Abstract
This study aims to explore the clinical effect of Arbidol (ARB) combined with adjuvant therapy on patients with coronavirus disease 2019 (COVID‐19). The study included 62 patients with COVID‐19 admitted to the First Hospital of Jiaxing from January to March 2020, and all patients were divided into the test group and the control group according to whether they received ARB during hospitalization. Various indexes in the two groups before and after treatment were observed and recorded, including fever, cough, hypodynamia, nasal obstruction, nasal discharge, diarrhea, C‐reactive protein (CRP), procalcitonin (PCT), blood routine indexes, blood biochemical indexes, time to achieve negative virus nucleic acid, and so on. The fever and cough in the test group were relieved markedly faster than those in the control group (P < .05); there was no obvious difference between the two groups concerning the percentage of patients with abnormal CRP, PCT, blood routine indexes, aspartate aminotransferase, and alanine aminotransferase (P > .05); the time for two consecutive negative nucleic acid tests in the test group were shorter than that in the control group; the hospitalization period of the patients in the test group and control group were (16.5 ± 7.14) days and (18.55 ± 7.52) days, respectively. ARB combined with adjuvant therapy might be able to relieve the fever of COVID‐19 sufferers faster and accelerate the cure time to some degree, hence it's recommended for further research clinically.
Keywords: adjuvant therapy, Arbidol, COVID‐19
Research Highlights
-
1.
Arbidol combined with adjuvant therapy could shorten the course of patient's basic symptoms;
-
2.
Arbidol combined with adjuvant therapy could normalize patient's temperature faster;
-
3.
Viral nucleic acid of patients treated with Arbidol combined with adjuvant therapy could turn negative within a shorter time.
1. INTRODUCTION
Coronaviruses (CoVs) (order Nidovirales, family Coronaviridae, subfamily Coronavirinae) are enveloped, positive single‐stranded RNA viruses, and they have the largest genomes for RNA viruses as their genome sizes range from 26 to 32 kilobases (kb) in length. 1 CoVs primarily infect birds and mammals, causing a variety of lethal diseases. They can also infect humans and cause diseases to vary degrees, from upper respiratory tract infections resembling the common cold to lower respiratory tract infections such as bronchitis, pneumonia, and even severe acute respiratory syndrome (SARS). 2 , 3 , 4 The virus is believed transmitted mainly via the respiratory tract, fecal‐oral transmission, or contact. 5 There are over 10 types of CoVs that have been already known so far. Historically, CoVs caused two serious infectious diseases including the SARS in 2003 and the Middle‐East Respiratory Syndrome (MERS) in 2012, 6 , 7 with a fatality rate of approximately 10% (916/8422) and 35% (750/2144), respectively. 8 , 9 Therefore, it is plain to see that CoVs pose a great threat to human life.
Since the outbreak of coronavirus disease 2019 (COVID‐19) in December 2019 in China, there were 80 303 confirmed cases by 3 March 2020, including 2949 deaths. Meanwhile, the disease spread overseas, with 10 059 confirmed cases and 160 deaths. Although the overall mortality rate of COVID‐19 is about 3.4%, which is much lower than that of SARS and MERS, COVID‐19 is extremely contagious, contributing to a fairly large infection base, hence the death toll surpassed that of SARS and MERS. The most common clinical symptoms of COVID‐19 sufferers are fever, dry cough, hypodynamia, while some patients develop nasal obstruction, nasal discharge, and diarrhea. 10 The current therapeutic scheme has mainly focused on symptomatic treatment as no specific medicine has been developed up to now. Based on the therapeutic experience of SARS and MERS, we have strived to research efficient drugs for COVID‐19 to improve patient's cure rate and survival quality.
Arbidol (ARB) is a kind of hemagglutinin (HA) inhibitor with high selectivity and is able to target HA fusion machinery and prevent CoVs from adsorbing cell surface and entering the cells. 11 Currently, ARB has been authorized for years in China and was reported by researchers to be a broad‐spectrum and multitarget antiviral drug, which plays an inhibitory effect on influenza virus, parainfluenza virus, and coxsackievirus. 12 , 13 , 14 Therefore, the application of ARB in SARS was reported and the result indicated that ARB could suppress SARS‐CoV, 14 which provides a new orientation for the treatment of COVID‐19 in our study.
In this study, we used ARB combined with adjuvant therapy for COVID‐19 sufferers and set up the control group to investigate the effect of ARB on COVID‐19 patients.
2. MATERIALS AND METHODS
2.1. Information collection
The study included 62 patients with COVID‐19 admitted to the First Hospital of Jiaxing from January to March, 2020, including 28 females and 34 males aged from 5 to 72, with most patients ranging from 48 to 63. All of them were confirmed cases of COVID‐19 by imaging examination and pathological examination upon admission. Ground‐glass opacity could be seen in imaging and virus nucleic acid (nCoV‐RNA) testing was positive. We divided them into two groups. The one who received ARB combined with adjuvant therapy was classified into the test group (n = 42), while the one who didn't was classified into the control group (n = 20). All clinical data of patients, including sex, hypertension, diabetes, CT, temperature, oxygen saturation, hemoglobin (HB) concentration, C‐reactive protein (CRP), and so on are listed in Table 1.
Table 1.
Patients' clinical data
| Features | Test group (n = 42) | Control group (n = 20) | P values |
|---|---|---|---|
| Sex | .60 | ||
| Male | 24 | 10 | |
| Female | 18 | 10 | |
| Hypertension | .70 | ||
| Yes | 8 | 3 | |
| No | 34 | 17 | |
| Diabetes | .82 | ||
| Yes | 5 | 2 | |
| No | 37 | 18 | |
| Computed tomography | .13 | ||
| Normal | 39 | 16 | |
| Abnormal | 3 | 4 | |
| Temperature, ℃ | 37.84 ± 0.78 | 37.47 ± 0.76 | .08 |
| Oxygen saturation | 97.50 ± 0.95 | 97.10 ± 1.80 | .26 |
| HB concentration, g/dL | 140.5 ± 18.04 | 136.59 ± 20.36 | .45 |
| CRP | .11 | ||
| <10 mg/L | 25 | 16 | |
| >10 mg/L | 17 | 4 | |
Abbreviations: CRP, C‐reactive protein; HB, hemoglobin.
2.2. Therapeutic scheme
2.2.1. Control group
This group primarily received symptomatic treatment. (a) Antiviral treatment: aerosol inhalation of interferon (Shering‐Plough, Shanghai, China), 5 million U or equivalent for adult, with 2 mL of sterile water for injection, twice per day. (b) For infection and inflammation (involving cough, expectoration, and wheeze): Asmeton (Compound Methoxyphenamine Capsules; Daiichi Sankyo, Shanghai, China). Usage and dosage: two pills by oral after a meal three times a day for 15 years and above; one pill by oral after a meal three times a day for children above 8 and under 15. Eucalyptol (Limonene and Pinene Enteric Soft Capsules; Johamu, Beijing, China). Usage and dosage for adult: one pill (0.3 g) by oral with cold boiled water 30 minutes before a meal three to four times a day for acute cases; one pill (0.3 g) by oral 30 minutes before a meal twice a day for chronic cases. Moxifloxacin (Moxifloxacin Hydrochloride Tablets; Bayer Healthcare, Beijing, China). Usage and dosage: 0.4 g by oral or intravenous injection every 24 hours. (c) For dyspnea: effective oxygenic therapy was given, including a nasal catheter, mask oxygen inhalation, and high‐flow nasal oxygen if necessary. (d) For fever: physical cooling therapy was chiefly adopted. Patients with a temperature of over 38.5℃ were asked to take 0.2 g of Ibuprofen sustained‐release capsules (SihuanPharm, Beijing, China) by oral. Patients with cough and expectoration were treated with Mucosolvan (ambroxol hydrochloride injection; Boehringer Ingelheim; Shanghai, China).
2.2.2. Test group
ARB was additionally added on the basis of symptomatic treatment. Patients started to take ARB as soon as they were admitted to the hospital. Arbidol Tablets (Jiangsu Wuzhong Pharmaceutical Group Corporation) was given for adults: two pills (0.2 g) by oral three times a day. β Receptor antagonists such as metoprolol and propranolol were not allowed to be used together. Drug withdrawal was recommended when the heart rate (HR) was lower than 60 beats/minutes. Symptomatic treatment would be applied if the digestive tract reaction appeared.
2.3. Therapeutic evaluation
Patient's clinical symptoms, such as fever, dry cough, nasal obstruction, nasal discharge, sore throat, hypodynamia, diarrhea, and some laboratory indexes including blood routine indexes, CRP, procalcitonin (PCT), blood biochemical indexes as well as the virus nucleic acid testing were observed and recorded during the treatment. Curative criteria: Patient's quarantine couldn't be abolished until their temperature returned to normal for over 3 days, with marked improvement in respiratory symptoms, significant absorption of inflammation showed by pulmonary imaging, and negative nucleic acid testing for two consecutive times (sampling interval was at least 1 day).
2.4. Statistical analysis
All statistical analyses were performed using SPSS 26.0. Measurement data were expressed as X ± s, and differences between the two groups were analyzed by Student's t test. Part of the enumeration data were represented as a percentage (%). Comparisons between the two groups were verified by means of the χ 2 test or Fisher's test. P < .05 was considered statistically significant.
3. RESULTS
3.1. Index changes
We recorded patient's temperature at the beginning of and during hospitalization as basically all of them had fever. The highest temperature of each patient was recorded every day. The results suggested that compared with the control group, the temperature in the test group dropped more significantly than that in the control group, and the time for back to normal was much shorter (4.98 ± 1.79 vs 6.01 ± 1.80; P = .021; Figure 1). Additionally, symptoms like dry cough of patients in the test group recovered faster than that of patients in the control group (4.39 ± 1.30 vs 5.08 ± 1.42; P = .040). While for other symptoms, such as nasal obstruction, nasal discharge, sore throat, hypodynamia, and diarrhea, there was no marked difference in recovery time between the two groups (P > .05). The details are listed in Table 2.
Figure 1.
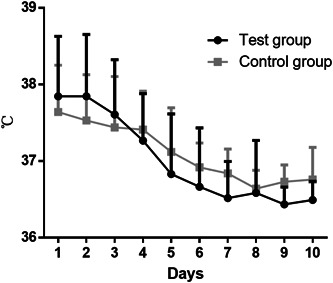
Comparison of temperature change tendency between the two groups
Table 2.
Indexes recovery time of the two groups
| Indexes | Time for recovery (d) | ||
|---|---|---|---|
| Test group (n = 42) | Control group (n = 20) | P values | |
| Fever | 4.98 ± 1.79 | 6.01 ± 1.80 | .021 |
| Dry cough | 4.39 ± 1.30 | 5.08 ± 1.42 | .040 |
| Nasal obstruction | 3.42 ± 0.85 | 3.36 ± 1.09 | .800 |
| Nasal discharge | 3.95 ± 0.27 | 3.88 ± 0.36 | .366 |
| Sore throat | 3.47 ± 1.14 | 3.91 ± 1.28 | .139 |
| Hypodynamia | 2.25 ± 0.56 | 2.11 ± 0.48 | .340 |
| Diarrhea | 3.39 ± 0.62 | 3.57 ± 0.75 | .284 |
3.2. Blood routine and blood biochemistry
In view of the fact that a number of patients had inflammation, we observed the changes in patient's blood routine indexes, CRP, PCT, and biochemical indexes and recorded the indexes which were higher or lower than the normal range. The percentage of patients with abnormal CRP and PCT in the test group were lower than those in the control group, yet the percentage of patients who developed abnormal lymphocytes in the test group was increased than that in the control group. In addition, there was no significant difference between the two groups with regard to the percentage of patients with abnormal blood routine indexes, including white blood cell (WBC), HB, platelet (PLT), and so on (P > .05). As for blood biochemical indexes, we mainly recorded alanine aminotransferase (ALT) and aspartate aminotransferase (AST) to observe whether the medication would damage the patient's liver. The result indicated that from admission to discharge, there was no significant difference between the two groups concerning the percentage of patients with abnormal ALT and AST (P > .05), which validated that adding ARB to the therapeutic scheme would cause no new damage to patient's liver. The detailed results for the above are seen in Figures 2 and 3.
Figure 2.
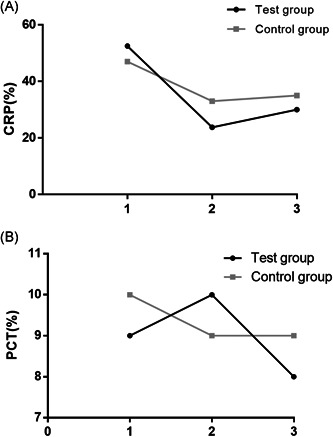
Comparison of CRP and PCT changes between the two groups. The percentage of patients with abnormal (A) CRP and (B) PCT in the test group and the control group, respectively. Normal range: CRP: (0‐10) mg/L; PCT: (0‐5) mg/L. CRP, C‐reactive protein; PCT, procalcitonin
Figure 3.
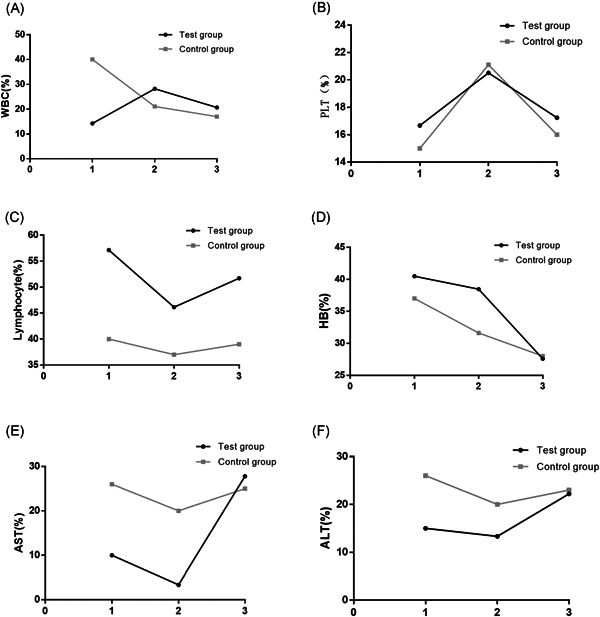
Comparison of blood routine indexes, AST and ALT changes between the two groups. The percentage of patients with abnormal (A) WBC, (B) PLT, (C) lymphocyte, (D) HB, (E) AST, and (F) ALT in the test group and the control group, respectively. Normal range: WBC: (3.5‐9.5) × 109/L; PLT: (125‐350) × 109/L; lymphocyte: (1.1‐3.2) × 109/L; HB: (115‐150) g/L; ALT: (9‐50) U/L (male), (7‐40) U/L (female); AST: (15‐40) U/L (male), (13‐35) U/L (female). ALT, alanine aminotransferase; AST, aspartate aminotransferase; HB, hemoglobin; PLT, platelet; WBC, white blood cells
3.3. Time to achieve negative nucleic acid testing and hospitalization period
Patient's virus nucleic acid was tested every day starting from their admission. We recorded every patient's time to achieve negative nucleic acid testing (including the 1st, 2nd, and 3rd time to achieve negative results, that is, the time for two consecutive negative nucleic acid tests was recorded) and their hospitalization period. The results revealed that compared with the control group, the time for patient's nucleic acid turning negative in the test group was shorter than that in the control group, especially the time to achieve 2nd and the 3rd negative result (Figure 4). The hospitalization period of the patients in the two groups was (16.5 ± 7.14) days (test group) and (18.55 ± 7.52) days (control group), respectively. Relatively, the hospitalization period in the test group was shorter, but there was no marked difference between the two groups in this aspect (P > .05).
Figure 4.
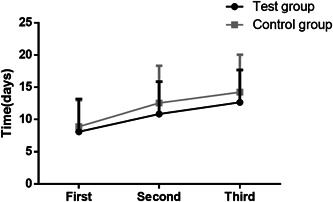
Comparison of the time for patient's nucleic acid turning negative between the two groups
3.4. Adverse drug reactions
During the treatment, patient's principal adverse drug reactions in the two groups included slowed HR (test group: n = 5, control group: n = 2), nausea (test group: n = 7, control group: n = 3), diarrhea (test group: n = 2, control group: n = 1), and dizziness (test group: n = 2, control group: n = 1). There was no significant difference between the two groups regarding the number of patients with adverse drug reactions (P > .05), which demonstrated that the application of ARB in COVID‐2019 is safe and would cause no marked adverse drug reactions.
4. CONCLUSION
Currently, antiviral, anti‐infectin, and supportive treatment are mainly applied in COVID‐19 sufferers clinically. Previously, a MERS‐related systematic review and meta‐analysis unveiled that the application of antiviral treatment at an early stage and a low age was able to reduce the mortality rate of MES patients. 15 Conducting antiviral treatment has always been thought of as the key to curation of MERS‐CoV infected sufferers. Consistently, the key to the treatment of COVID‐19 patients also lies in antiviral treatment. In this study, we mainly found that ARB combined with common systematic therapy could relieve patient's symptoms faster, including fever, cough, nasal obstruction, nasal discharge, hypodynamia, diarrhea, and so on, and accelerate patient's cure time without producing new toxic reaction and side effect.
ARB is a broad‐spectrum antiviral compound invented by Russian scientists from the Chemical‐Pharmaceutical Scientific Research Institute of Russia approximately 25 years ago and licensed in Russia and China for the prevention and treatment of human influenza and relevant postinfection complications. 16 Subsequently, it was proved to demonstrate inhibitory activity against a number of DNA/RNA viruses and enveloped/nonenveloped viruses. 17 ARB plays its antiviral role mainly by suppressing virus‐mediated fusion with the target membrane and consequently preventing virus from entering into target cells. 18 It has been found that ARB can interact with hemagglutinin (HA) to stabilize it against the low pH transition to its fusogenic state and therefore prevent HA‐mediated membrane fusion during influenza virus infection. 19 In the treatment of Hepatitis C virus (HCV), ARB interacts with HCV envelope protein and is capable of inhibiting membrane fusion to various extent. 13 , 20 Besides, the immunoregulation effect of ARB is able to interfere with macrophage activation. 21 ARB has become the candidate drug for the treatment of human virus infection due to its broad‐spectrum antiviral activity. Consistent with the prior research, ARB combined with systematic therapy in the treatment of COVID‐19 sufferers was able to relieve symptoms and shorten the course of many symptoms, and accelerate the patient's nucleic acid turning negative. It could be seen that ARB played a positive role in the treatment of COVID‐19. However, the results of this study are likely to be affected by other drugs as COVID‐19 patients applied extensive and messy medications during treatment. Consequently, more research needs to be done in the future so as to obtain more reliable results.
In conclusion, the application of ARB in the treatment of COVID‐19 sufferers is likely to shorten the course of the disease and is able to quicken patient's recovery. Hence, it's recommended that more studies should be further done clinically.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
WY and MY conducted the literature search. ZX and XD wrote the article. WY and MY performed data analysis and drafted. ZW revised the article. All authors gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
ETHICS STATEMENT
This study was conducted in accordance with Helsinki Declaration II the and was approved by the Institutional Review Boards of Affiliated Hospital of Jiaxing University/The First Hospital of Jiaxing.
ACKNOWLEDGMENTS
The authors gratefully acknowledge contributions from Jiaxing Fight Novel Coronavirus Pneumonia Emergency Technology Attack Special Project, the Key Discipline of Jiaxing Respiratory Medicine Construction Project, Jiaxing Key Laboratory of Lung Cancer Precise Treatment, Jiaxing Medical Key Discipline (infectious diseases) Project and Jiaxing Key Laboratory of Precision Treatment for Lung Cancer. The work was funded by Jiaxing Fight Novel Coronavirus Pneumonia Emergency Technology Attack Special Project in 2020 (2020GZ30001); the Key Discipline of Jiaxing Respiratory Medicine Construction Project (No.2019‐zc‐04); Jiaxing Medical Key Discipline (infectious diseases) Project (no.2019‐zc‐02); Jiaxing Key Laboratory of Precision Treatment for Lung Cancer.
Chen W, Yao M, Fang Z, Lv X, Deng M, Wu Z. A study on clinical effect of Arbidol combined with adjuvant therapy on COVID‐19. J Med Virol. 2020;92:2702–2708. 10.1002/jmv.26142
Wenyu Chen, Ming Yao, Zhixian Fang, and Xiaodong Lv contributed to the work equally.
Contributor Information
Min Deng, Email: dengmigaa@163.com.
Zhen Wu, Email: wuzhen1897621@163.com.
DATA AVAILABILITY STATEMENT
The data and materials in the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16:69. 10.1186/s12985-019-1182-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bande F, Arshad SS, Bejo MH, Moeini H, Omar AR. Progress and challenges toward the development of vaccines against avian infectious bronchitis. J Immunol Res. 2015;2015:424860. 10.1155/2015/424860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vabret A, Dina J, Gouarin S, et al. Human (non‐severe acute respiratory syndrome) coronavirus infections in hospitalised children in France. J Paediatr Child Health. 2008;44:176‐181. 10.1111/j.1440-1754.2007.01246.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gerna G, Percivalle E, Sarasini A, et al. Human respiratory coronavirus HKU1 versus other coronavirus infections in Italian hospitalised patients. J Clin Virol. 2007;38:244‐250. 10.1016/j.jcv.2006.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Special Expert Group for Control of the Epidemic of Novel Coronavirus Pneumonia of the Chinese Preventive Medicine . An update on the epidemiological characteristics of novel coronavirus pneumonia COVID‐19. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:139‐144. 10.3760/cma.j.issn.0254-6450.2020.02.002 [DOI] [PubMed] [Google Scholar]
- 6. Peiris JS, Lai ST, Poon LL, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319‐1325. 10.1016/s0140-6736(03)13077-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814‐1820. 10.1056/NEJMoa1211721 [DOI] [PubMed] [Google Scholar]
- 8. Cheng VC, Lau SK, Woo PC, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20:660‐694. 10.1128/CMR.00023-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hui DS, Azhar EI, Kim YJ, Memish ZA, Oh MD, Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18:e217‐e227. 10.1016/S1473-3099(18)30127-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID‐19 based on current evidence. J Med Virol. 2020. 10.1002/jmv.25722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blaising J, Polyak SJ, Pecheur EI. Arbidol as a broad‐spectrum antiviral: an update. Antiviral Res. 2014;107:84‐94. 10.1016/j.antiviral.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi L, Xiong H, He J, et al. Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo. Arch Virol. 2007;152:1447‐1455. 10.1007/s00705-007-0974-5 [DOI] [PubMed] [Google Scholar]
- 13. Boriskin YS, Pecheur EI, Polyak SJ. Arbidol: a broad‐spectrum antiviral that inhibits acute and chronic HCV infection. Virol J. 2006;3:56. 10.1186/1743-422X-3-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khamitov RA, Loginova S, Shchukina VN, Borisevich SV, Maksimov VA, Shuster AM. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr Virusol. 2008;53:9‐13. [PubMed] [Google Scholar]
- 15. Morra ME, Van Thanh L, Kamel MG, et al. Clinical outcomes of current medical approaches for Middle East respiratory syndrome: a systematic review and meta‐analysis. Rev Med Virol. 2018;28:e1977. 10.1002/rmv.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boriskin YS, Leneva IA, Pecheur EI, Polyak SJ. Arbidol: a broad‐spectrum antiviral compound that blocks viral fusion. Curr Med Chem. 2008;15:997‐1005. 10.2174/092986708784049658 [DOI] [PubMed] [Google Scholar]
- 17. Pécheur EI, Borisevich V, Halfmann P, et al. The synthetic antiviral drug Arbidol inhibits globally prevalent pathogenic viruses. J Virol. 2016;90:3086‐3092. 10.1128/JVI.02077-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Villalain J. Membranotropic effects of arbidol, a broad anti‐viral molecule, on phospholipid model membranes. J Phys Chem B. 2010;114:8544‐8554. 10.1021/jp102619w [DOI] [PubMed] [Google Scholar]
- 19. Leneva IA, Russell RJ, Boriskin YS, Hay AJ. Characteristics of arbidol‐resistant mutants of influenza virus: implications for the mechanism of anti‐influenza action of Arbidol. Antiviral Res. 2009;81:132‐140. 10.1016/j.antiviral.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 20. Pécheur EI, Lavillette D, Alcaras F, et al. Biochemical mechanism of hepatitis C virus inhibition by the broad‐spectrum antiviral arbidol. Biochemistry. 2007;46:6050‐6059. 10.1021/bi700181j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Q, Xiong HR, Lu L, et al. Antiviral and anti‐inflammatory activity of arbidol hydrochloride in influenza A (H1N1) virus infection. Acta Pharmacol Sin. 2013;34:1075‐1083. 10.1038/aps.2013.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials in the current study are available from the corresponding author on reasonable request.


