To the Editor
At the present time, the whole world is faced with coronavirus disease 2019 (COVID‐19). Cutaneous manifestations in these patients are being increasingly reported, including rash, acrocyanosis or urticaria. 1 Exanthemas in COVID‐19 patients are becoming frequent in our daily practice, and they pose a challenge regarding their pathogenesis.
We present a retrospective case series of twelve adult patients (6 male/6 female) with a mean age of 66,3 years (47–79). All patients had pneumonia and nasopharyngeal swab PCR positive for SARS‐CoV‐2 and had received treatment for COVID‐19 per protocol established. Table 1 shows the characteristics of these patients.
Table 1.
Characteristics of 12 patients with atypical exanthemas and eosinophilia
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | Case 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics |
Female, 64 y.o. Asthma, HT |
Male, 79 y.o. HT, DL, CKD |
Female, 74 y.o. | Male, 47 y.o. |
Female, 74 y.o. HT, DL |
Female, 61 y.o. HT, DL |
Male, 67 y.o. HT, DL, CKD |
Male, 76 y.o. HT |
Female, 61 y.o. Asthma |
Male, 71 y.o. HT |
Male, 59 y.o. |
Female 62 y.o. HT, DL |
| Treatment received for COVID‐19 | HC, LP/RT, IFN‐β, CF | HC, LP/RT, IFN‐β, CF | HC, LP/RT, IFN‐β, CF |
HC LP/RT, IFN‐β, CF, AZ, DXM, TZ |
HC, LP/RT, IFN‐β, AZ, MP | HC LP/RT, IFN‐β, CF, |
HC LP/RT, IFN‐β, MP, piperacillin/ tazobactam |
HC, LP/RT, CF, AZ, MP | HC, LP/RT, CF |
HC LP/RT, IFN‐β, CF, AZ, DXM TZ, RDS |
HC LP/RT, IFN‐β, CF, DXM, TZ |
HC LP/RT, CF, AZ, MP, TZ |
| Time from hospital admission to exanthema onset | 14 days | 28 days | 23 days | 24 days | 18 days | 10 days | 19 days | 19 days | 22 days | 21 days | 26 days | 21 days |
| COVID disease status at exanthema onset | Improvement |
Worsening. Reintroduction of drugs |
Improvement | Improvement | Improvement | Improvement | Worsening | Improvement | Improvement | Improvement | Improvement | Improvement |
| Clinical presentation |
Fever Generalized maculopapular confluent exanthema Targetoid lesions Facial oedema Itch +++ |
No fever Generalized maculopapular confluent exanthema Violaceous lesions Targetoid lesions Itch + |
Fever Generalized maculopapular confluent exanthema Violaceous lesions Targetoid lesions Facial oedema Itch ++ |
No fever Generalized maculopapular confluent exanthema Violaceous lesions Targetoid lesions Itch ++ |
No fever Generalized maculopapular confluent exanthema Itch ++ |
No fever Generalized maculopapular confluent exanthema Itch +++ |
No fever Generalized maculopapular confluent exanthema Itch ++ |
No fever Generalized maculopapular confluent exanthema Itch + |
Low‐grade fever Generalized maculopapular confluent exanthema Violaceous lesions Facial oedema Itch ++ |
No fever Generalized maculopapular confluent exanthema Violaceous lesions Itch + |
No fever Generalized maculopapular confluent exanthema Itch + |
No fever Generalized maculopapular confluent exanthema Violaceous lesions Itch ++ |
| Analytical results |
Eos 1500/μL Lymp 3100/μL ALTa 29 U/L |
Eos 1400/μL Lymp 1000/μL ALT 50 U/L |
Eos 1400/μL Lymp 2600/μL ALT 56 U/L |
Eos 2300/μL Lymp 2100/μL ALT 153 U/L |
Eos 1200/μL Lymp 2000/μL ALT 68 U/L |
Eos 800/μL Lymp 2000/μL ALT 34 U/L |
Eos 1000/μL Lymp 1300/μL ALT 32 U/L |
Eos 700/μL Lymp 900/μL ALT 30 U/L |
Eos 1600/μL Lymp 1800μL ALT 23 U/L |
Eos 900/μL Lymp 1100μL ALT 20 U/L |
Eos 1000/μL Lymp 800μL ALT 36 U/L |
Eos 600/μL Lymp 900/μL ALT 495 U/L |
| Treatment | MP (IV): 40mg BID | Prednisone (PO): 40 mg OD | MP (IV): 30 mg BID | MP (IV): 20mg OD | MP aceponate (top.) BID | MP aceponate (top.) BID | MP aceponate (top.) BID | MP aceponate (top.) BID | Prednisone (PO): 30 mg OD | MP aceponate (top.) BID | MP aceponate (top.) BID | MP (IV): 40mg BID |
Reference ranges are as follows: eosinophils 0 to 500 per microlitre; lymphocytes 1300 to 1500 per microlitre and ALT 5 to 41 U/L. y.o. denotes years old, HT hypertension, DL dyslipidemia, CKD chronic kidney disease, HC hydroxychloroquine, LP/RT Lopinavir/Ritonavir, CF ceftriaxone, AZ azithromycin, MP methylprednisolone, TZ tocilizumab, RDS remdesivir Eos eosinophils, Lymp lymphocytes, IV intravenous, BID Bis in die; PO Per os, OD Omnie die.
All patients developed an itching papular exanthema after an average of 20,4 days (10–28) from their admission. At the exanthema onset, all the drugs had already been discontinued; therefore, topical corticosteroids were prescribed. However, the exanthema showed a cephalocaudal progression and confluence with islands of sparing in all cases. Seven patients developed violaceous‐areas and/or target‐like (Fig. 1) lesions; of them, three developed fever and facial oedema. In one patient, the progression of the cutaneous lesions coincided with reintroduction of hydroxychloroquine and lopinavir/ritonavir. Cutaneous biopsy was performed in two of these patients: one of them showed a superficial perivascular inflammation with eosinophils and the other showed a lichenoid pattern with eosinophils. Both two were compatible with drug reaction.
Figure 1.
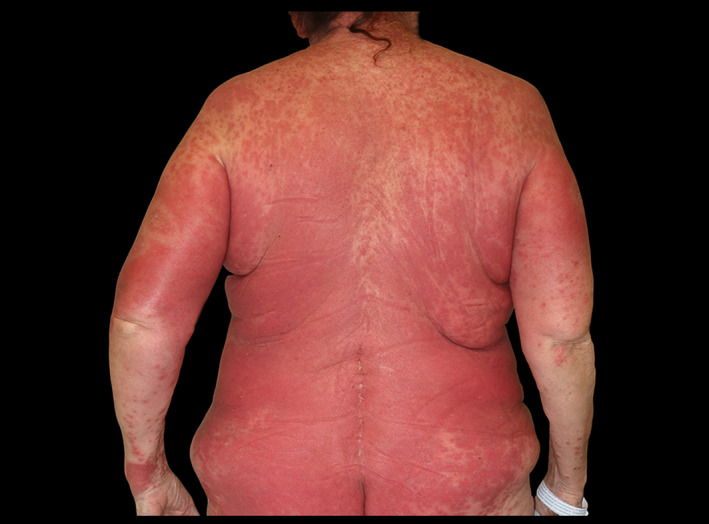
Generalized maculopapular confluent exanthema with targetoid lesions.
Systemic corticosteroids were prescribed in six patients with violaceous areas, starting at 0.5–1 mg/kg and then tapered over the ensuing 2–4 weeks with progressive improvement. The other cases improved with topical corticosteroids.
It has been suggested that underlying viral infections may increase the risk of adverse drug reactions. The association of viral infections and drug reactions has been described in many clinical situations, such as the ampicillin rash in infectious mononucleosis or the increased risk of drug reactions in AIDS patients. 2 In DRESS syndrome, viral reactivation (especially HHV‐6) is a characteristic feature. Antiviral immune responses may facilitate drug allergy development, and several biological mechanisms have been proposed for this effect, including excessive production of proinflammatory cytokines, which has been observed in COVID‐19. 3 , 4
The presence of exanthema and eosinophilia suggests a drug reaction in our patients. DRESS syndrome, although unusual, has been reported related to hydroxychloroquine but it has not been described with lopinavir/ritonavir. 5 Other drug reactions have also been reported with the treatments used for COVID‐19 management. However, the high frequency we are observing these reactions in the COVID‐19 pandemic make us think that SARS‐CoV‐2 infection may have a role in their pathogenesis. We suggest that several exanthemas may result from interaction between antiviral immune response and drugs. Nevertheless, more studies are needed to confirm this hypothesis. We must be cautious until then. It would be therefore strongly recommended that all COVID‐19 patients with exanthema and eosinophilia were investigated for drug sensitization.
We suggest that systemic corticosteroids should be considered in those exanthemas that progress to violaceous areas or target‐like lesions, since in our experience topical corticosteroids have not been able to achieve an improvement in these cases.
References
- 1. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. Journal of the European Academy of Dermatology and Venereology. 2020; 34: 5. 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 2. Shiohara T, Kano Y. A complex interaction between drug allergy and viral infection. Clin Rev Allergy Immunol 2007; 33:124–133. [DOI] [PubMed] [Google Scholar]
- 3. Shiohara T, Inaoka M, Kano Y. Drug‐induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int Off J Jpn Soc Allergol 2006; 55:1–8. [DOI] [PubMed] [Google Scholar]
- 4. Mehta P, McAuley DF, Brown M et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet Lond Engl 2020; 395:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Randhawa A. Wylie G. A case of an acute cutaneous drug reaction with hydroxychloroquine. Scott Med J 2018; 63:91–94. [DOI] [PubMed] [Google Scholar]
Acknowledgement
The patients in this manuscript have given written informed consent to publication of their case details.


