To the Editor,
Coronavirus disease 2019 (COVID‐19) is ongoing and spreading worldwide after cases of COVID‐19 were reported in Wuhan, China in December 2019. 1 Older age, diabetes, hypertension, and smoking have been reported as aggravating factors, 2 and human immunodeficiency virus (HIV) infection is considered to be a potentially aggravating factor. There are very few reports of COVID‐19 in untreated HIV patients 3 , 4 , 5 HIV patients are known to be susceptible to respiratory viruses and to have more severe symptoms, but the clinical course and prognosis of COVID‐19 in HIV patients are not known yet. We report a case of COVID‐19 with untreated HIV infection.
On 23 March 2020, a 28‐year‐old male living in Tokyo suffered from persistent fever and nonproductive cough for 8 days before coming to our hospital. He told us he had an HIV infection but had not received any antiretroviral therapy (ART). He was diagnosed with HIV infection 2 years prior and was then noted to be seropositive for syphilis and hepatitis B virus. However, he was lost to follow‐up by the hospital before starting ART. The HIV‐1 viral load and the CD4+ T lymphocyte count were 1.28 × 104 copies/mL and 491/μL, respectively, at the last visit. He had no other past medical history, but he was a heavy smoker of two packs per day and a heavy drinker. A patient is a man who has receptive sex with men and the last intercourse was 2 months prior. He had no recognized contact with any confirmed COVID‐19 patients and had not traveled abroad for a year.
Physical examination was as follows: body temperature, 39.2°C; pulse rate, 130 beats per minute; respiratory rate, 20 breaths per minute; blood pressure, 110/78 mm Hg; and oxygen saturation, 97% while breathing room air. There were no abnormalities in his bilateral lung sounds. However, chest computed tomography showed multiple ground‐glass opacities (Table 1). Blood testing revealed mild lymphopenia (981/μL), decreased CD4+ T lymphocyte counts (194/μL), elevated lactate dehydrogenase (529 U/L), and elevated C‐reactive protein (10.97 mg/dL). The HIV‐1 viral load was 1.00 × 102 copies/mL. Cytomegalovirus was not detected in peripheral blood. A salivary Pneumocystis jirovecii polymerase chain reaction (PCR) assay was negative. The nasopharyngeal specimen tested negative by a BioFire Diagnostics Respiratory Panel (BioFire Diagnostics; Salt Lake City, UT), however, positive for SARS‐CoV‐2 by reverse transcription PCR assay. Therefore, we confirmed that this patient was coinfected with HIV and SARS‐CoV‐2, and he was hospitalized. We administered 200 mg hydroxychloroquine twice a day for 14 days. On day 3, his fever subsided. However, on day 4, he needed additional oxygen due to difficulties in breathing without desaturation, but he recovered the next day. SARS‐CoV‐2 was not detected in nasopharyngeal specimens obtained on either day 7 or 8. He was discharged on day 9. One month after his discharge, the HIV‐1 viral load had increased (2.37 × 104 copies/mL). We started on ART (bictegravir/emtricitabine/tenofovir alafenamide fumarate) and he has had no particular problems for the next 2 months (Figure 1).
Table 1.
Comparison of previous cases and our case
| Characteristics | Chinese case | Turkish case | Spanish case | Our case |
|---|---|---|---|---|
| Age (years) | 61 | 34 | 31 | 28 |
| Sex | Male | Male | Transgender | Male |
| Underlying condition | Diabetes, Smoker | HBV infecition, Bipolar disorder | none | Smoker, Drinker, HBV infection |
| Day of illness admission | 8 | not available | 7 | 8 |
| Saturation on admission (%) | 80 | not available | <90% | 97 |
| CT findings on admission | multiple GGO | multiple GGO | not available | multiple GGO |
| Lymphocyte counts (/μL) | 560 | 360 | 900 | 981 |
| CD4+ lymphocyte counts (/μL) | 26.6 | 2.8 | 13 | 194 |
| HIV‐1 viral load (copies/mL) | not available | 434,782 | 45,500 | 100 |
| Lactate dehydrogenase (U/L) | not available | 308 | 1,149 | 529 |
| C reactive protein (mg/dL) | not available | 0 | 40 | 10.97 |
| Maximum oxygen supply (L/min) | 5 | 0 | not available | 0.6 |
| Possible anti SARS‐CoV‐2 agents | Lopinavir/ritonavir | Lopinavir/ritonavir | Darunavir/cobicistat | Hydroxychloroquine |
| γ‐globlin | Azithromycin | Hydroxychloroquine | ||
| Interferon beta‐1b | ||||
| Azithromycin | ||||
| Mechanical ventilation | none | none | non‐invasive | none |
| Day of SARS‐CoV‐2 negativity from admission | 11 | not available | not available | 8 |
GGO = ground‐glass opacities.
Figure 1.
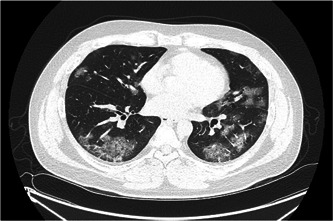
Thoracic computed tomography image on admission
This is a case report of COVID‐19 in an untreated HIV patient.
Neither our case nor the previous untreated HIV cases 3 , 4 , 5 required invasive mechanical ventilation although all cases had CD4+ T lymphocyte counts under 200 at the first visit (Table 1). Respiratory viral infections may become severe and prolonged in HIV patients. 5 CD4+ T lymphocyte counts may not affect the severity of COVID‐19 or delay SARS‐CoV‐2 clearance in the nasopharynx (Table 1).
In our case, ART was not given during the hospitalization because there can be a risk of developing immune reconstitution inflammatory syndrome (IRIS). P. jirovecii and Mycobacterium spp. are the pathogens causing IRIS in pneumonia in HIV patients. 6 The latest guideline recommends starting ART as soon as possible, except in cases of tuberculous meningitis. However, we did not administer ART to the patient during the acute phase for the following three reasons. First, SARS‐CoV‐2 exists more frequently in sputum than in the nasopharynx or oropharynx. 7 Second, a decrease in CD4+ T lymphocytes can slow SARS‐CoV clearance. 8 Finally, a relationship between COVID‐19 pneumonia and cytokine storms has been suggested. 9
The HIV‐1 viral load was significantly reduced on admission compared with the last visit 2 years prior. It is possible that the HIV‐1 virus was reduced by SARS‐CoV‐2 interference. Moss et al 10 reported that HIV replication is suppressed during acute measles.
Here, we describe a case of COVID‐19 pneumonia with untreated HIV infection. COVID‐19 pneumonia in untreated HIV patients requires careful follow‐up for IRIS with ART.
ACKNOWLEDGMENTS
The authors would like to thank Editage (http://www.editage.com) for English language editing. This study was partly supported by funding research grants from the Emerging/Re‐emerging Infectious Diseases Project of Japan from the Japan Agency for Medical Research and Development, AMED (20fk0108116h0001).
REFERENCES
- 1. Bedford J, Enria D, Giesecke J, et al. COVID‐19: towards controlling of a pandemic. Lancet. 2020;395:1015‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020:e200994. https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2763184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu F, Cao Y, Xu S, Zhou M. Co‐infection of SARS‐CoV‐2 and HIV in a patient in Wuhan city, China. J Med Virol. 2020;92:529‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aydin OA, Karaosmanoglu HK, Yasar KK. HIV/SARS‐CoV‐2 co‐infected patients in Istanbul, Turky. J Med Virol. 2020. https://onlinelibrary.wiley.com/doi/full/10.1002/jmv.25955 [Google Scholar]
- 5. Blanco JL, Ambrosioni J, Garcia F, et al. COVID‐19 in patients with HIV: clinical case series. Lancet HIV. 2020;7(5):e314‐e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. King JC Jr. Community respiratory viruses in individuals with human immunodeficiency virus infection. Am J Med. 1997;102(3):19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS‐CoV‐2 in infected patients. Clin Infect Dis. 2020:ciaa345. https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa345/5812997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moss WJ, Ryon JJ, Monze M, Cutts F, Quinn TC, Griffin DE. Suppression of human immunodeficiency virus replication furring acute measles. J Infect Dis. 2002;185(8):1035‐1042. [DOI] [PubMed] [Google Scholar]


