosis, and its ability to ameliorate associated chronic inflammation in interferonopathies is expected to show promising results in ongoing clinical trials of COVID‐19. 157 , 158
5.5.2. Hypersensitivity reactions
Few cases were reported: one with a morbiliform eruption and exfoliative dermatitis due to ruxolitinib, 159 another one with palmoplantar pustulosis due to baricitinib, 160 and cases of acute urticaria 161 and palmoplantar pustulosis 162 due to tofacitinib (Table 3).
5.6. Cyclosporine
5.6.1. Clinical use in COVID‐19
Cyclosporine A prevents the transcription of genes encoding cytokines like IL‐2 and inhibits the replication of diverse coronaviruses at noncytotoxic, low‐micromolar concentrations in vitro. 163
5.6.2. Hypersensitivity reactions
Rare cases of pruritus, urticaria, angioedema, and anaphylaxis were reported. 164 , 165 , 166 The possible mechanisms can be both immunologic and nonimmunologic, which seems to depend on the administration route and formulation. 164 In some cases, DHRs have been attributed to the additives such as castor oil 165 or Cremophor EL. 166 SPTs and IDTs or basophil activation test (BAT) can be used for the diagnosis of cyclosporine‐ and additive‐induced IgE‐mediated IHRs 18 , 164 , 166 (Table 3).
5.7. Colchicine
5.7.1. Clinical use in COVID‐19
It is a nonselective inhibitor of NLRP3 inflammasome which is thought to be a major pathophysiologic component of ARDS and/or acute lung injury seen in COVID‐19. 167
5.7.2. Hypersensitivity reactions
Rare cases of anaphylaxis, 151 confirmed FDE with DPT 168 and successfully desensitized MPE 169 were reported. For PTs, it is recommended to dilute colchicine to 1% in petrolatum 170 (Table 3).
5.8. Eculizumab
5.8.1. Clinical use in COVID‐19
Eculizumab, a humanized anti‐C5 monoclonal antibody, is under investigation as a candidate drug to play a role in the thrombotic microvascular injury mediated by complement activation causing lung injury either due to severe pneumonia or ARDS in severe COVID‐19. 21 , 36 , 171
5.8.2. Hypersensitivity reactions
Immediate hypersensitivity reactions or infusion reactions due to eculizumab are very rare. 172 , 173 A case of anaphylaxis diagnosed with STs was successfully desensitized with a rapid protocol 174 (Table 3).
5.9. Glucocorticoids
5.9.1. Clinical use in COVID‐19
In COVID‐19‐infected patients, the use of glucocorticoids (GCs) is rather controversial. 175 , 176 Early start of GCs could be helpful for patients who have an overly exuberant inflammatory response or are at high risk of developing ARDS, whereas the benefit of GCs as rescue treatment remains doubtful. 177
5.9.2. Hypersensitivity reactions
IHRs to GCs are overall rare and mostly IgE‐mediated. 178 , 179 , 180 , 181 , 182 , 183 In a review of the literature from 2004 to 2014, anaphylaxis was the most common manifestation reported (60.8%, 73/120 reactions) followed by urticaria and/or angioedema (26.7%). Methylprednisolone was implicated in 41% of reactions, followed by prednisolone (20%), triamcinolone (14%), and hydrocortisone (10%). 181
In most subjects with IHRs, it is possible to identify the culprit and safe alternative GCs by performing immediate‐reading STs. 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 In the aforementioned review, 74.1% of 112 STs carried out with GCs suspected of being responsible for reactions were positive. 181 In some subjects, positive STs were associated with positive serum‐specific IgE assays and BATs 181 , 182 (Table 3).
Immediate hypersensitivity reactions to medication components other than the GC itself, such as succinate ester used to enhance the solubility in parenteral preparations, have been described. 181 , 185 Hence, when evaluating a reaction to an esterified GC, it is advisable to include in STs the suspected GC and the same GC without the ester component, or with a different ester.
Immediate hypersensitivity reactions to excipients or preservatives in GC preparations, such as lactose, carboxymethylcellulose, polyethylene glycol, and hexylene glycol, have also been reported. 181 , 185 Therefore, testing should be performed with a preservative free GC, in addition to preservative testing per se if needed 185 (Table 3). A study proposed a comprehensive diagnostic algorithm to evaluate hypersensitivity reactions to GCs, as well as to their components and preservatives. 185 This algorithm included STs with Carmellose® eye drops in subjects who had reacted to carboxymethylcellulose‐containing GCs and with cow's milk proteins in those who had reacted to lactose‐containing GCs.
In the allergy workup, negative results in STs should be confirmed with DPTs. 180 , 181 , 182 , 183 , 184 , 185 DPTs are also recommended to ensure tolerance of alternative preparations. 184 Cross‐reactivity patterns based on structural characteristics have not been clearly established for IHRs as they have been for allergic contact dermatitis. 179 DPTs have shown that patients often tolerate alternative GCs belonging to the same chemical group as the responsible GC. 182 , 183 Desensitization to methylprednisolone has been successfully performed 186 , 187 (Table 3).
Nonimmediate hypersensitivity reactions following systemic administration of GCs have been more rarely reported than IHRs; most reports concerned isolated cases of eczematous or exanthematous skin eruptions 178 , 179 (Table 3). Some are systemic contact dermatitis, occurring in patients with previous contact dermatitis to GCs. They can be revealed by a Baboon syndrome, characterized by a buttock erythema associated to a symmetric, flexural erythema. 188
Most patients do not have a previous topical sensitization. In NIHRs, the main feature is MPE, but other clinical aspects can also occur such as annular erythema, erythroderma, SDRIFE, AGEP, FDE, and a few cases of SJS 188 (Table 3).
Nonimmediate hypersensitivity reactions can be T cell–mediated, and PTs, together with delayed‐reading IDTs, are useful tools for evaluating them. 17 PTs have to be read at 2, 4, and also 7 days. Even though delayed reading IDTs are more sensitive than PTs, the sensitivity of the former is limited. Therefore, DPTs are often necessary to diagnose NIHRs. In a study by Padial et al, only 2 of the 38 patients with NIHRs to GCs displayed positive delayed‐reading IDTs and PTs to the responsible GCs (ie, dexamethasone and betamethasone), while 21 of the 32 negative patients who agreed to undergo DPTs reacted to them, experiencing almost exclusively delayed‐appearing urticarial eruptions or MPEs 189 (Table 3).
6. ANTI‐COAGULANT AND ANTI‐AGGREGANT DRUGS USED FOR COAGULOPATHY
6.1. Heparin and low molecular weight heparins (LMWHs)
6.1.1. Clinical use in COVID‐19
Heparin [unfractionated heparin (UFH)] and LMWHs are administered for treatment or prophylaxis of thrombosis, and therefore, it is used for the coagulopathy observed during COVID‐19. 190
6.1.2. Hypersensitivity reactions
Unfractionated heparin may induce all types of DHRs, mostly type IV and type II. 191 Cutaneous NIHRs to subcutaneous heparin occur at the injection site as itchy erythematous or eczematous plaques usually on the 7th‐10th day of treatment, although they can appear on the 1‐3th day in case of antecedent sensitization. 192 Risk factors for NIHRs to heparin are obesity, female gender, old age, pregnancy, and repeated exposures. 193 , 194 If the treatment is continued regardless of a local reaction, the patient may develop generalized eczema or exanthem. 195 , 196 Patients with a NIHR to UFH or LMWH at injection site usually tolerate intravenous administration of UFH. 192 Cross‐reactivity among LMWHs has been reported in NIHRs. 197 However, fondaparinux is generally well tolerated in patients who react to LMWHs. 194 Heparin may induce DRESS 198 and SJS. 199
Immune‐mediated heparin‐induced thrombocytopenia (HIT) is induced by IgG antibodies against complex of heparin and platelet‐factor 4 tetramers. 200 HIT manifests as a more than 50% decrease in the platelet count in 5‐10 days after the onset of treatment. 201 The risk of HIT is increased exclusively with UFH. 202 Treatment includes the discontinuation of heparin and the introduction of an alternative anti‐coagulant such as argatroban, fondaparinux, danaparoid, or bivalirudin. 203
The IgE‐mediated reactions to heparin manifesting as urticaria, angioedema, and anaphylaxis are rare. 197 , 203 , 204 Positive STs with UFH and LMWHs have been reported 197 , 203 , 204 , 205 (Table 3). Cross‐reactivity in IHRs has been reported between UFH and LMWH and among LMWHs. 205
For IHRs with heparins, diagnostic approach primarily consists of SPTs and IDTs. 17 The results of BAT with UFH and LMWH are controversial. 204 , 206 , 207 Heparin itself may cause a release of histamine, leading to a false‐positive ST. Further serial dilutions of heparin (1:100, 1:1000, 1:10 000) might be needed. 204 IDTs and PTs with the culprit and alternative heparin are performed in NIHRs. 17 PTs, with tape stripping, are less sensitive but may be positive 191 (Table 3).
Drug provocation test is considered when the diagnosis is obscure, tissue pathology is unavailable, or an alternative anti‐coagulant needs to be determined. 208 Subcutaneous DPTs with UFC and LMWHs are performed with increasing doses reaching up to a daily dose on the first day, then are evaluated on three consecutive days and day 7 in case of NIHRs. Intravenous DPTs with UFC may also be necessary to prove tolerance for emergency situations both for IHRs and NIHRs. 191 , 192 A standard protocol for UFH desensitization has not been established yet and published as case reports 209 , 210 (Table 3).
6.2. Dipyridamole
6.2.1. Clinical use in COVID‐19
Dipyridamole is an inhibitor of phosphodiesterase 3 and 5; thereby, it increases intracellular cAMP and/or cGMP in platelets and inhibits platelet aggregation. 211 Besides, it has antiviral features against several viruses. 212 , 213 Dipyridamole as an adjunctive therapy was demonstrated to be associated with decreased D‐dimer levels in COVID‐19. 214
6.2.2. Hypersensitivity reactions
Drug hypersensitivity reactions related to dipyridamole are extremely rare. An adult patient with delayed eczematous lesions revealed positive PT results. 215 Anaphylaxis or anaphylaxis like reactions were described in two cases; however, they lack diagnostic tests 216 , 217 (Table 3).
7. DIAGNOSIS, DIFFERENTIAL DIAGNOSIS, AND MANAGEMENT OF DHRS DUE TO DRUGS INVESTIGATED FOR THE TREATMENT OF COVID‐19
Considering the severity of the disease and the emergent need for interventions, it is important to give accurate and quick diagnostic and therapeutic decisions in case of DHRs during COVID‐19 treatment. However, it is challenging considering the diverse spectrum of drugs introduced either for direct treatment of the disease or other accompanying conditions during the course of the disease especially in severe cases when the disease is prolonged. Consequently, multiple medications applied at a time make a clear‐cut association with one medication more difficult. Furthermore, disease‐related eruptions as an important reason of differential diagnosis can make the diagnosis even harder, considering that the majority of the drugs used are more associated with drug‐related cutaneous NIHRs.
Given the critical state of the disease, the diagnosis can mostly rely on clinical observations without performing in vivo tests which have possible contamination risks and time‐consuming in vitro tests. During a DHR, STs cannot be applied considering the possibility of aggravation and the low diagnostic accuracy expected during ongoing treatment with antihistamines and corticosteroids. When introducing an alternative drug, a DPT based on established methods may be preferred in order to reduce the risk of a possible DHR. 14
If alternative drugs are not available and underlying DHR is not severe, we can recommend that drugs can be applied with published or tailored desensitization protocols. 19 , 20 When mild, self‐limiting DHR is considered, “treating through” concept, the continued administration of a drug despite a suspected allergic hypersensitivity reaction, can be considered under strict surveillance measures. 218 Our recommendations for the diagnosis and management of DHRs due to drugs administered during COVID‐19 are listed in Box 1.
BOX 1. Recommendations for diagnosis and management of DHRs in COVID‐19.
No equivalent alternatives for the currently off‐label repurposed drugs or novel drugs used in COVID‐19 do exist.
We should extrapolate our knowledge on DHRs from other clinical situations to COVID‐19 considering the scarce experience for the DHRs during the disease.
Various drugs being used in different phases of the disease seem to cause rare but potentially severe DHRs, mostly nonimmediate cutaneous hypersensitivity reactions based on data from limited number of case reports.
The most important differential diagnosis of these DHRs is disease‐related exanthems, which can further be classified into the ones similar to those in other viral infections and the others related to thrombovascular events and vascular pathologies seen during COVID‐19.
Experience of diagnostic and management methods for DHRs due to the drugs used in COVID‐19 depend mostly on few case reports or series.
Knowledge of DHRs is urgently needed from pharmacovigilance registries and data from ongoing clinical trials ofCOVID‐19.
Quick diagnostic and therapeutic decisions in case of DHRs during COVID‐19 are mandatory.
Clinical diagnosis of DHRs during COVID‐19 might mostly rely on clinical observations and basic laboratory findings regarding the need of urgent treatment of COVID‐19.
If the risks of a DHR outweigh the benefits obtained from the drug administration, the offending drug should be discontinued.
When introducing an alternative drug, a DPT may be preferred in order to reduce the risk of a possible DHR.
If an alternative drug cannot be replaced, the offending drug can be administered via desensitization with published or tailored protocols when there are no contraindications.
“Treating through” concept, the continued administration of a drug despite a suspected allergic hypersensitivity reaction, can also be considered under strict surveillance measures if the underlying DHR is mild and self‐limiting, and an alternative drug does not exist.
8. CONCLUSION
This review brings together all the published information about DHRs due to current and candidate off‐label drugs to treat COVID‐19. The current knowledge depends mostly on previous clinical experience and few published studies or case reports. Hopefully, published literature reveals that most of these drugs rarely cause DHRs but severe reactions may also occur. One limitation of this review is that it includes extremely low number of reports of ADRs seen so far during COVID19 treatment. In near future, we need to obtain data about DHRs during the disease from ongoing clinical trials and DHR registries. Additionally as time passes, we will observe if SARS‐CoV‐2 can aggravate T cell–mediated reactions as some viruses do, 219 and if the hyperinflammation observed during the course of the disease may influence DHRs.
This review also highlights the presence of two different groups of disease‐related exanthems as an important cause of differential diagnosis of DHRs expected during the treatment of the disease. We think that it is extremely important to distinguish these disease‐related eruptions from true DHR‐related skin manifestations considering that the majority of the drugs used are more associated with drug‐related nonimmediate skin reactions.
In near future, further data from ongoing clinical studies and registries established in different countries will enlighten the obscure parts of our understanding on DHRs due to the drugs used in the treatment of COVID‐19 and will possibly enable us to establish accurate diagnostic and treatment approaches for these reactions.
CONFLICT OF INTEREST
None of the authors declare conflict of interest.
Supporting information
Figure S1
Gelincik A, Brockow K, Çelik GE, et al. Diagnosis and management of the drug hypersensitivity reactions in Coronavirus disease 19: An EAACI Position Paper. Allergy. 2020;75:2775–2793. 10.1111/all.14439
REFERENCES
- 1. Organization WHO . Clinical management of severe acute respiratory infection (SARI) when COVID‐19 disease is suspected. Interim guidance V 1.2. 2020.
- 2. Dong X, Cao YY, Lu XX, et al. Eleven faces of coronavirus disease 2019. Allergy 2020;75:1699‐1709. 10.1111/all.14289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS‐CoV‐2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26(4):502‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol 2020;34(5):e212‐e213. [DOI] [PubMed] [Google Scholar]
- 5. Monteagudo LA, Boothby A, Gertner E. Continuous intravenous Anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatol. 2020;2(5):276‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID‐19 and Interleukin‐6 receptor (IL‐6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents 2020;55(5):105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou M, Zhang X, Qu J. Coronavirus disease 2019 (COVID‐19): a clinical update. Front Med. 2020;14(2):126‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;323(18):1824‐1836. [DOI] [PubMed] [Google Scholar]
- 9. Du YX, Chen XP. Favipiravir: pharmacokinetics and Concerns about clinical trials for 2019‐nCoV infection. Clin Pharmacol Ther. 2020. [DOI] [PubMed] [Google Scholar]
- 10. Health TMo . Turkish Ministry of Health COVID‐19 Guideline 14.04.2020. 2020.
- 11. Direzione Generale Cura della Persona SeW . Indirizzi terapeutici della Regione E‐R per il trattamento della infezione da SARS‐CoV2 (COVID‐19). 2020.
- 12. Garvey LH, Ebo DG, Mertes PM, et al. An EAACI position paper on the investigation of perioperative immediate hypersensitivity reactions. Allergy. 2019;74(10):1872‐1884. [DOI] [PubMed] [Google Scholar]
- 13. Romano A, Atanaskovic‐Markovic M, Barbaud A, et al. Towards a more precise diagnosis of hypersensitivity to beta‐lactams ‐ an EAACI position paper. Allergy. 2019;75(6):1300‐1315. [DOI] [PubMed] [Google Scholar]
- 14. Aberer W, Bircher A, Romano A, et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003;58(9):854‐863. [DOI] [PubMed] [Google Scholar]
- 15. Brockow K, Ardern‐Jones MR, Mockenhaupt M, et al. EAACI position paper on how to classify cutaneous manifestations of drug hypersensitivity. Allergy. 2019;74(1):14‐27. [DOI] [PubMed] [Google Scholar]
- 16. Gomes ER, Brockow K, Kuyucu S, et al. Drug hypersensitivity in children: report from the pediatric task force of the EAACI Drug Allergy Interest Group. Allergy. 2016;71(2):149‐161. [DOI] [PubMed] [Google Scholar]
- 17. Brockow K, Garvey LH, Aberer W, et al. Skin test concentrations for systemically administered drugs ‐ an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013;68(6):702‐712. [DOI] [PubMed] [Google Scholar]
- 18. Mayorga C, Celik G, Rouzaire P, et al. In vitro tests for drug hypersensitivity reactions: an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2016;71(8):1103‐1134. [DOI] [PubMed] [Google Scholar]
- 19. Cernadas JR, Brockow K, Romano A, et al. General considerations on rapid desensitization for drug hypersensitivity ‐ a consensus statement. Allergy. 2010;65(11):1357‐1366. [DOI] [PubMed] [Google Scholar]
- 20. Scherer K, Brockow K, Aberer W, et al. Desensitization in delayed drug hypersensitivity reactions – an EAACI position paper of the Drug Allergy Interest Group. Allergy. 2013;68(7):844‐852. [DOI] [PubMed] [Google Scholar]
- 21. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020. 10.1111/bjd.19163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henry D, Ackerman M, Sancelme E, Finon A, Esteve E. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hunt M, Koziatek C. A case of COVID‐19 pneumonia in a young male with full body rash as a presenting symptom. Clin Pract Cases Emerg Med. 2020;4(2):219‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marzano AV, Genovese G, Fabbrocini G, et al. Varicella‐like exanthem as a specific COVID‐19‐associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83(1):280‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Genovese G, Colonna C, Marzano AV. Varicella‐like exanthem associated with COVID‐19 in an 8‐year‐old girl: A diagnostic clue? Pediatr Dermatol. 2020. 10.1111/pde.14201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verdoni L, Mazza A, Gervasoni A. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riphagen S, Gomez X, Gonzalez‐Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID‐19 pandemic. Lancet. 2020;395(10237):1607‐1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones VG, Mills M, Suarez D, et al. COVID‐19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10(6):537‐540. pii: hpeds.2020‐0123 [DOI] [PubMed] [Google Scholar]
- 30. Jimenez‐Cauhe J, Ortega‐Quijano D, Prieto‐Barrios M, Moreno‐Arrones OM, Fernandez‐Nieto D. Reply to "COVID‐19 can present with a rash and be mistaken for Dengue": Petechial rash in a patient with COVID‐19 infection. J Am Acad Dermatol. 2020;S0190‐9622(20)30556‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hedou M, Carsuzaa F, Chary E, Hainaut E, Cazenave‐Roblot F, Masson RM. Comment on "Cutaneous manifestations in COVID‐19: a first perspective " by Recalcati S. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gianotti R, Veraldi S, Recalcati S, et al. Cutaneous Clinico‐Pathological Findings in three COVID‐19‐Positive Patients Observed in the Metropolitan Area of Milan, Italy. Acta Derm Venereol. 2020;100(8):adv00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Joob B, Wiwanitkit V. COVID‐19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Manalo IF, Smith MK, Cheeley J, Jacobs R. A dermatologic manifestation of COVID‐19: transient livedo reticularis. J Am Acad Dermatol. 2020;S0190‐9622(20)30558‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection‐induced chilblains: a case report with histopathological findings. JAAD Case Rep. 2020;6:489‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mazzotta F, Troccoli T, Bonifazi E. A new vasculitis at the time of COVID‐19. Eur J Pediatr Dermatol ‐ pd online. 2020;30(2):75‐78. [Google Scholar]
- 37. Fernandez‐Nieto D, Jimenez‐Cauhe J, Suarez‐Valle A, et al. Characterization of acute acro‐ischemic lesions in non‐hospitalized patients: a case series of 132 patients during the COVID‐19 outbreak. J Am Acad Dermatol. 2020;S0190‐9622(20):30996‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID‐2019 pneumonia and acro‐ischemia. Zhonghua Xue Ye Xue Za Zhi 2020;41:E006. [DOI] [PubMed] [Google Scholar]
- 39. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: A report of five cases. Transl Res. 2020;220:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y. The epidemiology, diagnosis and treatment of COVID‐19. Int J Antimicrob Agents. 2020;55(5):105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019‐nCoV). Nat Rev Drug Discov. 2020;19(3):149‐150. [DOI] [PubMed] [Google Scholar]
- 42. Jean SS, Lee PI, Hsueh PR. Treatment options for COVID‐19: The reality and challenges. J Microbiol Immunol Infect. 2020;53(3):436‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Costanzo M, De Giglio MAR, Roviello GN. SARS‐CoV‐ 2: recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr Med Chem. 2020;27. [DOI] [PubMed] [Google Scholar]
- 44. Rosa SGV, Santos WC. Clinical trials on drug repositioning for COVID‐19 treatment. Rev Panam Salud Publica. 2020;44:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jefferson T, Jones M, Doshi P, Spencer EA, Onakpoya I, Heneghan CJ. Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ. 2014;348:g2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Simin M, Brok J, Stimac D, Gluud C, Gluud LL. Cochrane systematic review: pegylated interferon plus ribavirin vs. interferon plus ribavirin for chronic hepatitis C. Aliment Pharmacol Ther. 2007;25(10):1153‐1162. [DOI] [PubMed] [Google Scholar]
- 47. Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir‐Ritonavir in Adults Hospitalized with Severe COVID‐19. N Engl J Med. 2020;382(19):1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Madruga JV, Berger D, McMurchie M, et al. Efficacy and safety of darunavir‐ritonavir compared with that of lopinavir‐ritonavir at 48 weeks in treatment‐experienced, HIV‐infected patients in TITAN: a randomised controlled phase III trial. Lancet 2007;370(9581):49‐58. [DOI] [PubMed] [Google Scholar]
- 49. Zuo W, Wen LP, Li J, Mei D, Fu Q, Zhang B. Oseltamivir induced Stevens‐Johnson syndrome/toxic epidermal necrolysis‐case report. Medicine (Baltimore). 2019;98(19):e15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gonzalez‐Ramos J, Lamas C, Bellon T, et al. Oseltamivir‐induced toxic epidermal necrolysis in a patient with Cushing's disease. Indian J Dermatol Venereol Leprol. 2019. [DOI] [PubMed] [Google Scholar]
- 51. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID‐19. N Engl J Med. 2020;382(24):2327‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brillanti S, Mazzella G, Roda E. Ribavirin for chronic hepatitis C: and the mystery goes on. Dig Liver Dis. 2011;43(6):425‐430. [DOI] [PubMed] [Google Scholar]
- 53. Lorcy S, Gaudy‐Marqueste C, Botta D, et al. Cutaneous adverse events of telaprevir/peginterferon/ribavirin therapy for chronic hepatitis C: A multicenter prospective cohort study. Ann Dermatol Venereol. 2016;143(5):336‐346. [DOI] [PubMed] [Google Scholar]
- 54. Patrk I, Morovic M, Markulin A, Patrk J. Cutaneous reactions in patients with chronic hepatitis C treated with peginterferon and ribavirin. Dermatology. 2014;228(1):42‐46. [DOI] [PubMed] [Google Scholar]
- 55. Brok J, Gluud LL, Gluud C. Meta‐analysis: ribavirin plus interferon vs. interferon monotherapy for chronic hepatitic C ‐ an updated Cochrane review. Aliment Pharmacol Ther. 2010;32(7):840‐850. [DOI] [PubMed] [Google Scholar]
- 56. Barreira P, Cadinha S, Malheiro D, da Silva JP. Delayed hypersensitivity to ribavirin confirmed by provocation test. J Investig Allergol Clin Immunol. 2014;24(6):441‐442. [PubMed] [Google Scholar]
- 57. Shindo M, Terai I. Adverse skin reactions due to ribavirin in hepatitis C combination therapy with pegylated interferon‐alpha2a. Case Rep Dermatol. 2013;5(3):379‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ladd AM, Martel‐Laferriere V, Dieterich D. Successful desensitization to ribavirin in a patient with chronic hepatitis C. J Clin Gastroenterol. 2012;46(8):716‐717. [DOI] [PubMed] [Google Scholar]
- 59. Toker O, Tal Y, Daher S, Shalit M. Ribavirin desensitization in chronic hepatitis C. Isr Med Assoc J. 2015;17(9):583‐584. [PubMed] [Google Scholar]
- 60. Corbett AH, Lim ML, Kashuba AD. Kaletra (lopinavir/ritonavir). Ann Pharmacother. 2002;36(7–8):1193‐1203. [DOI] [PubMed] [Google Scholar]
- 61. Ghosn J, Duvivier C, Tubiana R, Katlama C, Caumes E. Acute generalized exanthematous pustulosis induced by HIV postexposure prophylaxis with lopinavir‐ritonavir. Clin Infect Dis. 2005;41(9):1360‐1361. [DOI] [PubMed] [Google Scholar]
- 62. Echeverria P, Negredo E, Carosi G, et al. Similar antiviral efficacy and tolerability between efavirenz and lopinavir/ritonavir, administered with abacavir/lamivudine (Kivexa), in antiretroviral‐naive patients: a 48‐week, multicentre, randomized study (Lake Study). Antiviral Res. 2010;85(2):403‐408. [DOI] [PubMed] [Google Scholar]
- 63. Sun L, Deng X, Chen X, et al. Incidence of adverse drug reactions in COVID‐10 patients in China: An active monitoring study by hospital pharmacovigilance system. Clin Pharmacol Ther. 2020. 10.1002/cpt.1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Molina JM, Cohen C, Katlama C, et al. Safety and efficacy of darunavir (TMC114) with low‐dose ritonavir in treatment‐experienced patients: 24‐week results of POWER 3. J Acquir Immune Defic Syndr. 2007;46(1):24‐31. [DOI] [PubMed] [Google Scholar]
- 65. Buijs BS, van den Berk GE, Boateng CP, Hoepelman AI, van Maarseveen EM, Arends JE. Cross‐reactivity between darunavir and trimethoprim‐sulfamethoxazole in HIV‐infected patients. AIDS. 2015;29(7):785‐791. [DOI] [PubMed] [Google Scholar]
- 66. Marcos Bravo MC, Ocampo Hermida A, Martinez Vilela J, et al. Hypersensitivity reaction to darunavir and desensitization protocol. J Investig Allergol Clin Immunol. 2009;19(3):250‐251. [PubMed] [Google Scholar]
- 67. Lorber M, Haddad S. Hypersensitivity and desensitization to darunavir in a case of HIV infection with triple‐class drug resistance: case description and review of the literature. J Int Assoc Provid AIDS Care. 2013;12(6):378‐379. [DOI] [PubMed] [Google Scholar]
- 68. Hirschfeld G, Weber L, Renkl A, Scharffetter‐Kochanek K, Weiss JM. Anaphylaxis after Oseltamivir (Tamiflu) therapy in a patient with sensitization to star anise and celery‐carrot‐mugwort‐spice syndrome. Allergy. 2008;63(2):243‐244. [DOI] [PubMed] [Google Scholar]
- 69. Tran DH, Sugamata R, Hirose T, et al. Azithromycin, a 15‐membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process. J Antibiot (Tokyo). 2019;72(10):759‐768. [DOI] [PubMed] [Google Scholar]
- 70. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents 2020;105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71. Araujo L, Demoly P. Macrolides allergy. Curr Pharm Des. 2008;14(27):2840‐2862. [DOI] [PubMed] [Google Scholar]
- 72. Mori F, Pecorari L, Pantano S, et al. Azithromycin anaphylaxis in children. Int J Immunopathol Pharmacol. 2014;27(1):121‐126. [DOI] [PubMed] [Google Scholar]
- 73. Barni S, Butti D, Mori F, et al. Azithromycin is more allergenic than clarithromycin in children with suspected hypersensitivity reaction to macrolides. J Investig Allergol Clin Immunol. 2015;25(2):128‐132. [PubMed] [Google Scholar]
- 74. Schissel DJ, Singer D, David‐Bajar K. Azithromycin eruption in infectious mononucleosis: a proposed mechanism of interaction. Cutis. 2000;65(3):163‐166. [PubMed] [Google Scholar]
- 75. Milkovic‐Kraus S, Macan J, Kanceljak‐Macan B. Occupational allergic contact dermatitis from azithromycin in pharmaceutical workers: a case series. Contact Dermatitis. 2007;56(2):99‐102. [DOI] [PubMed] [Google Scholar]
- 76. Mendes‐Bastos P, Bras S, Amaro C, Cardoso J. Non‐occupational allergic contact dermatitis caused by azithromycin in an eye solution. J Dtsch Dermatol Ges. 2014;12(8):729‐730. [DOI] [PubMed] [Google Scholar]
- 77. An I, Demir V, Akdeniz S. Fixed drug eruption probably induced by azithromycin. Australas J Dermatol. 2017;58(4):e253‐e254. [DOI] [PubMed] [Google Scholar]
- 78. Campanon‐Toro MV, Sierra O, Moreno E, Sobrino‐Garcia M, Gracia‐Bara MT, Davila I. Acute generalized exanthematous pustulosis (AGEP) induced by azithromycin. Contact Dermatitis. 2017;76(6):363‐364. [DOI] [PubMed] [Google Scholar]
- 79. Sriratanaviriyakul N, Nguyen LP, Henderson MC, Albertson TE. Drug reaction with eosinophilia and systemic symptoms syndrome (DRESS) syndrome associated with azithromycin presenting like septic shock: a case report. J Med Case Rep. 2014;8:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Aihara Y, Ito S, Kobayashi Y, Aihara M. Stevens‐Johnson syndrome associated with azithromycin followed by transient reactivation of herpes simplex virus infection. Allergy. 2004;59(1):118. [DOI] [PubMed] [Google Scholar]
- 81. Xu L, Zhu Y, Yu J, Deng M, Zhu X. Nursing care of a boy seriously infected with Steven‐Johnson syndrome after treatment with azithromycin: A case report and literature review. Medicine (Baltimore). 2018;97(1):e9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Odemis E, Kalyoncu M, Okten A, Yildiz K. Azithromycin‐induced leukocytoclastic vasculitis. J Rheumatol. 2003;30(10):2292. [PubMed] [Google Scholar]
- 83. Pursnani A, Yee H, Slater W, Sarswat N. Hypersensitivity myocarditis associated with azithromycin exposure. Ann Intern Med. 2009;150(3):225‐226. [DOI] [PubMed] [Google Scholar]
- 84. Empedrad R, Darter AL, Earl HS, Gruchalla RS. Nonirritating intradermal skin test concentrations for commonly prescribed antibiotics. J Allergy Clin Immunol. 2003;112(3):629‐630. [DOI] [PubMed] [Google Scholar]
- 85. Won HK, Yang MS, Song WJ, et al. Determination of nonirritating concentrations of antibiotics for intradermal skin tests in Korean adults. J Allergy Clin Immunol Pract 2017;5(1):192‐194. e192. [DOI] [PubMed] [Google Scholar]
- 86. Seitz CS, Brocker EB, Trautmann A. Suspicion of macrolide allergy after treatment of infectious diseases including Helicobacter pylori: results of allergological testing. Allergol Immunopathol (Madr) 2011;39(4):193‐199. [DOI] [PubMed] [Google Scholar]
- 87. Unal D, Demir S, Gelincik A, et al. Diagnostic value of oral challenge testing in the diagnosis of macrolide hypersensitivity. J Allergy Clin Immunol Pract 2018;6:521‐527. [DOI] [PubMed] [Google Scholar]
- 88. Staso P, Leonov A. Drug desensitization in 17‐year‐old male with Mast Cell Activation Syndrome, pneumonia, and antibiotic hypersensitivities. AME Case Rep 2017;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sinha N, Balayla G. Hydroxychloroquine and COVID‐19. Postgrad Med J. 2020;postgradmedj‐2020‐137785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Soria A, Barbaud A, Assier H, et al. Cutaneous adverse drug reactions with antimalarials and allergological skin tests. Dermatology 2015;231(4):353‐359. [DOI] [PubMed] [Google Scholar]
- 91. Matsuda T, Ly NTM, Kambe N, et al. Early cutaneous eruptions after oral hydroxychloroquine in a lupus erythematosus patient: A case report and review of the published work. J Dermatol. 2018;45(3):344‐348. [DOI] [PubMed] [Google Scholar]
- 92. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)‐results of a multinational case‐control study (EuroSCAR). Br J Dermatol. 2007;157(5):989‐996. [DOI] [PubMed] [Google Scholar]
- 93. Volpe A, Marchetta A, Caramaschi P, Biasi D, Bambara LM, Arcaro G. Hydroxychloroquine‐induced DRESS syndrome. Clin Rheumatol. 2008;27(4):537‐539. [DOI] [PubMed] [Google Scholar]
- 94. Schmutz JL, Barbaud A, Trechot P. Hydroxychloroquine and DRESS. Ann Dermatol Venereol. 2008;135(12):903. [DOI] [PubMed] [Google Scholar]
- 95. Girijala RL, Siddiqi I, Kwak Y, Wright D, Patel DB, Goldberg LH. Pustular DRESS syndrome secondary to hydroxychloroquine with EBV reactivation. J Drugs Dermatol. 2019;18(2):207‐209. [PubMed] [Google Scholar]
- 96. Perez‐Ezquerra PR, de Barrio FM, de Castro Martinez FJ, Ruiz Hornillos FJ, Prieto GA. Delayed hypersensitivity to hydroxychloroquine manifested by two different types of cutaneous eruptions in the same patient. Allergol Immunopathol (Madr). 2006;34(4):174‐175. [DOI] [PubMed] [Google Scholar]
- 97. Phillips‐Howard PA, Warwick BJ. Idiosyncratic reaction resembling toxic epidermal necrolysis caused by chloroquine and maloprim. Br Med J (Clin Res Ed). 1988;296(6636):1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kanny G, Renaudin JM, Lecompte T, Moneret‐Vautrin DA. Chloroquine hypersensitivity syndrome. Eur J Intern Med. 2002;13(1):75‐76. [DOI] [PubMed] [Google Scholar]
- 99. Murphy M, Carmichael AJ. Fatal toxic epidermal necrolysis associated with hydroxychloroquine. Clin Exp Dermatol. 2001;26(5):457‐458. [DOI] [PubMed] [Google Scholar]
- 100. Cameron MC, Word AP, Dominguez A. Hydroxychloroquine‐induced fatal toxic epidermal necrolysis complicated by angioinvasive rhizopus. Dermatol Online J. 2014;20(11). 13030/qt1q90q0h5 [PubMed] [Google Scholar]
- 101. Lisi P, Assalve D, Hansel K. Phototoxic and photoallergic dermatitis caused by hydroxychloroquine. Contact Dermatitis. 2004;50(4):255‐256. [DOI] [PubMed] [Google Scholar]
- 102. Meier H, Elsner P, Wuthrich B. Occupationally‐induced contact dermatitis and bronchial asthma in a unusual delayed reaction to hydroxychloroquine. Hautarzt. 1999;50(9):665‐669. [DOI] [PubMed] [Google Scholar]
- 103. Charfi O, Kastalli S, Sahnoun R, Lakhoua G. Hydroxychloroquine‐induced acute generalized exanthematous pustulosis with positive patch‐testing. Indian J Pharmacol. 2015;47(6):693‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mates M, Zevin S, Breuer GS, Navon P, Nesher G. Desensitization to hydroxychloroquine–experience of 4 patients. J Rheumatol. 2006;33(4):814‐816. [PubMed] [Google Scholar]
- 105. Caramaschi P, Barbazza R, Tinazzi I, Biasi D. Desensitization to hydroxychloroquine: 4 cases. J Rheumatol. 2011;38(10):2267; author reply 2267. [DOI] [PubMed] [Google Scholar]
- 106. Tal Y, Maoz Segal R, Langevitz P, Kivity S, Darnizki Z, Agmon‐Levin N. Hydroxychloroquine desensitization, an effective method to overcome hypersensitivity‐a multicenter experience. Lupus. 2018;27(5):703‐707. [DOI] [PubMed] [Google Scholar]
- 107. Barailler H, Milpied B, Chauvel A, et al. Delayed hypersensitivity skin reaction to hydroxychloroquine: Successful short desensitization. J Allergy Clin Immunol Pract. 2019;7(1):307‐308. [DOI] [PubMed] [Google Scholar]
- 108. Rowane M, Schend J, Patel J, Hostoffer R Jr. Rapid desensitization of hydroxychloroquine. Ann Allergy Asthma Immunol. 2020;124(1):97‐98. [DOI] [PubMed] [Google Scholar]
- 109. Donado CD, Diez EM. Successful desensitization for hydroxychloroquine anaphylaxis. J Rheumatol. 2010;37(9):1975‐1976. [DOI] [PubMed] [Google Scholar]
- 110. Perez‐Sanchez N, Esponda‐Juarez K, Cimarra Alvarez M, Aleo Lujan E, Toledano Martinez E, Fernandez‐Rivas MM. Short desensitization in an adolescent with hydroxychloroquine anaphylaxis. Pediatr Allergy Immunol. 2014;25(8):819‐821. [DOI] [PubMed] [Google Scholar]
- 111. Rothan HA, Stone S, Natekar J, Kumari P, Arora K, Kumar M.The FDA‐ approved gold drug Auranofin inhibits novel coronavirus (SARS‐COV‐2) replication and attenuates inflammation in human cells. https://www.biorxiv.org/content/10.1101/2020.04.14.041228v1 [DOI] [PMC free article] [PubMed]
- 112. Morgenstern B, Michaelis M, Baer PC, Doerr HW, Cinatl J Jr. Ribavirin and interferon‐beta synergistically inhibit SARS‐associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun. 2005;326(4):905‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS‐CoV. Nat Commun. 2020;11(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Vazquez‐Lopez F, Manjon‐Haces JA, Perez‐Alvarez R, Perez‐Oliva N. Eczema‐like lesions and disruption of therapy in patients treated with interferon‐alfa and ribavirin for chronic hepatitis C: the value of an interdisciplinary assessment. Br J Dermatol. 2004;150(5):1046‐1047; author reply 1047. [DOI] [PubMed] [Google Scholar]
- 115. Kerl K, Negro F, Lubbe J. Cutaneous side‐effects of treatment of chronic hepatitis C by interferon alfa and ribavirin. Br J Dermatol. 2003;149(3):656. [DOI] [PubMed] [Google Scholar]
- 116. Calabresi PA, Kieseier BC, Arnold DL, et al. Pegylated interferon beta‐1a for relapsing‐remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double‐blind study. Lancet Neurol. 2014;13(7):657‐665. [DOI] [PubMed] [Google Scholar]
- 117. Milkiewicz P, Yim C, Pache I, Heathcote J. Diffuse skin reaction in patient with hepatitis B, treated with two different formulations of pegylated interferon. Can J Gastroenterol. 2005;19(11):677‐678. [DOI] [PubMed] [Google Scholar]
- 118. Cottoni F, Bolognini S, Deplano A, et al. Skin reaction in antiviral therapy for chronic hepatitis C: a role for polyethylene glycol interferon? Acta Derm Venereol. 2004;84(2):120‐123. [DOI] [PubMed] [Google Scholar]
- 119. Poreaux C, Bronowicki JP, Debouverie M, Schmutz JL, Waton J, Barbaud A. Clinical allergy. Managing generalized interferon‐induced eruptions and the effectiveness of desensitization. Clin Exp Allergy. 2014;44(5):756‐764. [DOI] [PubMed] [Google Scholar]
- 120. Sidhu‐Malik NK, Kaplan AL. Multiple fixed drug eruption with interferon/ribavirin combination therapy for hepatitis C virus infection. J Drugs Dermatol. 2003;2(5):570‐573. [PubMed] [Google Scholar]
- 121. Conroy M, Sewell L, Miller OF, Ferringer T. Interferon‐beta injection site reaction: review of the histology and report of a lupus‐like pattern. J Am Acad Dermatol. 2008;59(2 Suppl 1):S48‐49. [DOI] [PubMed] [Google Scholar]
- 122. Brown DL, Login IS, Borish L, Powers PL. An urticarial IgE‐mediated reaction to interferon beta‐1b. Neurology 2001;56(10):1416‐1417. [DOI] [PubMed] [Google Scholar]
- 123. Kalpaklioglu FA, Baccioglu Kavut A, Erdemoglu AK. Desensitization in interferon‐beta1a allergy: a case report. Int Arch Allergy Immunol. 2009;149(2):178‐180. [DOI] [PubMed] [Google Scholar]
- 124. Cortellini G, Amadori A, Comandini T, Corvetta A. Interferon beta 1a anaphylaxis, a case report. Standardization of non‐irritating concentration for allergy skin tests. Eur Ann Allergy Clin Immunol. 2013;45(5):181‐182. [PubMed] [Google Scholar]
- 125. Sakatani A, Doi Y, Matsuda T, et al. Protracted anaphylaxis developed after peginterferon alpha‐2a administration for chronic hepatitis C. World J Gastroenterol. 2015;21(9);2826‐2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Meller S, Erhardt A, Auci A, Neumann NJ, Homey B. Drug‐induced exanthema caused by pegylated interferon‐alpha 2b. Hautarzt. 2003;54(10):992‐993. [DOI] [PubMed] [Google Scholar]
- 127. Taghavi SA, Eshraghian A. Successful interferon desensitization in a patient with chronic hepatitis C infection. World J Gastroenterol. 2009;15(33):4196‐4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA‐approved drug ivermectin inhibits the replication of SARS‐CoV‐2 in vitro. Antiviral Res. 2020;178:104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ngwasiri CA, Abanda MH, Aminde LN. Ivermectin‐induced fixed drug eruption in an elderly Cameroonian: a case report. J Med Case Rep. 2018;12(1):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kerneuzet I, Blind E, Darrieux L, Moreau S, Safa G. Ivermectin‐induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. JAAD Case Rep. 2018;4(6):524‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Aroke D, Tchouakam DN, Awungia AT, Mapoh SY, Ngassa SN, Kadia BM. Ivermectin induced Steven‐Johnsons syndrome: case report. BMC Res Notes. 2017;10(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Seegobin K, Bueno E, Maharaj S, Ashby T, Brown M, Jones L. Toxic epidermal necrolysis after ivermectin. Am J Emerg Med. 2018;36(5):887‐889. [DOI] [PubMed] [Google Scholar]
- 133. Kelleni MT. Nitazoxanide/azithromycin combination for COVID‐19: A suggested new protocol for COVID‐19 early management. Pharmacol Res. 2020;30:104874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Regnier Galvao V, Castells MC. Hypersensitivity to biological agents‐ Updated diagnosis, management and treatment. J Allegy Clin Immunol Pract. 2015;3:174‐185. [DOI] [PubMed] [Google Scholar]
- 135. Nakamura M, Tokura Y. Tocilizumab‐induced erythroderma. Eur J Dermatol. 2009;19(3):273‐274. [DOI] [PubMed] [Google Scholar]
- 136. Yoshiki R, Nakamura M, Tokura Y. Drug eruption induced by IL‐6 receptor inhibitor tocilizumab. J Eur Acad Dermatol Venereol. 2010;24(4):495‐496. [DOI] [PubMed] [Google Scholar]
- 137. Soyer O, Demir S, Bilginer Y, et al. Severe hypersensitivity reactions to biological drugs in children with rheumatic diseases. Pediatr Allergy Immunol. 2019;30(8):833‐840. [DOI] [PubMed] [Google Scholar]
- 138. Koc R, Sonmez HE, Cakan M, et al. Drug reactions in children with rheumatic diseases receiving parenteral therapies: 9 years' experience of a tertiary pediatric rheumatology center. Rheumatol Int. 2020;40(5):771‐776. [DOI] [PubMed] [Google Scholar]
- 139. Rocchi V, Puxeddu I, Cataldo G, et al. Hypersensitivity reactions to tocilizumab: role of skin tests in diagnosis. Rheumatology (Oxford). 2014;53(8):1527‐1529. [DOI] [PubMed] [Google Scholar]
- 140. Tetu P, Hamelin A, Moguelet P, Barbaud A, Soria A. Management of hypersensitivity reactions to Tocilizumab. Clin Exp Allergy. 2018;48(6):749‐752. [DOI] [PubMed] [Google Scholar]
- 141. Cortellini G, Mascella F, Simoncelli M, et al. Effective desensitization to tocilizumab in delayed hypersensitivity reaction. Pharmacology. 2018;102(1–2):114‐116. [DOI] [PubMed] [Google Scholar]
- 142. Ben Said B, Gerfaud‐Valentin M, Seve P. Fatal DRESS syndrome under tocilizumab treatment for seronegative polyarthritis. J Allergy Clin Immunol Pract. 2018;6(3):1048‐1049. [DOI] [PubMed] [Google Scholar]
- 143. Zuelgaray E, Domont F, Peiffer‐Smadja N, Saadoun D, Cacoub P. Tocilizumab‐induced drug reaction with eosinophilia and systemic symptoms syndrome in adult‐onset still disease. Ann Intern Med. 2017;167(2):141‐142. [DOI] [PubMed] [Google Scholar]
- 144. Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double‐blind, placebo‐controlled trial. Lancet. 2016;387(10031):1921‐1927. [DOI] [PubMed] [Google Scholar]
- 145. Izquierdo JH, Bonilla‐Abadia F, Ochoa CD, Agualimpia A, Tobon GJ, Canas CA. Acute generalized exanthematous pustulosis due to tocilizumab in a rheumatoid arthritis patient. Case Rep Rheumatol. 2012;2012:517424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Yasuoka R, Iwata N, Abe N, et al. Risk factors for hypersensitivity reactions to tocilizumab introduction in systemic juvenile idiopathic arthritis. Mod Rheumatol. 2019;29(2):324‐327. [DOI] [PubMed] [Google Scholar]
- 147. den Broeder AA, de Jong E, Franssen MJ, Jeurissen ME, Flendrie M, van den Hoogen FH. Observational study on efficacy, safety, and drug survival of anakinra in rheumatoid arthritis patients in clinical practice. Ann Rheum Dis. 2006;65(6):760‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kaiser C, Knight A, Nordström D, Petterson T, Fransson J, Robertsson EF. Injection‐site reactions upon Kineret (anakinra) administration: experiences and explanations. Rheumatol Int. 2012;32:295‐299. 10.1007/s00296-011-2096-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Soyyigit S, Kendirlinan R, Aydin O, Celik GE. Successful desensitization with anakinra in a case with immediate hypersensitivity reaction. Ann Allergy Asthma Immunol. 2014;113(3):325‐326. [DOI] [PubMed] [Google Scholar]
- 150. Desai D, Goldbach‐Mansky R, Milner JD, et al. Anaphylactic reaction to anakinra in a rheumatoid arthritis patient intolerant to multiple nonbiologic and biologic disease‐modifying antirheumatic drugs. Ann Pharmacother. 2009;43(5):967‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Yılmaz I, Turk M, Nazik BS. Successful rapid subcutaneous desensitization to anakinra in a case with a severe immediate‐type hypersensitivity reaction. Eur Ann Allergy Clin Immunol. 2018;50(2):94‐96. [DOI] [PubMed] [Google Scholar]
- 152. Emmi G, Silvestri E, Cantarini L, et al. Rapid desensitization to anakinra‐related delayed reaction: Need for a standardized protocol. J Dermatol. 2017;44(8):981‐982. [DOI] [PubMed] [Google Scholar]
- 153. Bendele A, Colloton M, Vrkljan M, Morris J, Sabados K. Cutaneous mast cell degranulation in rats receiving injections of recombinant human interleukin‐1 receptor antagonist (rhIL‐1ra) and/or its vehicle: possible clinical implications. J Lab Clin Med. 1995;125(4):493‐500. [PubMed] [Google Scholar]
- 154. Lu CC, Chen MY, Chang YL. Potential therapeutic agents against COVID‐19: What we know so far? J Chin Med Assoc. 2020;83(6):534‐536. 10.1097/JCMA.0000000000000318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wells AF, Parrino J, Mangan EK, et al. Immunogenicity of sarilumab monotherapy in patients with rheumatoid arthritis who were inadequate responders or intolerant to disease‐modifying antirheumatic drugs. Rheumatol Ther. 2019;6:339‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Grom AA, Horne A, De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol. 2016;12(5):259‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Favalli EG, Biggioggero M, Maioli G, Caporali R. Baricitinib for COVID‐19: a suitable treatment? Lancet Infect Dis. 2020; 10.1016/S1473-3099(20)30262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Stebbing J, Phelan A, Griffin I, et al. COVID‐19: combining antiviral and anti‐inflammatory treatments. Lancet Infect Dis. 2020;20(4):400‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Fournier JB, Cummings F, Cannella J. Drug‐associated skin lesions in a patient with myelofibrosis receiving ruxolitinib. Dermatol Online J. 2014;20(10). pii: 13030/qt2jg3q02x. [PubMed] [Google Scholar]
- 160. Koumaki D, Koumaki V, Lagoudaki E, Bertsias G. Palmoplantar pustulosis‐like eruption induced by baricitinib for treatment of rheumatoid athritis. Eur J Case Rep Intern Med. 2019;7(1):001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Doohan BJ, Cranwell WC, Varigos GA, De Cruz R. An urticarial drug eruption caused by tofacitinib for alopecia universalis. Dermatol Ther. 2019;32:e12933. [DOI] [PubMed] [Google Scholar]
- 162. Shibata T, Muto J, Hirano Y, et al. Palmoplantar pustulosis‐like eruption following tofacitinib therapy for juvenile idiopathic arthritis. Ohshima, JAAD Case Rep. 2019;5:518‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. de Wilde AH, Zevenhoven‐Dobbe JC, van der Meer Y, et al. Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol. 2011;92:2542‐2548. 10.1099/vir.0.034983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Volcheck GW, Van Dellen RG. Anaphylaxis to intravenous cyclosporine and tolerance to oral cyclosporine: case report and review. Ann Allergy Asthma Immunol. 1998;80(2):159‐163. [DOI] [PubMed] [Google Scholar]
- 165. Kang SY, Sohn KH, Lee JO, Kim SH, Cho SH, Chang YS. Intravenous tacrolimus and cyclosporine induced anaphylaxis: what is next? Asia Pac Allergy. 2015;5(3):181‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ebo DG, Piel GC, Conraads V, Stevens WJ. IgE‐mediated anaphylaxis after first intravenous infusion of cyclosporine. Ann Allergy Asthma Immunol. 2001;87(3):243‐245. [DOI] [PubMed] [Google Scholar]
- 167. Deftereos SG, Siasos G, Giannopoulos G, et al. The Greek study in the Effects of Colchicine in Covid‐19 complications prevention (GRECCO‐19 study): rationale and study design. Hellenic J Cardiol J. 2020; S1109‐9666(20)30061‐0. 10.1016/j.hjc.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Mochida K, Teramae H, Hamada T. Fixed drug eruption due to colchicine. Dermatology. 1996;192:61. [DOI] [PubMed] [Google Scholar]
- 169. Levinger U, Monselise A. Reporting a desensitization protocol for colchicine treatment. Clin Exp Rheumatol. 2001;19(5 Suppl 24):S79. [PubMed] [Google Scholar]
- 170. Barbaud A, Trechot P, Reichert‐Penetrat S, Commun N, Schmutz JL. Relevance of skin tests with drugs in investigating cutaneous adverse drug reactions. Contact Dermatitis. 2001;45(5):265‐268. [DOI] [PubMed] [Google Scholar]
- 171. Diurno F, Numis FG, Porta G, et al. Eculizumab treatment in patients with COVID‐19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24(7):4040‐4047. [DOI] [PubMed] [Google Scholar]
- 172. Misawa S, Kuwabara S, Sato Y, et al. Safety and efficacy of eculizumab in Guillain‐Barre syndrome: a multicentre, double‐blind, randomised phase 2 trial. Lancet Neurol. 2018;17(6):519‐529. [DOI] [PubMed] [Google Scholar]
- 173. Rondeau E, Cataland SR, Al‐Dakkak I, Miller B, Webb NJA, Landau D. Eculizumab safety: five‐year experience from the global atypical hemolytic uremic syndrome registry. Kidney Int Rep. 2019;4(11):1568‐1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lo R, Alexander S, Moss J, Siddiqi A, Liu A. Eculizumab hypersensitivity and desensitization in a toddler with atypical hemolytic uremic syndrome. J Allergy Clin Immunol Pract. 2019;7(7):2409‐2410. [DOI] [PubMed] [Google Scholar]
- 175. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet 2020;395(10223):473‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhou W, Liu Y, Tian D, et al. Potential benefits of precise corticosteroids therapy for severe 2019‐nCoV pneumonia. Signal Transduct Target Ther. 2020;5(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Li T, Lu H, Zhang W. Clinical observation and management of COVID‐19 patients. Emerg Microbes Infect. 2020;9(1):687‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ventura MT, Calogiuri GF, Muratore L, et al. Cross‐reactivity in cell‐mediated and IgE‐mediated hypersensitivity to glucocorticoids. Curr Pharm Des. 2006;12(26):3383‐3391. [DOI] [PubMed] [Google Scholar]
- 179. Baeck M, Marot L, Nicolas JF, Pilette C, Tennstedt D, Goossens A. Allergic hypersensitivity to topical and systemic corticosteroids: a review. Allergy. 2009;64(7):978‐994. [DOI] [PubMed] [Google Scholar]
- 180. Baker A, Empson M, The R, Fitzharris P. Skin testing for immediate hypersensitivity to corticosteroids: a case series and literature review. Clin Exp Allergy. 2015;45(3):669‐676. [DOI] [PubMed] [Google Scholar]
- 181. Patel A, Bahna SL. Immediate hypersensitivity reactions to corticosteroids. Ann Allergy Asthma Immunol. 2015;115(3):178‐182. e173. [DOI] [PubMed] [Google Scholar]
- 182. Aranda A, Mayorga C, Ariza A, et al. IgE‐mediated hypersensitivity reactions to methylprednisolone. Allergy. 2010;65(11):1376‐1380. [DOI] [PubMed] [Google Scholar]
- 183. Venturini M, Lobera T, del Pozo MD, Gonzalez I, Blasco A. Immediate hypersensitivity to corticosteroids. J Investig Allergol Clin Immunol. 2006;16(1):51‐56. [PubMed] [Google Scholar]
- 184. Rachid R, Leslie D, Schneider L, Twarog F. Hypersensitivity to systemic corticosteroids: an infrequent but potentially life‐threatening condition. J Allergy Clin Immunol. 2011;127(2):524‐528. [DOI] [PubMed] [Google Scholar]
- 185. Li PH, Wagner A, Thomas I, Watts TJ, Rutkowski R, Rutkowski K. Steroid allergy: clinical features and the importance of excipient testing in a diagnostic algorithm. J Allergy Clin Immunol Pract. 2018;6(5):1655‐1661. [DOI] [PubMed] [Google Scholar]
- 186. Angel‐Pereira D, Berges‐Gimeno MP, Madrigal‐Burgaleta R, Urena‐Tavera MA, Zamora‐Verduga M, Alvarez‐Cuesta E. Successful rapid desensitization to methylprednisolone sodium hemisuccinate: a case report. J Allergy Clin Immunol Pract. 2014;2(3):346‐348. [DOI] [PubMed] [Google Scholar]
- 187. Guvenir H, Misirlioglu ED, Aydin F, Ece D, Cakar N, Kocabas CN. Successful methylprednisolone desensitization in a pediatric patient. Pediatr Allergy Immunol. 2017;28(3):305‐306. [DOI] [PubMed] [Google Scholar]
- 188. Barbaud A, Waton J. Systemic allergy to corticosteroids: clinical features and cross reactivity. Curr Pharm Des. 2016;22(45):6825‐6831. [DOI] [PubMed] [Google Scholar]
- 189. Padial A, Posadas S, Alvarez J, et al. Nonimmediate reactions to systemic corticosteroids suggest an immunological mechanism. Allergy. 2005;60(5):665‐670. [DOI] [PubMed] [Google Scholar]
- 190. Zhai Z, Li C, Chen Y, et al. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020;120:937‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Bircher AJ, Harr T, Hohenstein L, Tsakiris DA. Hypersensitivity reactions to anticoagulant drugs: diagnosis and management options. Allergy. 2006;61(12):1432‐1440. [DOI] [PubMed] [Google Scholar]
- 192. Trautmann A, Seitz CS. Heparin allergy: delayed‐type non‐IgE‐mediated allergic hypersensitivity to subcutaneous heparin injection. Immunol Allergy Clin North Am. 2009;29(3):469‐480. [DOI] [PubMed] [Google Scholar]
- 193. Bank I, Libourel EJ, Middeldorp S, Van Der Meer J, Buller HR. High rate of skin complications due to low‐molecular‐weight heparins in pregnant women. J Thromb Haemost. 2003;1(4):859‐861. [DOI] [PubMed] [Google Scholar]
- 194. Tan E, Thompson G, Ekstrom C, Lucas M. Non‐immediate heparin and heparinoid cutaneous allergic reactions: a role for fondaparinux. Intern Med J. 2018;48(1):73‐77. [DOI] [PubMed] [Google Scholar]
- 195. Kim KH, Lynfield Y. Enoxaparin‐induced generalized exanthem. Cutis. 2003;72(1):57‐60. [PubMed] [Google Scholar]
- 196. Seitz CS, Brocker EB, Trautmann A. Management of allergy to heparins in postoperative care: subcutaneous allergy and intravenous tolerance. Dermatol Online J. 2008;14(9):4. [PubMed] [Google Scholar]
- 197. Rodriguez‐Fernandez A, Sanchez‐Dominguez M, Torrado‐Espanol I, Noguerado‐Mellado B, Rojas‐Perez‐Ezquerra P. Clinical patterns of heparin allergy: cross‐reactivity between low‐molecular‐weight heparins and unfractionated heparins. J Investig Allergol Clin Immunol. 2019;29(2):132‐134. [DOI] [PubMed] [Google Scholar]
- 198. Ronceray S, Dinulescu M, Le Gall F, Polard E, Dupuy A, Adamski H. Enoxaparin‐induced DRESS syndrome. Case Rep Dermatol. 2012;4(3):233‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Bidaki R, Saeidi SA, Zarch MB. Delirious state and agitation following heparin induced Stevens‐Johnson syndrome. J Clin Diagn Res. 2017;11(5):VL01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Greinacher A, Potzsch B, Amiral J, Dummel V, Eichner A, Mueller‐Eckhardt C. Heparin‐associated thrombocytopenia: isolation of the antibody and characterization of a multimolecular PF4‐heparin complex as the major antigen. Thromb Haemost. 1994;71(2):247‐251. [PubMed] [Google Scholar]
- 201. Greinacher A. Heparin‐Induced Thrombocytopenia. N Engl J Med. 2015;373(19):1883‐1884. [DOI] [PubMed] [Google Scholar]
- 202. Martel N, Lee J, Wells PS. Risk for heparin‐induced thrombocytopenia with unfractionated and low‐molecular‐weight heparin thromboprophylaxis: a meta‐analysis. Blood. 2005;106(8):2710‐2715. [DOI] [PubMed] [Google Scholar]
- 203. Berkun Y, Haviv YS, Schwartz LB, Shalit M. Heparin‐induced recurrent anaphylaxis. Clin Exp Allergy. 2004;34(12):1916‐1918. [DOI] [PubMed] [Google Scholar]
- 204. Anders D, Trautmann A. Allergic anaphylaxis due to subcutaneously injected heparin. Allergy Asthma Clin Immunol. 2013;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Cesana P, Scherer K, Bircher AJ. Immediate type hypersensitivity to Heparins: two case reports and a review of the literature. Int Arch Allergy Immunol. 2016;171(3–4):285‐289. [DOI] [PubMed] [Google Scholar]
- 206. Leguisamo S, Prados Castano M, Pinero Saavedra M, Cimbollek S. Recurrent anaphylaxis due to enoxaparin. J Invest Allergol Clin Immunol. 2015;25(4):297‐299. [PubMed] [Google Scholar]
- 207. Caballero MR, Fernandez‐Benitez M. Allergy to heparin: a new in vitro diagnostic technique. Allergol Immunopathol (Madr). 2003;31(6):324‐328. [DOI] [PubMed] [Google Scholar]
- 208. Schindewolf M, Lindhoff‐Last E, Ludwig RJ, Boehncke WH. Heparin‐induced skin lesions. Lancet. 2012;380(9856):1867‐1879. [DOI] [PubMed] [Google Scholar]
- 209. Parekh K, Burkhart HM, Hatab A, Ross A, Muller BA. Heparin allergy: successful desensitization for cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2005;130(5):1455‐1456. [DOI] [PubMed] [Google Scholar]
- 210. Patriarca G, Rossi M, Schiavino D, et al. Rush desensitization in heparin hypersensitivity: a case report. Allergy. 1994;49(4):292‐294. [DOI] [PubMed] [Google Scholar]
- 211. Gresele P, Momi S, Falcinelli E. Anti‐platelet therapy: phosphodiesterase inhibitors. Br J Clin Pharmacol. 2011;72(4):634‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Tonew E, Indulen MK, Dzeguze DR. Antiviral action of dipyridamole and its derivatives against influenza virus A. Acta Virol. 1982;26(3):125‐129. [PubMed] [Google Scholar]
- 213. Fata‐Hartley CL, Palmenberg AC. Dipyridamole reversibly inhibits mengovirus RNA replication. J Virol 2005;79(17):11062‐11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Liu X, Li Z, Liu S, et al. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID‐19. Acta Pharm Sin B. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Salava A, Alanko K, Hyry H. Dipyridamole‐induced eczematous drug eruption with positive patch test reaction. Contact Dermatitis. 2012;67(2):103‐104. [DOI] [PubMed] [Google Scholar]
- 216. Angelides S, Van der Wall H, Freedman SB. Acute reaction to dipyridamole during myocardial scintigraphy. N Engl J Med. 1999;340(5):394. [DOI] [PubMed] [Google Scholar]
- 217. Weinmann P, Moretti JL, Leynadier F. Anaphylaxis‐like reaction induced by dipyridamole during myocardial scintigraphy. Am J Med. 1994;97(5):488. [DOI] [PubMed] [Google Scholar]
- 218. Pichler WJ. Immune pathomechanism and classification of drug hypersensitivity. Allergy. 2019;74(8):1457‐1471. [DOI] [PubMed] [Google Scholar]
- 219. Trautmann A, Benoit S, Goebeler M, Stoevesandt J. !Treating through! Decision and follow‐up in antibiotic therapy‐associated exanthemas. J Allergy Clin Immunol Pract. 2017;5(6):1650‐1656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


