Abstract
COVID‐19 is a new viral infection that has a significant impact on global health and economy. Because of its rapid spread worldwide, it may influence the prognosis of other medical conditions, such as ST‐segment elevation myocardial infarction (STEMI). We report a case of a 58‐year female patient admitted with an infero‐posterior STEMI on the background of recently positive COVID‐19 swab. Reperfusion was attempted through primary PCI but unfortunately failed to restore coronary blood flow due to massive thrombotic burden despite several attempts of balloon dilatation and aspiration thrombectomy. She sadly died later on because of hemodynamic deterioration. This scenario raises concerns about Neutrophil Extracellular Traps (NETS) which might potentially have propagated inflammation and thrombosis via platelets' aggregation leading to enhanced coagulopathy and massive coronary thrombosis. Therefore, we suggest primary PCI as the first‐choice of revascularization in patients with combined COVID‐19 and STEMI. Additionally, we emphasize on the importance of using the potent new generation P2Y12 inhibitors along with GPIIb/IIIa inhibitors in every STEMI patient with COVID‐19 to achieve favorable conditions for primary PCI as well as favorable outcomes after stent implantation.
Keywords: acute myocardial infarction, antithrombotic treatment, neutrophil extracellular traps, primary percutaneous coronary intervention, viral infection
1. INTRODUCTION
COVID‐19 is a new mysterious viral infection that is rapidly spreading worldwide and has deleterious impacts on global health and economy. It has recently been published that COVID‐19 may directly affect the cardiovascular system. 1 There is evidence that myocardial injury may contribute to increased mortality of infected patients. 2 Impact of COVID‐19 on ST‐segment elevation myocardial infarction (STEMI) patients could be either direct with a high incidence of developing acute heart failure or indirect through the increased delay between first medical contact and primary percutaneous coronary intervention (PCI) due to the contamination measures that have recently been followed by hospitals. 3 However, it is believed that the worst prognosis would be for a patient with combined COVID‐19 and STEMI. NETS in COVID‐19 patients was believed to be the culprit behind the propagation of inflammation, cytokine release and thrombosis . 4 We report a case of a patient contracted SARS‐CoV‐2 infection then developed infero‐posterior STEMI but failed PCI due to massive coronary thrombosis.
1.1. Case series
On April 30, a 58‐year old female patient presented to the hospital with a 1 hr acute onset chest pain on the background of recently positive SARS‐CoV‐2 nose swab. ECG revealed infero‐posterior STEMI and, the patient immediately received loading doses of dual antiplatelet therapy (ticagrelor 180 mg and aspirin 300 mg) and 2.5 mg of subcutaneous fondaparinux. Revascularization was planned through primary PCI, and the patient was immediately transferred to the catheterization lab. Coronary angiography showed a massive thrombus occluding right coronary artery (Figure 1). An intra‐arterial weight‐adjusted dose of heparin was administrated. Wiring was successfully performed; however, multiple balloon dilatation failed to restore blood flow (Figure 2). Therefore, aspiration thrombectomy was performed thorough aspiration catheter (export catheter), but also failed despite severe attempts. Intravenous bolus dose injection of GP IIb/IIIa inhibitor (eptifibatide) followed by continuous infusion due to heavy thrombotic burden. Finally, a trial of thrombus aspiration was attempted using 6Fr Guideliner but was unfortunately unsuccessful (Figure 3). The decision was made to abandon the procedure and transfer the patient back to the intensive therapeutic unit (ITU) with continuous 48 hr intravenous infusion of eptifibatide as well as activated coagulation time guided therapeutic intravenous heparin followed by repeat coronary angiography. Also, ticagrelor was replaced by prasugrel (with a 60 mg loading dose). In the ITU intravenous fluids were commenced for low blood pressure.
FIGURE 1.
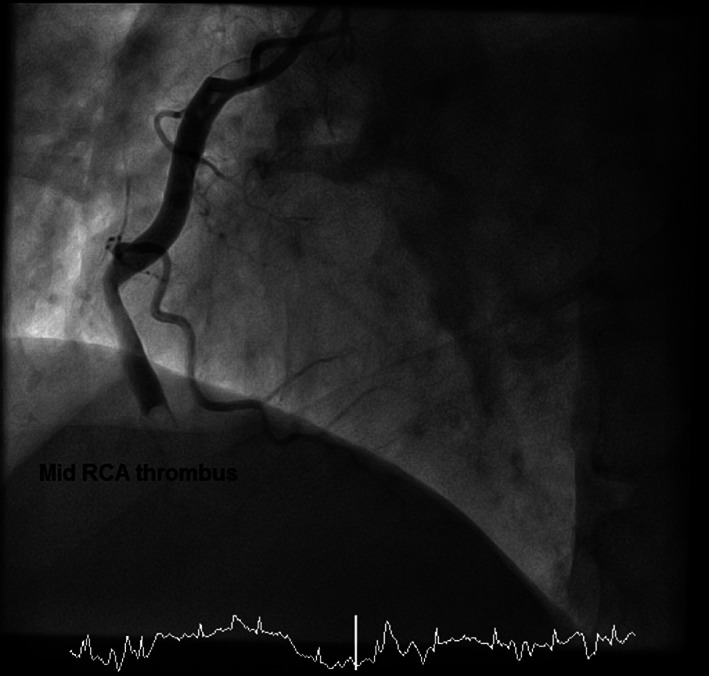
Coronary angiography image showing extensive mid segment right coronary artery (RCA) thrombus (left‐anterior‐oblique view)
FIGURE 2.
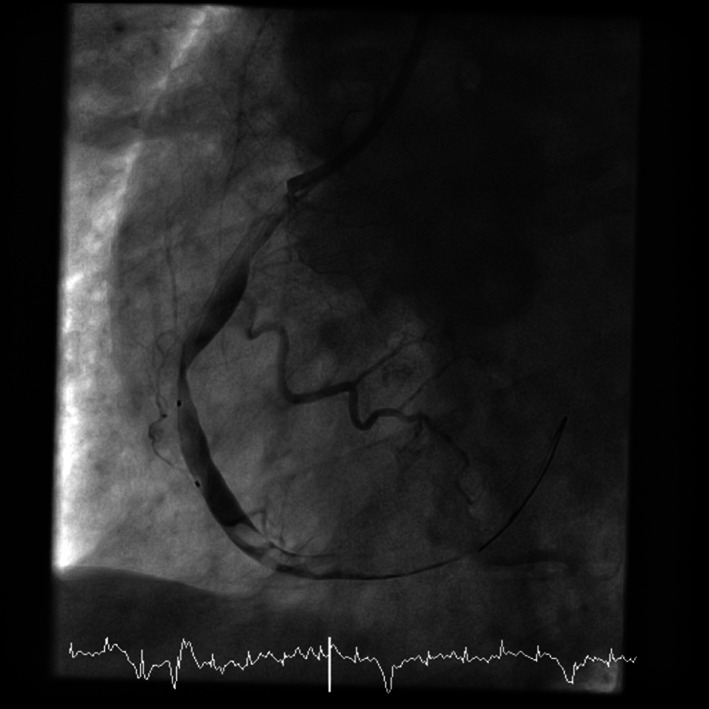
Coronary angiography image showing failed attempts of balloon dilatation to restore blood flow (left‐anterior‐oblique view)
FIGURE 3.
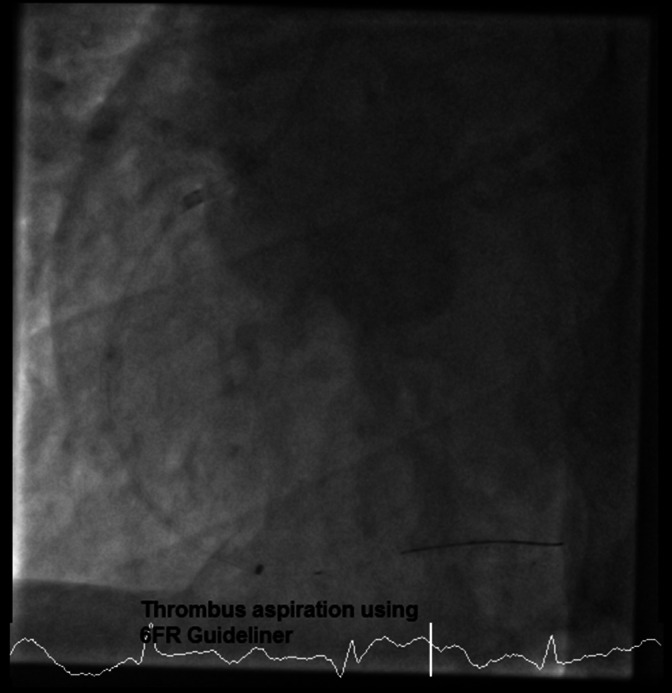
Coronary angiography image showing a trial of thrombus aspiration using a 6Fr Guideliner (left‐anterior‐oblique view)
Additionally, patient's ECG showed atrial tachycardia for which amiodarone infusion was started. Left ventricular ejection fraction was estimated at 40%. Blood results illustrated acute kidney injury (eGFR 28 ml/min, urea 32 mmol/L and creatinine 164 mmol/L), elevated C‐reactive protein elevation (225 mg/L) and mild leucocytosis (12.3 G/L, N < 10). Unfortunately, the patient finally died 48 hr later from hemodynamic and respiratory deterioration.
2. DISCUSSION
Previous observational studies have illustrated that severe bacterial or viral infection may be associated with intravascular disseminated coagulopathy with thrombocytopenia. 2 NETS are an extracellular web of chromatin, oxidant enzymes and mitochondrial proteins released by neutrophils to contain the infection; however, it might induce microvascular thrombosis and therefore have an impact on patients' coagulation. 4 Nevertheless, there is no evidence of direct action for COVID‐19 on platelet function. NETS were noticed in patients with severe ARDS and COVID‐19; it is associated with the worst prognosis. It was observed that high levels of NETS' highly specific markers were in the sera of COVID‐19 patients. 4 Both arterial and venous thrombosis will significantly increase as a result of NETS; therefore, we assume that infection with SARS‐CoV‐2 has exerted a significant role in this massive intracoronary thrombosis and unfavorable outcome. 5 The acute viral infection is associated with a significant rise in the incidence of acute myocardial infarction as a result of the acute inflammatory response and endothelial dysfunction. 6 , 7 Besides, with surging numbers of COVID‐19 patients worldwide, concomitantly with the high prevalence of coronary artery disease, it is expected to experience a significant rise in the numbers of patients with combined STEMI and COVID‐19. 8
3. CONCLUSION
Accordingly, our case raises concerns about the best strategy to deal with COVID‐19 patients presented with STEMI. We believe that the main issue is the high tendency of an infected patient to develop the highly thrombotic field and we assume exaggeration of platelet aggregation with COVID‐19 leading to enhanced coagulopathy which could be secondary to NETS. Therefore, we suggest that:
Primary PCI is the first choice in STEMI patients over fibrinolytic therapy that we believe will not be able to deal with such massive thrombus.
The potent new generation of P2Y12 inhibitors such as prasugrel should be preferred.
Upstream administration of GP IIb/IIIa inhibitors could be considered in every patient with STEMI and suspected or proved COVID‐19 infection planned for primary PCI in an attempt to achieve favorable conditions at the time of intervention for primary PCI.
We recommend continuing GP IIb/IIIa inhibitors infusion postprimary PCI to prevent acute stent thrombosis and get favorable outcomes after stent implantation.
Of note, we recommend further investigations to confirm this proposed high thrombotic tendency in patients combining STEMI and COVID‐19 and our report aims to draw attention toward this specific situation that may be frequently seen in our cardiology cath labs.
ACKNOWLEDGMENT
We would like to acknowledge our senior radiographers: Anthony Miccoli, Gina Matheson, and Judith Winnard for their assistance in the management of the patient and in gathering angiographic data for our article.
Seif S, Ayuna A, Kumar A, Macdonald J. Massive coronary thrombosis caused primary percutaneous coronary intervention to fail in a COVID‐19 patient with ST‐elevation myocardial infarction. Catheter Cardiovasc Interv. 2021;97:E667–E669. 10.1002/ccd.29050
Case was performed at Royal Albert Edward infirmary.
REFERENCES
- 1. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nature Rev Cardiol. 2020;17:259‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonow RO, Fonarow GC, O'Gara PT, Yancy CW. Association of Coronavirus Disease 2019 (COVID‐19) with myocardial injury and mortality. JAMA Cardiol. 2020;5(7):571‐573. [DOI] [PubMed] [Google Scholar]
- 3. Tam CCF, Cheung KS, Lam S, et al. Impact of coronavirus disease 2019 (COVID‐19) outbreak on ST‐segment‐elevation myocardial infarction Care in Hong Kong, China [internet]. Circ: Cardiovasc Quality Outcomes. 2020;13(4):e006631. 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zuo Y, Yalavarthi S, Shi H, et al. Neutropil extracellular traps in COVID‐19. JCI Inshight. 2020;5(11):e138999. 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prominent changes in blood coagulation of patients with SARS‐CoV‐2 infection. Clinical Chemistry and Laboratory Medicine (CCLM) 2020;58(7):1116‐1120. [DOI] [PubMed] [Google Scholar]
- 6. Lacour T, Semaan C, Genet T, Ivanes F. Insights for increased risk of failed fibrinolytic therapy and stent thrombosis associated with COVID‐19 in ST‐segment elevation myocardial infarction patients. Catheter Cardiovasc Interv. 2020;97(2):E241‐E243.. 10.1002/ccd.28948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mattila KJ. Viral and bacterial infections in patients with acute myocardial infarction. J Intern Med. 1989;225(5):293‐296. [DOI] [PubMed] [Google Scholar]
- 8. Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611‐2618. [DOI] [PubMed] [Google Scholar]


