Abstract
The coronavirus disease 2019 (COVID-19) pandemic caused by SARS coronavirus 2 (SARS-CoV-2) has caused significant morbidity and mortality for patients and stressed healthcare systems worldwide. The clinical features, disease course, and serologic response of COVID-19 among immunosuppressed patients such as solid organ transplant (SOT) recipients, who are at presumed risk for more severe disease, are not well characterized. We describe our institutional experience with COVID-19 among 10 SOT patients, including the clinical presentation, treatment modalities, and outcomes of 7 renal transplant recipients, 1 liver transplant recipient, 1 heart transplant recipient, and 1 lung transplant recipient. In addition, we report the serologic response in SOT recipients, documenting a positive IgG response in all 7 hospitalized patients. We also review the existing literature on COVID-19 in SOT recipients to consolidate the current knowledge on COVID-19 in the SOT population for the transplant community.
KEYWORDS: antibody biology, clinical research/practice, infection and infectious agents – viral, infectious disease
Abbreviations: ACE/ARB, ACE inhibitor/angiotensin receptor blocker; ALF, assisted living facility; BMI, body mass index; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CRRT, continuous renal replacement therapy; CTX, ceftriaxone; CVD, cerebrovascular disease; DDRT, deceased donor renal transplant; DM, diabetes mellitus; EUA, emergency use authorization; GGO, ground-glass opacities; HCQ, hydroxychloroquine; HFrEF, heart failure with reduced ejection fraction; HLD, hyperlipidemia; HTN, hypertension; LDH, lactate dehydrogenase; LDLT, living donor liver transplant; LPV/r, lopinavir/ritonavir; LRRT, living related renal transplant; LURT, living unrelated renal transplant; MMF, mycophenolate mofetil; MPS, mycophenolate sodium; NH/PI, Native Hawaiian or Pacific Islander; OHT, orthotopic heart transplant; RA, rheumatoid arthritis; RCT, randomized controlled trial; rRT-PCR, real-time reverse transcriptase–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SNF, skilled nursing facility; SOT, solid organ transplant; UCSF, University of California San Francisco; URI, upper respiratory symptoms
1. INTRODUCTION
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to a global pandemic with > 11 million reported cases and > 500 000 deaths.1 , 2 Clinical coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 varies from asymptomatic infection to critical illness with acute respiratory distress syndrome.3 , 4 Clinical manifestations include fever, fatigue, myalgias, dry cough, dyspnea, anosmia, and dysgeusia.5 , 6
Along with comorbidities such as hypertension, diabetes, cardiovascular disease, and chronic lung or kidney disease, malignancy is an identified risk factor for severe COVID-19 disease.4 , 7 , 8 However, the clinical presentation and disease course among other immunocompromised patients, including solid organ transplant (SOT) recipients, are not well characterized. Although SOT recipients with other respiratory virus infections often exhibit severe lower respiratory tract infection,9 the association between COVID-19 and intense cytokine release10 raises the possibility that immunosuppression may actually temper the exuberant inflammatory response in severe disease. Furthermore, despite interest in using SARS-CoV-2 serology to improve diagnosis and predict immunity, it is unknown whether SOT recipients will mount an antibody response against SARS-CoV-2.
This study aims to build our understanding of COVID-19 disease in the SOT population. We present the clinical features of COVID-19 in 10 SOT recipients at our institution and describe the SARS-CoV2 serologic response in the 7 hospitalized SOT recipients.
2. MATERIALS AND METHODS
2.1. Study subjects and setting
Adult SOT recipients (age ≥18 years) cared for at the University of California San Francisco (UCSF) and diagnosed with COVID-19 by RNA testing were identified via comprehensive standard clinical reporting to the UCSF SOT Program from March 9, 2020, to April 28, 2020. This study was approved under UCSF IRB protocols #20-30629 and #10-02598. Data on demographics, medical history, clinical results, treatment, and outcomes were extracted from the electronic medical record.
During the study period, our institutional treatment approach was to enroll patients with COVID-19 lower respiratory tract infection into clinical trials, if possible. The main trial has been the National Institute of Allergy and Infectious Diseases phase 2 adaptive, randomized, double-blind, placebo-controlled trial of the investigational antiviral drug remdesivir (NCT04280705). Patients not qualifying for the study with moderate-to-severe hypoxemia were considered for either compassionate use remdesivir, hydroxychloroquine, or convalescent plasma. All admitted COVID-19 patients were provided aggressive supportive care.
2.2. Laboratory testing
COVID-19 RNA testing of nasopharyngeal and pooled nasopharyngeal/oropharyngeal swab samples was performed using a real-time reverse transcriptase–polymerase chain reaction (rRT-PCR) assay based on a US Centers for Disease Control and Prevention assay approved by U.S. Food and Drug Administration Emergency Use Authorization (EUA).11
SARS-CoV-2 IgG serology was performed using an Abbott chemiluminescent microparticle immunoassay detecting IgG antibodies to the nucleocapsid protein of SARS-CoV-2 approved under FDA EUA12 where a chemiluminescent reaction measured as relative light units is used to calculate an index value. At a predefined index value threshold of 1.4 for positivity, this assay performs with an analytical specificity of 99.5%. Compared to SARS-CoV-2 PCR, the assay has a positive percent agreement of 91.2% and 100.0% at >7 and ≥14 days from symptom onset, respectively, and a negative percent agreement of 99.6%. Results of serologic testing were not reported clinically. Other laboratory and microbiology testing were conducted as part of standard medical care at the discretion of the clinical team.
3. CASE SERIES
We identified 10 SOT recipients at our institution with COVID-19 infection ( Table 1).
TABLE 1.
Demographics and clinical details of the 10 solid organ transplant recipients with COVID-19
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||||
| Age (y) | 47 | 73 | 77 | 61 | 71 | 52 | 50 | 42 | 44 | 80 |
| Gender | Male | Male | Male | Female | Female | Male | Female | Male | Male | Female |
| Race/ethnicity | Black | Unknown | Black | Latino | Asian | Asian | Latino | NH/PI | Black | White |
| Transplant details | ||||||||||
| Type | Kidney (LRRT) | Kidney (LRRT) | Kidney (DDRT) | Kidney (DDRT) | Kidney (DDRT) | Kidney (LURT) | Bilateral Lung | Heart (OHT) | Kidney (DDRT) | Liver (LDLT) |
| Years from transplant | 7.4 | 4.8 | 10.8 | 0.33 | 0.42 | 1.1 | 4.8 | 7.8 | 9.3 | 14.2 |
| Rejection last 3 mo | No | No | No | No | No | No | No | No | No | No |
| Comorbidities | DM, HTN, CVD | CAD, DM, HTN, HLD | CAD, HFrEF, sarcoidosis | DM, HLD | CAD, DM, CVD | DM, HTN, HLD, hypothyroid | RA, DM, hypothyroid | CKD, HTN, OSA, gout, hypothyroid | HTN, CKD | CAD, DM, HTN, CKD, asthma, hypothyroid, dementia |
| BMI (kg/m2) | 27.8 | 27.4 | 18.0 | 20.4 | 20.0 | 28.6 | 36.1 | 49.4 | 23.6 | 36.6 |
| Medications | ||||||||||
| ACE/ARB use | No | No | No | No | No | No | No | No | No | No |
| Immunosuppression | Tac 0.5 mg bid, MMF 1 g bid, pred 5 mg qd | Tac 1 mg bid, MMF 750 mg bid | Tac 3 mg bid, MMF 500 mg bid, pred 5 mg qd | Tac 1.5/2 g bid, MMF 500 mg bid, pred 5 mg qd (thymo induction) | Tac 0.5/1 mg bid, MMF 500 mg bid, pred 5 mg qd (thymo induction) | Tac 1 mg bid, MMF 1 g bid, pred 5 mg qd | Tac 1.5 mg bid, MMF 360 mg bid, pred 7.5 qd | Tac 9 mg bid, MMF 250 mg bid | Tac 6 mg bid, MPS 540 mg bid, pred 5 mg qd | Tac 0.5 mg bid, MMF 500 mg bid |
| Clinical presentation | ||||||||||
| Recent travel | No | No | No | No | No | No | No | No | No | No |
| COVID contacts | No | Maybe (SNF) | No | No | No | No | Yes (family) | No | No | Maybe (ALF) |
| Symptom duration(d) | 14 | 21 | 2 | 3 | 14 | 3 | 5 | 4 | 6 | 1 |
| Fever (subjective) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | |
| Fever (°C, initial) | 38.3 | 36.5 | 36.2 | n/a | 38.3 | 36.9 | 37.5 | 37.7 | 37.7 | 36.8 |
| Fever (°C, Tmax) | 39.4 | 38.6 | 38.2 | n/a | 39.5 | n/a | 39.5 | 39.1 | 39.3 | n/a |
| Other symptoms | Dry cough, dyspnea, myalgia | Dry cough, dyspnea, myalgia, chest pain, fatigue, diarrhea, anosmia, dysgeusia | Fatigue | Productive cough | Productive cough, fatigue, anosmia, dysgeusia | Dry cough, myalgia, fatigue, nasal congestion | Dry cough, dyspnea, myalgia | Dry cough, dyspnea, myalgia, fatigue, diarrhea | Dry cough, dyspnea, diarrhea | Dyspnea |
| Laboratory findingsa | ||||||||||
| WBC count (×109/L) | 5.8 | 3.6 | 5.6 | 5.3 | 2.8 | 4.0 | 4.9 | 4.6 | 4.3 | 4.9 |
| Lymphocyte (×109/L) | 0.70 | 1.48 | 0.73 | 0.16 | 0.08 | 0.16 | 0.57 | 1.11 | 0.2 | 0.54 |
| Platelets (×109/L) | 275 | 89 | 226 | 335 | 174 | 280 | 133 | 117 | 141 | 103 |
| Creatinine (mg/dL) | 1.10 | 1.06 | 3.21 | 0.75 | 0.82 | 1.14 | 1.10 | 5.23 | 2.93 | 1.53 |
| AST/ALT (U/L) | 15/10 | 43/37 | 52/58 | 21/16 | 23/20 | 16/17 | 14/16 | 24/16 | 19/15 | 21/14 |
| Troponin (µg/L) | <0.02 | 0.03 | 0.14 | n/a | n/a | n/a | <0.02 | <0.02 | n/a | 0.02 |
| CRP (mg/L) | 176.9 | 48.6 | 35.6 | n/a | 22.6 | n/a | 208.9 | 92.5 | 135 | n/a |
| LDH (U/L) | 275 | n/a | 340 | n/a | n/a | 145 | 353 | 234 | n/a | 172 |
| Procalcitonin (µg/L) | 0.11 | 0.03 | 0.31 | n/a | 0.07 | n/a | 0.05 | 0.30 | n/a | n/a |
| D-dimer (ng/mL) | n/a | n/a | n/a | n/a | 1276 | n/a | 9725 | 408 | 543 | 1020 |
| Microbiology testing | ||||||||||
| COVID RNA | d14(+), d33(+), d38(−), d39(+), d42(−) | d21(+) | d2(+), d11(−) | d4(+) | d14(+) | d3(+) | d5(+), d10(+), d33(+), d37(−), d39(−) | d4(+), d10(−), d11(−) | d7(+) | d-4(+) |
| Influenza/RSV PCR | neg | n/a | neg | n/a | n/a | n/a | neg | n/a | n/a | n/a |
| Extended viral panelb | neg | neg | n/a | n/a | neg | n/a | neg | neg | n/a | n/a |
| Sputum culture | neg | n/a | n/a | n/a | n/a | n/a | neg | n/a | Klebsiella | n/a |
| Blood cultures | neg | neg | neg | n/a | neg | n/a | neg | neg | neg | n/a |
| Imaging | ||||||||||
| Chest X-ray | Bilat nodular opacities | Clear | Clear | n/a | Bilat patchy opacities | Clear | Clear | Chronic bilat opacities | Patchy infiltrate left | Left midlung opacity |
| Chest CT | Bilat GGO, nodular consolidation | n/a | n/a | n/a | n/a | n/a | Bilateral GGO, consolidation | Bilateral GGO, consolidation | n/a | n/a |
| Complications | ||||||||||
| Hospital admission | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | No |
| ICU (time from sx, d) | Yes (14) | No | No | No | No | No | Yes (10) | No | Yes (11) | No |
| Other complications | ARDS, shock, AKI (resolved) | None | AKI (resolved) | None | None | None | ARDS, shock, DVT | AKI (persistent) | ARDS, shock, AKI (CRRT), DVT | AKI |
| Therapies | ||||||||||
| Supplemental O2 (L) | Yes | No | No | No | Yes (2L) | No | Yes | Yes (3L) | Yes | Yes (2L) |
| Mech ventilation | Yes | No | No | No | No | No | Yes | No | Yes | No |
| Time from sx (d) | 16 | 17 | 19 | |||||||
| Duration (d) | 11 | 13 | 8+ | |||||||
| Required proning | No | Yes | Yes | |||||||
| Extubated | Yes | Yes | No | |||||||
| Is decreased | Yes - tac and MMF held | Yes - tac decreased, MMF held | Yes - MMF held | Yes - tac decreased | Yes - MMF decreased | No | Yes - MMF held | Yes - MMF held | Yes - tac, MMF held | No |
| Antivirals | RCT | None | None | None | None | None | RCT, then HCQ | HCQ | HCQ/azithro, LPV/r | None |
| Antibiotics | cefepime/doxy 5d, mero 3d | vanc/pip-tazo/azithro 1d | CTX/doxy 1d | None | None | None | CTX/azithro 6d, vanc/mero 5d | CTX/doxy x 5d | vanc/CTX/doxy 7d, vanc/mero 7d | Cefuroxime/doxy x 7d |
| Steroids or biologics | None | None | None | None | None | None | Stress dose steroids (shock) | None | Tocilizumab, methylpred | None |
| Convalescent plasma | No | No | No | No | No | No | No | No | Yes | No |
| Outcomes | ||||||||||
| Discharged ICU | Yes | n/a | n/a | n/a | n/a | n/a | Yes | n/a | No | n/a |
| Discharged hospital | Yes (LOS 29d) | Yes (LOS 7 d) | Yes (LOS 11 d) | n/a | Yes (LOS 10 d) | n/a | No | Yes (LOS 16) | No | n/a |
| Died | No | No | No | No | No | No | No | No | No | No |
| Duration follow-up (d) | 39 | 34 | 32 | 32 | 21 | 37 | 39 | 27 | 21 | 4 |
Abbreviations: ACE/ARB, ACE inhibitor/angiotensin receptor blocker; ALF, assisted living facility; BMI, body mass index; CRP, C-reactive protein; CRRT, continuous renal replacement therapy; CTX, ceftriaxone; CVD, cerebrovascular disease; DDRT, deceased donor renal transplant; DM, diabetes mellitus; GGO, ground-glass opacities; HCQ, hydroxychloroquine; HFrEF, heart failure with reduced ejection fraction; HLD, hyperlipidemia; HTN, hypertension; LDH, lactate dehydrogenase; LDLT, living donor liver transplant; LPV/r, lopinavir/ritonavir; LRRT, living related renal transplant; LURT, living unrelated renal transplant; MMF, mycophenolate mofetil; MPS, mycophenolate sodium; NH/PI, Native Hawaiian or Pacific Islander; OHT, orthotopic heart transplant; RA, rheumatoid arthritis; RCT, randomized controlled trial; SNF, skilled nursing facility; URI, upper respiratory symptoms.
Findings are the values from the day of diagnosis (for most tests) or the first available during the hospitalization.
Extended viral panel includes testing for influenza A (including subtypes H1 and H3) and B, RSV A and B, parainfluenza 1-3, metapneumovirus, adenovirus, and rhinovirus.
3.1. Demographics, transplant details, comorbidities
The median age was 56.5 years (range 42-80 years), and 6 were men. Three patients were African American, 2 were Hispanic/Latino, 2 were Asian, 1 was Native Hawaiian/Pacific Islander, and 1 was white. There were 7 kidney (cases 1-6, 9), 1 lung (case 7), 1 heart (case 8), and 1 liver (case 10) transplant recipient. The median time from transplant to presentation was 6.1 years: 2 patients (cases 4 and 5) underwent transplant within 6 months before presentation; the others were between 1.1 and 14.2 years posttransplant. None experienced recent rejection and 7 were receiving triple immunosuppression (cases 2, 8, and 10 had weaned off steroids). No patients were taking an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. All patients had underlying comorbidities (most commonly hypertension, diabetes, and cardiovascular disease), and 3 were obese (body mass index ≥30 kg/m2).
3.2. Clinical presentation
No patient had recent travel, although 3 had known or possible contact with a COVID-19 case. The median duration of symptoms at presentation was 4.5 days (range 1-21 days). Of the 7 hospitalized patients, all had objective fevers during admission, but only 2 had fever >38.0°C on presentation. Other common symptoms in these 10 patients were subjective fever (n = 8), cough (n = 8), dyspnea (n = 6), myalgias (n = 5), and fatigue (n = 5). Less common symptoms were diarrhea (n = 3) and anosmia/dysgeusia (n = 2).
3.3. Laboratory studies and microbiology
Two patients had leukopenia and 8 had lymphopenia, although 5 had baseline lymphopenia during the 3 previous months. Only 1 patient (case 3) had a marginally elevated alanine aminotransferase. Four patients (2 of whom were kidney transplant recipients) had an elevated creatinine. Of the 7 admitted patients, 2 had slightly elevated procalcitonin (0.30 and 0.31 µg/mL) and all had elevated C-reactive protein levels. Other laboratory values are reported in Table 1. No patient had a documented viral coinfection based on standard microbiology testing; case 8 had a sputum culture positive for Klebsiella late in his admission, thought to represent a ventilator associated pneumonia.
3.4. COVID-19 RNA testing
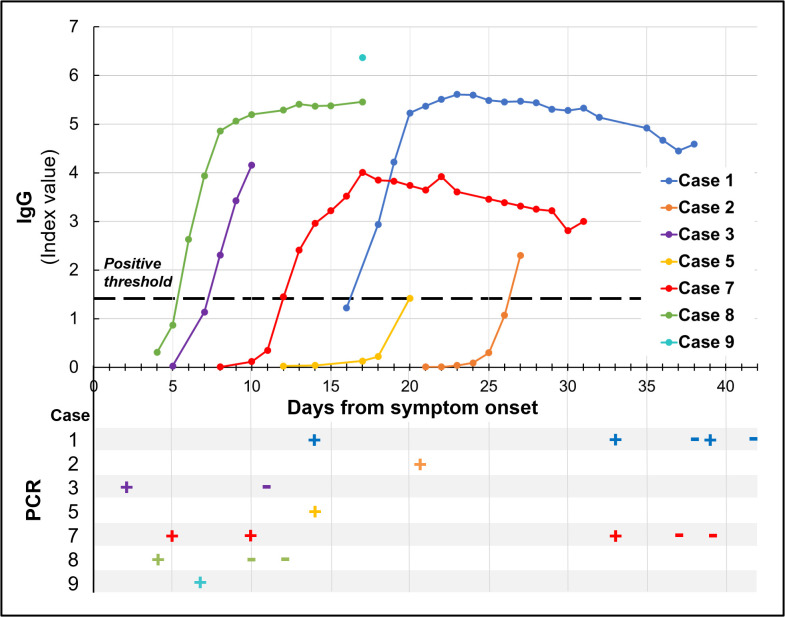
All patients had positive COVID-19 RNA testing on nasopharyngeal or pooled nasopharyngeal/oropharyngeal samples ( Figure 1). One patient (case 10) underwent asymptomatic screening at her assisted living facility where she was found to be positive; 4 days later she presented to the emergency department with dyspnea and hypoxia. All other patients were tested on presentation to care with acute symptoms. Four patients underwent repeat testing (cases 1, 3, 7, 8) as indicated in Table 1; 2 of the critically ill patients (cases 1 and 7) had repeat positive tests out to days 39 and 33, respectively. Two patients (cases 7 and 8) met our institutional criteria for discontinuation of isolation precautions (at least 14 days from symptom onset, at least 72 hours fever free without antipyretics and 2 consecutive negative COVID-19 swabs collected at least 24 hours apart) near the end of their hospitalization.
FIGURE 1.
SARS-COV-2 IgG serology and PCR results for all 7 solid organ transplant recipients requiring hospitalization. IgG serology was conducted using Abbott chemiluminescent microparticle immunoassay detecting IgG antibodies to the SARS-CoV-2 nucleocapsid protein with index value of 1.4 (dotted line) set as positive threshold. PCR was conducted on nasopharyngeal and pooled nasopharyngeal/oropharyngeal swab samples
3.5. Imaging findings
Chest X-ray (CXR) was performed in 9 patients: 5 were abnormal, with 3 showing bilateral opacities and 2 showing unilateral opacities. Computer tomography (CT) of the chest was performed in 3 patients (cases 1, 7, and 8) and all showed bilateral ground-glass opacities and consolidations.
3.6. Complications and therapies
Seven patients required hospitalization, 6 required supplemental oxygen, and 3 (cases 1, 7, and 9) required ICU admission (between days 10 and 14 after symptom onset). All intensive care unit patients developed acute respiratory distress syndrome requiring mechanical ventilation (between days 16 and 19 after symptom onset) and shock requiring vasopressors. Five patients had acute kidney injury (1 requiring renal replacement therapy) and 2 patients had a deep venous thrombosis. Cases 1 and 7 were enrolled in the randomized controlled trial of remdesivir versus placebo; case 7 was also subsequently treated with hydroxychloroquine in the setting of critical illness; and case 8 was treated with hydroxychloroquine alone. Case 9 was treated at a referring hospital with hydroxychloroquine/azithromycin, lopinavir/ritonavir, methylprednisolone, and tocilizumab; he was then treated with convalescent plasma at our institution. No other patients received antivirals, steroids, or biologics for COVID-19 (although case 7 received stress dose steroids for shock). Six of the 7 hospitalized patients received antibiotics, as did 1 outpatient. Immunosuppressive therapy was decreased in all except 2 patients when COVID-19 was diagnosed (Table 1).
3.7. Outcomes
The duration of follow-up ranged from 4 to 39 days (median 32 days). Five of the 7 hospitalized patients were discharged (median length of stay 11 days, range 7-29) and the other 2 remain hospitalized. Two of the 3 patients requiring mechanical ventilation have been successfully extubated. No patient has died as of the time of this report.
4. SEROLOGIC ANALYSIS
SARS-CoV-2 IgG serology was performed on all 7 of 10 SOT recipients with COVID-19 who were hospitalized (cases 1, 2, 3, 5, 7, 8, and 9) (Figure 1, Table S1). Patients were tested serially over 1-22 timepoints throughout the course of their illness, ranging from 4 to 38 days after symptom onset. All 7 patients had a positive SARS-CoV-2 IgG serology result, with 6 seroconverting from negative to positive at timepoints ranging from day 6 (case 8) to day 27 (case 2) from symptom onset (median 15 days). The only patient who did not seroconvert was tested once on day 17 of illness (case 9).
5. LITERATURE REVIEW
Existing literature on COVID-19 among SOT recipients is accumulating rapidly and currently consists of case series and case reports. Among these, 5 studies from China, Spain, and the United States (New York City) included > 10 patients each.13, 14, 15, 16, 17
Focusing on these 5 large series, SOT recipients were older (median age 51-72 years) and predominantly male (59%-80%). In the U.S. studies reporting race/ethnicity,16 , 17 significant proportions of patients were Hispanic (42%) or African American (22%-39%). Comorbidities including hypertension, diabetes, cardiovascular disease, chronic kidney disease, and obesity were highly prevalent. Common presenting symptoms were fever (58%-90%), dry cough (53%-90%), and diarrhea (22%-31%), with most patients exhibiting lymphopenia (67%-80%) and elevated CRP (49%-100%) on presentation. Rates of complications including intubation and intensive care unit level of care were high is most reports, including up to 39% in a New York City report of 36 kidney transplant recipients.16 Mortality among SOT recipients ranged from 7% to 28%, with the largest study of 90 SOT recipients (kidney, lung, liver, heart, heart–kidney) from New York City reporting a mortality rate of 18%.17 Although treatment of COVID-19 among SOT recipients varied significantly by study, decreased immunosuppression was a mainstay of treatment. The majority of patients had antimetabolite therapy held (53%-90%), and a smaller proportion had calcineurin inhibitor held or decreased (18%-70%). Other therapies administered included hydroxychloroquine (4 of 5 studies, 86%-91% of patients), tocilizumab (4 of 5 studies, 6%-16% of patients), boosted protease inhibitors (1 of 5 studies, 50% of patients), and IVIG (3 of 5 studies, 3%-70%).
Among the smaller case series and individual case reports, notable findings included SOT recipients with COVID-19 who were early in their posttransplant course and had favorable outcomes.18, 19, 20 Authors from Italy describe their experience with 6 liver transplant patients, among whom 3 were less than 2 years posttransplant and had mild disease, whereas the 3 who were > 10 years from transplant died.20 In addition to the significant variability in treatment, patients who received boosted protease inhibitors experienced significant drug–drug interactions and toxicity.21 Most cases of COVID-19 among SOT recipients were managed with immunosuppression reduction, but there were several case reports describing patients where immunosuppression was maintained and patients recovered.22, 23, 24
6. DISCUSSION
We report our institutional experience with 10 SOT recipients with COVID-19, who demonstrated a wide spectrum of disease from mild infection successfully managed as outpatients to severe disease requiring mechanical ventilation. We also reviewed the existing literature on COVID-19 in SOT recipients and found significant variability in clinical features and management.
Considering our cases and those reported in the literature, it is notable that the symptoms, laboratory values, and imaging in SOT recipients were similar to those of immunocompetent patients.3, 4, 5, 6 The majority of patients described here were African American, Hispanic/Latino, or Native Hawaiian/Pacific Islander. While our observations are derived from a small cohort, the racial/ethnic distribution of SOT patients in additional, larger studies will be of interest given emerging data from the United States, indicating that African American race may predispose to severe COVID-19 disease.25 Two of our SOT recipients, including 1 who was critically ill, were obese. This is slightly increased compared to the 11% prevalence of obesity among SOT recipients in Spain with COVID-19.13 Obesity is a recently described potentially risk factor for severe disease in COVID-19,26 , 27 and more research is needed to determine if this association is seen in SOT recipients as well.
It is notable that 30% of our patients required mechanical ventilation, which may indicate an increased risk of severe disease. However, despite being immunosuppressed with significant comorbidities associated with poor outcomes in COVID-19,3, 4, 5, 6 there have been no deaths among SOT recipients with COVID-19 at our institution. This result, taken in the context of high mortality rates reported among larger case series of COVID-19 in SOT recipients from the epicenters of the pandemic, may suggest the potential contribution of healthcare resource availability on patient outcomes. Further research is needed to establish the true mortality rate in SOT recipients and the role of immunosuppressive therapy in disease modulation.
We also describe SARS-CoV-2 serology in SOT recipients. Despite poor performance of many infectious disease serologic tests in this population, we found that all 7 patients assayed displayed an IgG serologic response, including a patient less than 6 months posttransplant. For 3 patients, seroconversion was documented, and occurred between days 6 and 27 after symptom onset. In the immunocompetent population, recent data suggest that most (78%-100%) patients develop a detectable IgG response 10-21 days after symptom onset.28, 29, 30, 31 A study from China reported a liver transplant patient diagnosed with COVID-19 in the immediate posttransplant period who had a “higher than baseline” COVID-19 IgG 9 days after symptom onset, and a kidney transplant recipient with IgG production 59 days after symptom onset.18 Further investigation into the dynamics of the SARS-CoV-2 serologic response is required in SOT recipients, but this early report suggests that SOT patients are able to mount an antibody response to SARS-CoV-2, which may have diagnostic and prognostic implications.
Our study has several limitations. First, this is a single-center analysis of a limited number of patients and may not be generalizable to other centers with different patient populations or treatment approaches. Second, the duration of follow-up is limited for some cases, as it has been in many clinical COVID-19 studies given the need to rapidly share knowledge in this quickly evolving pandemic. Last and most important, the role of serologic testing as a diagnostic tool and measure of immunity among SOT and other populations is currently not well defined. There is a risk of false positives, particularly with IgM testing, and a lack of clear evidence on which antibodies are surrogates of protection or potent at neutralizing virus.32
In conclusion, we report our experience with 10 SOT patients with COVID-19 and found that, despite immunosuppression, their clinical features and serologic response seem to mirror immunocompetent patients. Notably, no SOT recipients at our institution have died despite existing literature documenting increased mortality in this population, emphasizing the importance of further studies to determine SOT subgroups who may have more favorable outcomes. Additional research is urgently needed to close the knowledge gap regarding COVID-19 among SOT recipients.
ACKNOWLEDGMENTS
The authors would like to acknowledge Wei Gu, MD, and Elaine Hsu of the UCSF Department of Pathology for biobanking and John Hackett of Abbott Biosciences for running serology testing. Serologic testing and analysis were funded in part by National Institutes of Health grant R01-HL105704 (Dr Chiu) from the National Heart, Blood, and Lung Institute, the Charles and Helen Schwab Foundation (Dr Chiu), and Abbott Laboratories. No funding was provided for clinical aspects of study (case and literature review).
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr Chiu is the director of the UCSF-Abbott Viral Diagnostics and Discovery Center and receives research support in pathogen discovery from Abbott Laboratories, Inc. Dr Doernberg is a co-investigator for the Adaptive COVID-19 Treatment Trial funded by the National Institute of Allergy and Infectious Diseases (NIAID). She also receives grant support from the NIAID unrelated to this study under Award UM1AI104681. She is a consultant for Genentech and Basilea Pharmaceutica, unrelated to this report. Drs Prostko and Taylor are employees of Abbott Laboratories, Inc. The remaining authors have no conflicts of interest to disclose.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
Funding information National Institutes of Health, Grant/Award Number: R01-HL105704; National Heart, Blood, and Lung Institute; Charles and Helen Schwab Foundation; Abbott Laboratories
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Supplementary Material
REFERENCES
- 1.World Health Organization. WHO statement regarding cluster of pneumonia cases in Wuhan, China. https://www.who.int/china/news/detail/09-01-2020-who-statement-regarding-cluster-of-pneumonia-cases-in-wuhan-china. Published 2019. Accessed August 4, 2020
- 2.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow N, Fleming-Dutra K, Gierke R, et al. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 — United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manuel O, Estabrook M. RNA respiratory viral infections in solid organ transplant recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9) doi: 10.1111/ctr.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). Real-Time RT-PCR Panel for Detection 2019-Novel Coronavirus. Atlanta, GA: Centers for Disease Control and Prevention.
- 12.Abbott Laboratories. SARS-CoV-2 IgG [package insert]. 2020.
- 13.Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20(7):1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu L, Gong N, Liu B, et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77(6):748–754. doi: 10.1016/j.eururo.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Columbia University Kidney Transplant Program. Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020. 10.1681/ASN.2020030375 [DOI] [PMC free article] [PubMed]
- 16.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020:NEJMc2011117. 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed]
- 17.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US Epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong Z, Zhang Q, Xia H, et al. Clinical characteristics and immunosuppressants management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. 2020;20(7):1916–1921. doi: 10.1111/ajt.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arpali E, Akyollu B, Yelken B, Tekin S, Turkmen A, Case KB. Report: a kidney transplant patient with mild COVID-19. Transpl Infect Dis. 2020:e13296. 10.1111/tid.13296 [DOI] [PMC free article] [PubMed]
- 20.Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5(6):532–533. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ning L, Liu L, Li W, et al. Novel coronavirus (SARS-CoV-2) infection in a renal transplant recipient: case report. Am J Transplant. 2020;20(7):1864–1868. doi: 10.1111/ajt.15897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Li X, Cao G, Wu X, Wang Z, Yan T. COVID-19 in a kidney transplant patient. Eur Urol. 2020;77(6):769–770. doi: 10.1016/j.eururo.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bussalino E, De Maria A, Russo R, Paoletti E. Immunosuppressive therapy maintenance in a kidney transplant recipient SARS-CoV-2 pneumonia: a case report. Am J Transplant. 2020;20(7):1922–1924. doi: 10.1111/ajt.15920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seminari E, Colaneri M, Sambo M, et al. SARS Cov2 infection in a renal transplanted patients. A case report. Am J Transplant. 2020;20(7):1882–1884. doi: 10.1111/ajt.15902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020;1-29. 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed]
- 27.Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020. 10.1002/oby.22831 [DOI] [PMC free article] [PubMed]
- 28.Guo LI, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis. 2020;1-28. 10.1093/cid/ciaa310 [DOI] [PMC free article] [PubMed]
- 29.To K-W, Tsang O-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV- 2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease. SSRN Electron J. 2020;1-22. 10.2139/ssrn.3546052 [DOI] [PMC free article] [PubMed]
- 31.Du Z, Zhu F, Guo F, Yang B, Wang T. Detection of antibodies against SARS-CoV-2 in patients with COVID-19. J Med Virol. 2020. 10.1002/jmv.25820 [DOI] [PMC free article] [PubMed]
- 32.Infectious Diseases Society of America. IDSA COVID-19 Antibody Testing Primer. 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.