Abstract
Objective
To determine health perceptions of patients with rheumatic diseases in the early phase of the coronavirus disease 2019 (COVID‐19) pandemic.
Methods
Rheumatology patients at a single center received via text message the Australian Rheumatology Association COVID‐19 information sheet and an invitation to participate in a deidentified survey. Patient concerns regarding risks conferred by their rheumatologic disease or medications, impact of receiving the information sheet on the likelihood of staying on medication, and acceptance of telehealth were ascertained.
Results
A total of 2,630 patients received the text message, and the survey response rate was 21% (n = 550). The mean ± SD age of the participants was 52 ± 15.2 years, and 75.3% were female. Participants’ highest ranked concern was that their medications would increase the severity of their COVID‐19 symptoms (76.1%). The highest levels of concern were seen in patients taking combination conventional synthetic disease‐modifying antirheumatic drugs (DMARDs) and/or a biologic/targeted synthetic DMARD. There was no association between prednisolone dose and concern. While 63% of patients planned to continue their antirheumatic medications, a further 30% were more likely to continue taking their medications because of receiving the information. Telehealth was acceptable to 98.4% of patients, but 28.1% felt this was only appropriate while infection control measures were in place.
Conclusion
Concerns regarding the risk of COVID‐19 among patients taking antirheumatic drugs are common. Proactive dissemination of information is needed to address misconceptions related to medication risk, improve medication adherence, and minimize the risk of flares. Telehealth is acceptable to most patients during the COVID‐19 pandemic.
INTRODUCTION
The impact on the health care system of coronavirus disease 2019 (COVID‐19) has been tempered in some regions by the early adoption of public health measures and extensive testing 1. Health care systems have instituted major changes to achieve pandemic preparedness, such as deferment of elective surgery and nonurgent medical care, and widespread adoption of telehealth. Despite rapid developments in vaccines and therapeutics, infection control measures are likely to be in place in for a prolonged period.
Significance & Innovations.
Patients with rheumatic diseases are concerned about the risk posed by their medications, particularly those taking combination conventional synthetic disease‐modifying antirheumatic drugs (DMARDs) or a biologic/targeted synthetic DMARD.
Providing patients with information has the potential to improve adherence to immunosuppressive medication.
There is a widespread acceptance of telehealth rheumatology consultation in lieu of face‐to‐face appointments in the early phase of the coronavirus disease 2019 pandemic.
Evidence is lacking regarding the benefit of a proactive approach in communicating relevant advice regarding COVID‐19 to patients to minimize preventable adverse outcomes (2). The need to understand the perceptions of patients with rheumatic disease is integral to developing a strategy of communication. The objectives of the current study were to investigate the concerns of rheumatology patients regarding COVID‐19, to evaluate the potential impact of proactive dissemination of patient advice, and to assess the acceptance of telehealth.
PATIENTS AND METHODS
Patients and recruitment
Monash Health is a large tertiary hospital system in Australia. Patients of the rheumatology service who had an appointment in the preceding or subsequent 12 months were identified. Mobile telephone numbers of patients were extracted without identifying information. Patients were sent a text message identified as being from the Monash Health Department of Rheumatology, which directed them to the Australian Rheumatology Association (ARA) COVID‐19 information sheet (version 3, March 25, 2020) and an invitation to complete an anonymous online survey.
ARA COVID‐19 information sheet
The information sheet describes the known prognostic factors for COVID‐19, advises patients against stopping their medications unless they are unwell, reassures patients that there has been no data to date demonstrating increased mortality in rheumatology patients, and provides advice on next steps if patients were to become unwell. The information sheet also provided health advice for the general public and reinforced the importance of vaccinations.
Patient survey
Baseline demographics that were collected included age, sex, rheumatologic diagnosis, disease duration, current medications, current dose of prednisolone, and patient perception of disease control (reported on a visual analog scale [VAS], range 0–100). The VAS was annotated with anchoring instructions, where 0 = poorly controlled, 50 = reasonable, but could be better, and 100 = well‐controlled).
Patients were asked if they believed that their rheumatologic disease or medications increased their risk of contracting or becoming more unwell with COVID‐19. Patients who reported being concerned about their medications were asked to identify the medications of concern.
The survey assessed whether patients had already obtained advice regarding their medications and, if so, from what source. Patients were asked if they found the ARA patient information to be relevant and/or helpful, if receiving the information affected their intention to stay on their medications, and what information they would like included in future iterations. Acceptance of telehealth was assessed by asking patients about the situations in which a telehealth consultation was acceptable to them. Patients were able to check as many options as applicable and suggest alternatives.
Statistical analysis
Univariate binomial regression analysis was used to determine associations between exposure variables and patient perception regarding the COVID‐19 risk associated with their rheumatologic diagnosis and treatment. Exposure variables were age, sex, patient perception of disease control, disease duration, and diagnosis or treatment strategy. Exposure variables with a P value less than 0.25 (predetermined threshold) were included in multivariate analyses. Descriptive analysis was used to report concerns regarding specific medications, perceptions of telehealth, sources of information regarding COVID‐19, and impact of the ARA patient information sheet.
Statistical analyses were conducted using SPSS, version 23.0. Ethics approval was obtained from Monash Health (RES‐20‐0000‐217Q).
RESULTS
Of the eligible patients, 4% were not able to be contacted via text message as their mobile telephone numbers were not available. The survey response rate was 21% (n = 550 of 2,630 respondents) with a mean completion time of 11 minutes. The mean ± SD age of respondents was 52 ± 15.2 years, and 75.3% were female. The most common self‐reported diagnoses were rheumatoid arthritis (RA) (29.7%) and systemic lupus erythematosus (SLE) (19.2%) (Table 1). Median (interquartile range) disease duration and patient perception of disease control were 6 years (3–14) and 77 of 100 (53–93), respectively. A total of 63.8% of patients were taking ≥1 conventional synthetic disease‐modifying antirheumatic drugs (csDMARDs), and 17.8% were taking a biologic or targeted synthetic DMARD. Prednisolone and nonsteroidal antiinflammatory drug (NSAID) use was reported by 26.7% and 22.4% of patients, respectively (Table 1).
Table 1.
Characteristic of eligible patients (n = 550)a
| Valueb | |
|---|---|
| Age, mean ± SD years | 52 ± 15.2 |
| Female | 400 (75.3) |
| Diagnoses | |
| RA | 155 (29.7) |
| SLE | 100 (19.2) |
| Scleroderma | 51 (9.8) |
| Psoriatic arthritis | 47 (9.0) |
| Spondyloarthritis | 21 (4.0) |
| Vasculitis | 31 (5.9) |
| RA/SLE overlap | 17 (3.3) |
| Other (inflammatory)c | 80 (15.3) |
| Other (noninflammatory)d | 20 (3.8) |
| No diagnosis or missing | 28 (5.1) |
| Disease duration, median (IQR) years | 6 (3.0–14.0) |
| Disease control, median (IQR) years (range 0–100) | 77 (53.0–93.0) |
| Medications | |
| Prednisolone, median 1 (IQR 0–1) mg | 147 (26.7) |
| csDMARDs | 349 (63.8) |
| Methotrexate | 165 (30) |
| Hydroxychloroquine | 203 (36.9) |
| Sulfasalazine | 25 (4.5) |
| Mycophenolate | 50 (9.1) |
| Azathioprine | 28 (5.1) |
| Leflunomide | 16 (2.9) |
| Others (tacrolimus, cyclosporin) | 5 (1.0) |
| Cyclophosphamide | 2 (0.4) |
| bDMARDs or tsDMARDs | 98 (17.8) |
| TNF inhibitors | 53 (9.6) |
| Rituximab | 12 (2.2) |
| Secukinumab | 11 (2) |
| Tocilizumab | 9 (1.6) |
| JAK inhibitor | 6 (1.1) |
| Abatacept | 4 (0.7) |
| Ustekinumab | 3 (0.5) |
| NSAIDs | 123 (22.4) |
| No medications | 104 (19.8) |
Values are the number (%) of patients unless indicated otherwise. RA = rheumatoid arthritis; SLE = systemic lupus erythematosus; IQR = interquartile range; csDMARDs = conventional synthetic disease‐modifying antirheumatic drugs; bDMARDs = biologic DMARDs; tsDMARDs = targeted synthetic DMARDs; TNF = tumor necrosis factor; NSAIDs = nonsteroidal antiinflammatory drugs.
Data missing for age (n = 22), sex (n = 19), disease duration (n = 27), disease control (n = 15), medications (n = 24), and diagnosis (n = 28).
Other inflammatory diseases include mixed/undifferentiated connective tissue disease, sarcoidosis, IgG4‐related disease, seronegative or palindromic arthritis, Sjögren's syndrome, adult‐onset Still’s disease, juvenile idiopathic arthritis, reactive arthritis, Behçet's disease, inflammatory myopathies, relapsing polychondritis, polymyalgia rheumatica, gout, eosinophilic fasciitis, autoimmune liver disease, immune‐mediated pericarditis.
Noninflammatory diseases include fibromyalgia, osteoarthritis, and tendinopathies.
Patient concerns regarding risk associated with diagnosis and medications
A total of 41% of patients were concerned that their rheumatologic disease increased the risk of contracting COVID‐19, while 52.3% were concerned that their rheumatologic disease increased the risk of severity of their COVID‐19 symptoms. Univariate analysis demonstrated that female patients were more concerned about the disease‐associated risk of contracting or being more unwell with COVID‐19 (odds ratio [OR] 1.50 [95% confidence interval (95% CI) 1.008–2.246]; P = 0.046 and OR 1.59 [95% CI 1.016–2.480]; P = 0.043). Patients with SLE (OR 4.42 [95% CI 1.512–12.898]; P = 0.007) and scleroderma (OR 3.82 [95% CI 1.169–12.471]; P = 0.027) were more likely to be concerned about being more unwell with COVID‐19. These associations were attenuated in multivariate analyses (see Supplementary Table 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24347/abstract).
A total of 55.7% of patients were concerned that their medications increased their risk of contracting COVID‐19, while 76.1% were concerned that medications increased the severity. All patients (100%) taking cyclophosphamide were concerned about medication‐related risk, followed by 70% of patients taking mycophenolate, 62% of patients taking a biologic or targeted synthetic DMARDs, 57% of patients taking azathioprine, and 55% of patients taking methotrexate. Patients were least concerned about hydroxychloroquine (20%) and NSAIDs (28%) (Figure 1).
Figure 1.
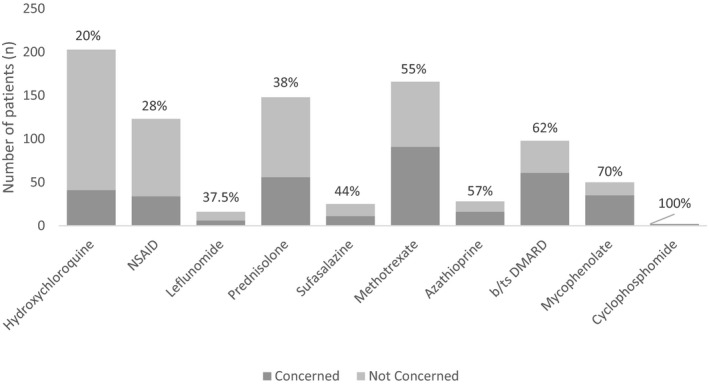
Level of patient concern regarding specific rheumatologic medications.
In multivariate analysis, there was a small but significant negative association between age and patient concern that their medications could increase their risk of contracting or having severe COVID‐19 (OR 0.98 [95% CI 0.967–0.996]; P = 0.014 and OR 0.98 [95% CI 0.965–0.993]; P = 0.003) (Tables 3 and 2). There was also an association with patient perception of poorer disease control (OR 0.99 [95% CI 0.976–0.994]; P = 0.002 and OR 0.99 [95% CI 0.998–0.998]; P = 0.016). Treatment regimens containing combination csDMARDs or biologic or targeted synthetic DMARDs were associated with increased concern regarding contracting COVID‐19, while all treatments were associated with increased concern regarding severity of COVID‐19 (Tables 3 and 2).
Table 3.
Univariate analysis findings showing participant beliefs regarding the risks posed by their rheumatologic medicationsa
| Belief that medication increases risk of contracting COVID‐19 | Belief that medication increases severity of COVID‐19 | |||||
|---|---|---|---|---|---|---|
| % (no./total no.)median (range) | OR (95% CI) | P | % (no./total no.)median (range) | OR (95% CI) | P | |
| Age, years | 49.0 (19.00–82.00) | 0.98 (0.971–0.994) | 0.003 | 50.0 (19.00–82.00) | 0.98 (0.970–0.993) | 0.002 |
| Female | 41.3 (161/390) | 1.07 (0.709–1.604) | 0.758 | 53.6 (206/384) | 1.18 (0.787–1.757) | 0.430 |
| Disease duration, years | 7.0 (0.00–53.00) | 1.00 (0.986–1.021) | 0.704 | 7.0 (0.00–53.00) | 1.00 (0.982–1.017) | 0.958 |
| Disease control (0–100) | 52.0 (18.00–87.00) | 1.00 (0.988–1.002) | 0.177 | 75.0 (0.00–100.00) | 1.00 (0.990–1.004) | 0.456 |
| Medications | ||||||
| No medication | 25.2 (29/115) | 1.00 | 30.2 (35/115) | 1.00 | ||
| Prednisolone only | 37.0 (10/27) | 5.33 (1.183–24.042) | 0.029 | 46.2 (12/26) | 1.98 (0.834–4.721) | 0.122 |
| csDMARD monotherapy | 31.3 (43/137) | 7.06 (1.538–32.393) | 0.012 | 47.1 (64/135) | 2.06 (1.223–3.461) | 0.007 |
| Combination csDMARDs | 61.1 (44/72) | 4.65 (0.956–22.565) | 0.057 | 76.1 (54/71) | 7.35 (3.747–14.424) | <0.001 |
| csDMARD + prednisolone | 41.9 (18/43) | 4.27 (0.870–20.928) | 0.074 | 54.5 (24/44) | 2.78 (1.360–5.669) | 0.005 |
| csDMARDs + prednisolone | 51.2 (21/41) | 18.67 (3.232–107.817) | 0.001 | 53.7 (22/41) | 2.68 (1.360–5.669) | 0.008 |
| b/tsDMARD monotherapy | 60.6 (20/33) | 5.78 (1.128–29.605) | 0.035 | 63.6 (21/33) | 4.05 (1.797–9.127) | 0.001 |
| b/tsDMARD + csDMARD(s) or prednisolone | 45.2 (19/42) | 6.14 (1.318–28.592) | 0.021 | 65.9 (27/41) | 4.46 (2.092–9.520) | <0.001 |
| b/tsDMARD + csDMARD(s) + prednisolone | 73.7 (14/19) | 4.36 (0.740–25.744) | 0.104 | 85.0 (17/20) | 13.11 (3.611–47.633) | <0.001 |
| Prednisolone dose (mg) | 0.0 (0.00–37.50) | 1.04 (1.002–1.085) | 0.039 | 0.0 (0.00–37.50) | 1.03 (0.990–1.074) | 0.135 |
Data missing for patients with concern regarding contracting COVID‐19 (n = 21, 10 female and 11 male) and for concern regarding more severe COVID‐19 (n = 24, 16 female and 8 male). OR = odds ratio; 95% CI = 95% confidence interval; csDMARD = conventional synthetic disease‐modifying antirheumatic drug; b/tsDMARD = biologic or targeted synthetic DMARD.
Table 2.
Multivariate analysis findings showing participant beliefs regarding the risks posed by their rheumatologic medicationsa
| Belief that medication increases risk of contracting COVID‐19 | Belief that medication increases severity of COVID‐19 | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age, years | 0.98 (0.967–0.996) | 0.014 | 0.98 (0.965–0.993) | 0.016 |
| Disease control (0–100) | 0.99 (0.976–0.994) | 0.002 | 0.99 (0.998–0.998) | 0.016 |
| Medications | ||||
| No medication | 1.00 | 1.00 | ||
| Prednisolone only | 1.64 (0.50–5.356) | 0.241 | 3.38 (1.081–10.572) | 0.036 |
| csDMARD monotherapy | 1.53 (0.751–3.119) | 0.241 | 2.54 (1.311–4.924) | 0.006 |
| Combination csDMARDs | 8.44 (3.752–18.966) | <0.001 | 12.06 (5.161–28.186) | <0.001 |
| csDMARD + prednisolone | 2.26 (0.829–6.174) | 0.111 | 3.80 (1.445–10.007) | 0.007 |
| csDMARDs + prednisolone | 3.40 (1.241–9.300) | 0.017 | 3.45 (1.289–0.242) | 0.014 |
| b/tsDMARD monotherapy | 5.82 (2.235–15.170) | <0.001 | 4.21 (1.658–10.716) | 0.003 |
| b/tsDMARD + csDMARD(s) or prednisolone | 3.67 (1.452–9.270) | 0.006 | 6.53 (2.581–16.521) | <0.001 |
| b/tsDMARD + csDMARD(s) + prednisolone | 15.12 (3.612–63.284) | <0.001 | 22.29 (4.403–112.881) | <0.001 |
| Prednisolone dose (mg) | 1.05 (0.980–1.114) | 0.097 | 1.01 (0.954–1.079) | 0.650 |
OR = odds ratio; 95% CI = 95% confidence interval; csDMARD = conventional synthetic disease‐modifying antirheumatic drug; b/tsDMARD = biologic or targeted synthetic DMARD.
COVID‐19 patient information
Sixty‐one percent of respondents (n = 277) had already obtained patient information. The most common sources of information were the rheumatology department (31%), general practice (30%), or an internet search (17%). Nine percent of patients had obtained information from the Arthritis Australia website, while 5% had obtained information from the Arthritis Foundation website.
Most patients (92%) reported that they found the ARA patient information sheet helpful and elected to receive updated information via email (n = 300) or text message (n = 193). Many patients (63%) reported that the information sheet had not changed their existing plan to continue their medications, but 30% of patients reported that they were more likely to stay on their medications as a result of receiving the information.
Additional information requested by patients included specific information regarding the impact of rheumatic disease, its activity, and immunosuppression (n = 29), general information regarding COVID‐19 (n = 10), specific advice regarding work (n = 4), support and reassurance regarding access to medications, particularly hydroxychloroquine (n = 8), community services for immunosuppression (n = 4), advice regarding well‐being practices while distancing (n = 2), and management following exposure to or diagnosis of COVID‐19 (n = 2).
Patient opinions on telehealth
The majority of patients (98.4%) considered the current use of telehealth to be appropriate. Factors that were important to patients in accepting telehealth were if their condition was well‐controlled (60%), if their rheumatologist felt it was appropriate (56.2%), if the consultation was with a rheumatologist who knew their case well (55.1%), and if they were unwell and were unable to attend (34%). Additional reasons provided were work and family commitments and transport limitations. A minority of patients felt that telehealth was only appropriate in times of strict infection control (28.1%) or that it was never appropriate (1.6%).
DISCUSSION
Dealing with uncertainty is an important aspect of the practice of rheumatology, where patient uncertainty is common and associated with poorer health outcomes 3, 4, 5. Our charge as clinicians is to minimize the experience of uncertainty in our patients, but our ability to do this has been challenged by the uncertainty surrounding the COVID‐19 pandemic. Our aim in this study was to ascertain the perceptions and concerns of patients in order to better address them, thereby minimizing patient uncertainty and possibly improving outcomes. The major findings were that concern regarding the impact of disease and/or treatment on COVID‐19 risk and/or severity was common, that a proactive intervention could increase adherence, and that telehealth was broadly acceptable.
A significant proportion of patients were concerned that their underlying diagnosis increased their risk of contracting or becoming more unwell with COVID‐19. There are robust data to support concerns that immune dysregulation in rheumatic diseases such as RA and SLE, particularly in high disease activity states, confers an increased infection risk 6, 7, 8, 9, 10. In multivariate analysis, we did not identify any predictive factors for concern, highlighting the importance of outreach with information to all patients with rheumatic diseases.
We found that 76% of respondents were concerned that their medications would increase their risk of becoming more unwell if they were to contract COVID‐19. Exploring specific drugs, biologic or targeted synthetic DMARDs, mycophenolate, and cyclophosphamide were associated with the most concern. It could be conjectured that this heightened awareness reflects efforts taken by clinical staff to inform patients on these medications, but this was not assessed in the present study, and there have been no previously published data on patient perceptions regarding infection risks associated with immunosuppression. In contrast, only 28% of patients were concerned regarding NSAIDs, which may reflect an understanding that these agents have not been shown to be associated with an increased risk of infection. Twenty percent were concerned regarding the infection risks posed by hydroxychloroquine despite the well‐publicized interest in this drug as a possible preventive or therapeutic for COVID‐19 11, 12.
In multivariate analyses conducted to identify patients who were most concerned about the risk posed by their immunosuppression, therapeutic regimens containing combination csDMARDs and biologic or targeted synthetic DMARDs appeared to generate the most concern. This may reflect higher levels of clinician education provided to such patients. Such treatment may indicate a history of more severe disease and a higher risk of requiring rescue glucocorticoids in the event of a flare caused by drug cessation, underlining the importance of dissemination of information to these patients. Only 38% of patients on prednisolone were concerned regarding the risk it posed, despite its consistent associations with infection risk in observational studies 7, 8, 10, 13. This may reflect the fact that 70% of patients taking prednisolone were on dosages of ≤5 mg/day, although there was no relationship between prednisolone dose and patient concern.
The exponential rise in COVID‐19 cases in Australia began in March 2020, leading to the distribution of the ARA patient information sheet in late March 2020 1. A total of 61% of respondents had already obtained COVID‐19 patient information, predominantly from their general practitioner or rheumatology service. One‐third of patients reported that receiving the ARA information sheet made them more likely to continue their antirheumatic medication. We assessed the characteristics of patients who were more likely to stay on treatment as a result of education (31%) compared to patients who had already planned to stay on treatment (63%) but found no convincing predictors. The impact of the information sheet on adherence intentions was no different in patients who had already received information, irrespective of the source.
We recorded patient suggestions for future iterations of an information sheet and found that patients largely wanted more specific information regarding the risks posed by their disease and treatment. When considered together with the other results, these findings highlight that the need for patient information among patients with rheumatic diseases is high. In regard to future iterations of patient information, patient concerns such as services available for immunosuppressed patients (e.g., pathology collection at home, delivery of medications, priority access to grocery shopping) and concerns regarding medication access were reported back to the ARA.
In Australia, although telehealth is used to support remote and regional areas, its potential to improve the care of urban patients by complementing face‐to‐face services has been untapped 14. This is largely due to limitations in funding models that subsidize only face‐to‐face care and the lack of technology infrastructure within clinics. The COVID‐19 pandemic has resulted in huge increases in telehealth consultations. There are limited data regarding the impact of telehealth on diagnostic accuracy and patient satisfaction outside of rural and regional settings, particularly in models of care that do not involve a primary care physician and in the assessment of new patients 14, 15. We found that the overall acceptance of telehealth was high, but one‐fourth of patients considered that this was only appropriate while strict infection control measures were in place, and nearly half did not feel it was appropriate if their condition was not well‐controlled or if their consultation was not with a clinician familiar with their case.
This is the first descriptive study to assess the concerns of rheumatology patients in the era of COVID‐19. Although a single‐center, cross‐sectional study, the sample size of this study is large, and it provides insights into patient perceptions in the early months of the pandemic. The key finding is that the need for information in patients with rheumatic diseases is high, particularly in regard to the risk posed by their diseases and medications. While there is a high level of concern in patients being treated with combination csDMARDs and biologic or targeted synthetic DMARDs, concern regarding prednisolone is low and discordant with its risks. We ascertained a high level of acceptance of telehealth while strict infection controls are in place. Remaining research questions include capturing the actions that patients have taken in regard to their immunosuppression and the impact of this on disease, the impact of a history of infections or comorbidities on patient beliefs and behaviors, and the outcomes of patients who have had telehealth consultations.
The major limitation of our study is response bias. The demographics and medications of all patients who received the invitation were not collected, and it is therefore difficult to hypothesize on the degree and direction of any response bias. We also did not collect data regarding the primary language, medical literacy, education status, and socioeconomic status of participants, which may be relevant confounders or effect modifiers.
The survey response rate does raise concerns regarding the method of information dissemination. Contributing factors may include the optional nature of the survey, lack of a reminder text, incorrect contact details, language or technological barriers, concerns regarding phishing, and lack of interest or relevance for patients with noninflammatory conditions. A more expansive strategy using mail and email with translations of the information sheet where appropriate would be important in order to adequately reach all patients. This strategy was not feasible at the time this study was conducted given the urgency to disseminate patient information quickly.
In conclusion, patients with rheumatic diseases have significant concerns regarding their risk of contracting or being more unwell with COVID‐19, and misconceptions relating to medication risk are common and the need for information is high. The dissemination of patient information has the potential to avoid unnecessary patient‐directed changes to therapy, which may minimize the risk of disease flare. Moreover, there is overwhelming acceptance of telehealth substituting face‐to‐face consultations during the COVID‐19 pandemic.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Antony had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Antony, Connelly, Ayoub, Morand.
Acquisition of data
Antony, Eades, De Silva.
Analysis and interpretation of data
Antony, Tillett, Ayoub, Morand.
Supporting information
ACKNOWLEDGMENTS
The authors thank Daniel Boulos, Kathleen Elford, Michael Gingold, Vera Golder, Molly Grant, Emma Guymer, Alberta Hoi, Leo Kyi, Michelle Leech, Susan Morton, Gene Siew Ng, Melissa Northcott, Bita Omdivar, Sudha Raghunathan, Lynden Roberts, Joanne Sahhar, Dorothy Wang, and Ai Li Yeo.
No potential conflicts of interest relevant to this article were reported.
References
- 1. O'Brien J. Coronavirus (COVID‐19) in Australia. 2020. URL: https://www.covid19data.com.au/.
- 2. Venerito VG, Lopalco G, Iannone F. COVID‐19, rheumatic diseases and immunosuppressive drugs: an appeal for medication adherence. Rheumatol Int 2020;40:827–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cleanthous S, Cano S, Isenberg D, Isenberg D, Newman S. Is patient uncertainty associated with outcomes in systemic lupus erythematosus? Rheumatology (Oxford) 2014;53 Suppl 1:i126. [Google Scholar]
- 4. Cleanthous S, Newman SP, Shipley M, Isenberg DA, Cano SJ. What constitutes uncertainty in systemic lupus erythematosus and rheumatoid arthritis? Psychol Health 2013;28:171–88. [DOI] [PubMed] [Google Scholar]
- 5. Cleanthous S, Isenberg DA, Newman SP, Cano SJ. Patient Uncertainty Questionnaire‐Rheumatology (PUQ‐R): development and validation of a new patient‐reported outcome instrument for systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) in a mixed methods study. Health Qual Life Outcomes 2016;14:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population‐based study. Arthritis Rheum 2002;46:2287–93. [DOI] [PubMed] [Google Scholar]
- 7. Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, et al. The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol 2008;35:387–93. [PubMed] [Google Scholar]
- 8. Au K, Reed G, Curtis JR, Kremer JM, Greenberg JD, Strand V, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis 2011;70:785–91. [DOI] [PubMed] [Google Scholar]
- 9. Duffy KN, Duffy CM, Gladman DD. Infection and disease activity in systemic lupus erythematosus: a review of hospitalized patients. J Rheumatol 1991;18:1180–4. [PubMed] [Google Scholar]
- 10. Danza A, Ruiz‐Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus 2013;22:1286–94. [DOI] [PubMed] [Google Scholar]
- 11. Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS‐CoV-2 infection in vitro. Cell Discov 2020;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents 2020;105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Youssef J, Novosad S, Winthrop K. Infection risk and safety of corticosteroid use. Rheum Dis Clin North Am 2016;42:157–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poulsen KA, Millen CM, Lakshman UI, Buttner PG, Roberts LJ. Satisfaction with rural rheumatology telemedicine service. Int J Rheum Dis 2015;18:304–14. [DOI] [PubMed] [Google Scholar]
- 15. Leggett P, Graham L, Steele K, Gilliland A, Stevenson M, O’Reilly D, et al. Telerheumatology: diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract 2001;51:746–8. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


