Abstract
Background and purpose
We systematically reviewed available evidence for reports of neurological signs and symptoms in patients with COVID‐19 to identify cases with severe acute respiratory syndrome coronavirus (SARS‐CoV)‐2 infection or immune‐mediated reaction in the nervous system.
Methods
We followed PRISMA guidelines and used the MEDLINE, EMBASE, Google Scholar, MedRxiv and ChinaXiv databases to search for articles on COVID‐19 and nervous system involvement that were published from 1 January to 24 April 2020. Data on design, sample size, neurological assessment and related work‐up were extracted. Biases were assessed with the Newcastle–Ottawa scale.
Results
We analysed 27 publications on potential neuroinvasive or parainfectious neurological complications of COVID‐19. The reports focused on smell and taste (n = 5) and evaluation of neurological symptoms and signs in cohorts (n = 5). There were cases of Guillain‐Barré syndrome/Miller‐Fisher syndrome/cranial neuropathy (seven cases), meningitis/encephalitis (nine cases) and various other conditions (five cases). The number of patients with examination of cerebrospinal fluid and, in particular, SARS‐CoV‐2 polymerase chain reaction was negligible. Two had a positive SARS‐CoV‐2 polymerase chain reaction examination of cerebrospinal fluid specimen. Study of potential parenchymal involvement with magnetic resonance imaging was rare. Only four reports received a rating of the highest quality standards.
Conclusions
This systematic review failed to establish comprehensive insights into nervous system manifestations of COVID‐19 beyond immune‐mediated complications in the aftermath of respiratory symptoms. The authors therefore provide guidance for more careful clinical, diagnostic and epidemiological studies to characterize the manifestations and burden of neurological disease caused by SARS‐CoV‐2 on behalf of the Infectious Disease Panel of the European Academy of Neurology.
Keywords: cerebrospinal fluid, COVID‐19, encephalitis, neuroinvasion, neurological complications, SARS‐CoV‐2
Introduction
The clinical spectrum of severe acute respiratory syndrome coronavirus (SARS‐CoV)‐2 infection is wide and encompasses asymptomatic infection, mild upper respiratory tract illness and severe viral pneumonia with respiratory failure and sometimes death. From a neurobiological and translational viewpoint, neurological manifestations can be expected in COVID‐19. This is substantiated, on the one hand, by a few cases with neurological signs and symptoms and detectable virus load in cerebrospinal fluid (CSF) during the SARS‐CoV‐1 epidemic in 2003 [1]. SARS‐CoV‐1 and SARS‐CoV‐2 share genetic sequences but SARS‐CoV‐2 has a 10–20 times higher binding affinity to angiotensin‐converting enzyme‐2 [2]. On the other hand, angiotensin‐converting enzyme‐2, the functional receptor utilized by SARS‐CoV‐1 and SARS‐CoV‐2 for cell entry, is not only expressed in the lungs but also in the central nervous system (CNS) [3, 4]. Expression of angiotensin‐converting enzyme‐2 is found in neurons and non‐neuronal cells, the latter including astrocytes, oligodendrocytes and olfactory support cells [5, 6]. Moreover, infection of neurons with SARS‐CoV‐1 has been proven in transgenic mice and several presumed routes of CNS entry were described in pre‐clinical models [7]. In a report from Wuhan, China, more than one‐third of the hospitalized patients with COVID‐19 had some sort of nervous system‐related clinical signs or symptoms [8]. These included more specific conditions such as loss of sense of smell or taste, myopathy and stroke. There were also non‐specific symptoms such as headache, impaired level of consciousness, dizziness or seizure. From a neuroinfectious viewpoint, the relevance of these findings is limited as these conditions can be coincidental, secondary to systemic complications or a side‐effect of therapy. Only further diagnostic details such as focused neuroimaging, evaluation of cardiovascular risk factors and comorbidities, assessment of prothrombotic or systemic hyperinflammatory states, presence of intrathecal inflammation and systematic exclusion of differentials would enable a placement within the spectrum of COVID‐19 complications.
This study therefore aimed to identify clinical cases of confirmed nervous system invasion or post‐infectious neurological disease in the available COVID‐19 literature on the basis of a systematic review. Members of the Infectious Disease Panel of the European Academy of Neurology (EAN) compiled guidance for the diagnostic approach, which emphasizes the need for precise case definitions and standards for reporting.
Methods
A systematic review was carried out to study all cases reporting nervous system involvement in patients with proven SARS‐CoV‐2 infection. The protocol followed the PRISMA guidance for reporting of systematic reviews. MEDLINE, EMBASE, Google Scholar, MedRxiv and ChinaXiv databases were searched for articles published from 1 January 2020 to 24 April 2020 regarding the nervous system and COVID‐19. The search strings for PubMed were as follows: (("COVID"[All Fields] OR “coronavirus”[All Fields] OR “SARS‐CoV‐2”[All Fields]) AND (("neurology"[MeSH Terms] OR "neurolog*"[All Fields]) OR ("brain"[MeSH Terms] OR "brain"[All Fields]) OR ("neuro"[All Fields]) OR ("nervous system"[MeSH Terms] OR "nervous system"[All Fields])) AND ("2020/01/01"[PDAT]: "2020/04/24"[PDAT])). We also hand‐searched reference lists of all articles identified in the electronic search using common search engines (e.g. Google, Bing). Ethical approval was not required.
The search selected studies reporting neurological features of patients with SARS‐CoV‐2 infection. Studies were identified after search and data were extracted regarding: study design, sample size, neurological assessment and diagnostic work‐up including brain imaging and CSF analysis. Biases were assessed with the Newcastle–Ottawa scale [9].
Results
The systematic search yielded 102 papers, of which 30 were eligible for full‐text assessment (see Fig. 1 for PRISMA flow‐chart). Four were excluded; these were commentaries, response letters and review articles proposing SARS‐CoV‐2 nervous system invasion but not comprising clinical findings.
Figure 1.
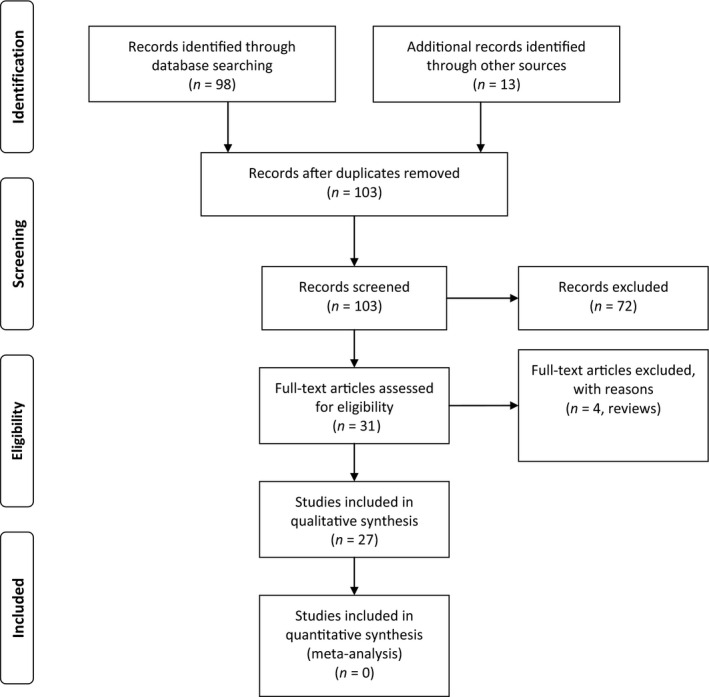
PRISMA flow‐chart.
A total of 26 publications reporting neurological disturbances in patients with SARS‐CoV‐2 infection were evaluated; the major readouts are shown in Table 1. Bias assessment revealed low to fair quality in more than half of the studies (14/27, 52%), as shown in Fig. 2. This was mainly due to selection and reporting bias, as well as on the basis of uncertain exposure and lack of testing for SARS‐CoV‐2 with polymerase chain reaction (PCR) in CSF. Only four reports reached a rating for high quality; one study evaluated neurological diagnoses of deceased patients with COVID‐19 [10], another studied neurological signs and symptoms in a cohort of hospitalized patients with COVID‐19 [8] and the third was a case series of peripheral nervous system dysfunction with in‐depth phenotyping and diagnostic work‐up [11]. The fourth study described the clinical characteristics, laboratory features, treatment and outcomes of cerebrovascular disease complicating SARS‐CoV‐2 infection [12]. Among the 221 consecutively admitted patients with COVID‐19, 11 (5%) had acute ischaemic stroke, 1 (0.5%) had cerebral venous sinus thrombosis and 1 (0.5%) had cerebral hemorrhage.
Table 1.
Reports on neurological disorders in association with respiratory severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection
| Author | Theme | Design | n | SARS‐CoV‐2 testing | Neurological assessment | CSF | Brain imaging | Other neurological work‐up | Main findings | Potential limitations |
|---|---|---|---|---|---|---|---|---|---|---|
| Bagheri [15] | Smell | Cross‐sectional | 10 069 | No neurological assessment; smell loss evaluated with a self‐reported online questionnaire | Not performed | NA | NA | 48.2% of patients reported anosmia/hyposmia | No neurological/diagnostic work‐up, no data on CSF | |
| Beltrán‐Corbellini [16] | Smell/taste | Case‐control study | 119 | Nasopharyngeal swab | No neurological assessment; smell and taste loss evaluated with a patient‐completed questionnaire | Not performed | NA | NA | 39.2% of patients reported smell/taste dysfunction vs. 12.5% of patients with influenza (controls) | No neurological/diagnostic work‐up, no data on CSF |
| Bernard‐Valnet [28] | Encephalitis | Case series | 2 | Nasopharyngeal swab (positive) CSF (negative) |
Case 1: NCSE, no meningism Case 2: headache, mental status changes, focal signs, no meningism |
Negative viral RNA testing, high proteins | MRI: normal | EEG (case 1): anterior spikeand waves, irregular slow theta background | ‘Encephalitic symptoms’ in patients with SARS‐CoV‐2 | Negative viral RNA testing on CSF, normal neuroimaging |
| Chen [10] | Neurological disease | Retrospective case series | 274 | Nasopharyngeal swab | No specific work‐up; data extracted from charts | Not performed | NA | NA | Hypoxic encephalopathy in 20% of deceased patients, occurrence in later disease stage; cerebrovascular disease in 1% of patients | No data on neurological assessment and work‐up |
| Duong [29] | Encephalitis | Case report | 1 | Nasopharyngeal swab | Headache, fever, seizure, meningism | High proteins, 70 cells/µL | CT: normal | EEG: unspecific slowing | Meningoencephalitis due to SARS‐CoV‐2 | No brain MRI, no CSF viral testing |
| Filatov [34] | Encephalopathy | Case report | 1 | Unspecified | Encephalopathy (patient non‐verbal, unable to follow any commands, no motor/sensory deficit, no meningism) | Normal glucose, normal protein, no cells, no viral RNA testing | CT: no acute lesions | EEG: bilateral slowing and focal slowing in the left temporal region with sharply countered waves | Encephalopathy due to SARS‐CoV‐2. No abnormalities in CSF | No SARS‐CoV‐2 PCR in CSF |
| Gutierrez‐Ortiz [21] | PNS | Case series | 2 | Nasopharyngeal swab (positive) CSF (negative) |
Case 1: right internuclear ophthalmoparesis, ataxia Case 2: multiple cranial neuropathies |
Negative viral RNA testing. Case 1 had positive GD1b‐IgG, case 2 not tested |
NA | No | Miller‐Fisher syndrome 5 days after COVID‐19 onset; cranial neuropathies 3 days after COVID‐19 onset | Negative viral RNA testing on CSF; no brain imaging or nerve conduction studies |
| Helms [18] | Neurological disease | Multicenter observational prospective | 58 | Nasopharyngeal swab | 65% of patients with COVID‐19‐related ARDS in ICU had confusion, 69% agitation, 67% pyramidal signs, 36% dysexecutive syndrome | n = 7: elevated proteins (n = 1), negative PCR for SARS‐CoV‐2 (n = 7) | MRI (n = 13): focal leptomeningeal enhancement (n = 8), perfusion abnormalities (n = 11), diffusion‐weighted ischaemic lesions (n = 3) | EEG: unspecific changes | Encephalopathy is common in patients with ARDS due to COVID‐19; single acute DWI ischaemic lesions can be seen on MRI. | All patients had negative viral RNA testing on CSF; only 8% assessed before sedation/neuromuscular blockade |
| Lechien [14] | Smell/taste | Multicenter observational prospective | 417 | Nasopharyngeal swab | No neurological assessment; smell and taste loss evaluated with a patient‐completed questionnaire | Not performed | No | No | 85% of patients reported olfactory dysfunction, 88% gustatory dysfunction; both associated with fever | No neurological/diagnostic work‐up, no data on CSF |
| Li [12] | Acute cerebrovascular disease | Retrospective observational | 221 | Nasopharyngeal swab | No specific work‐up; data extracted from charts | Not performed | MRI consistent with cerebrovascular disease | No | 5% of patients developed acute ischaemic stroke, 0.5% cerebral venous sinus thrombosis, 0.5% cerebral hemorrhage | No neurological/diagnostic work‐up, no data on CSF |
| Lu [19] | Seizure | Multicenter retrospective | 304 | Nasopharyngeal swab | 8 (2.6%) encephalopathic; 2 had ‘seizure‐like’ presentation, diagnosed as acute stress reaction (1) and electrolyte disturbance (1) | Not performed | NA | NA | No patient admitted with COVID‐19 had a seizure or status epilepticus | No routine or continuous EEG performed |
| Mao [8] | Neurological disease | Retrospective observational case series | 216 | Nasopharyngeal swab | CNS manifestations (dizziness, headache, impaired consciousness, cerebrovascular disease, ataxia, seizure), PNS (taste impairment, smell impairment, vision impairment and nerve pain) and skeletal muscular injury manifestations were assessed | Not performed | NA | NA | 36.4% of patient with COVID‐19 had neurological manifestations, which were more prevalent in patients with more severe disease course | No neurological/diagnostic work‐up, no data on CSF |
| Moein [17] | Smell | Case‐control study | 120 | Nasopharyngeal swab | No neurological assessment; smell and taste loss evaluated with a objective validated test | Not performed | NA | NA | 85.0% of patients had moderate hyposmia to anosmia vs. 0% of healthy controls | Healthy controls picked from previous database; no data on brain imaging and CSF |
| Moriguchi [27] | Encephalitis | Case report | 1 | Nasopharyngeal swab (negative) CSF (positive) | Coma (Glasgow Coma Scale score 6), neck stiffness, generalized seizures during transport and in later disease stage (treated with levetiracetam) | High opening pressure, colorless and clear, 12 cells/µL (10 mononuclear cells, 2 polymorphonuclear cells); RT‐PCR positive for SARS‐CoV‐2 RNA | MRI: peri‐ventricular temporal DWI hyperintensity, hyperintense right mesial temporal and hippocampal region on FLAIR, no contrast enhancement | No | SARS‐CoV‐2 can cause ventriculitis and encephalitis; viral RNA was detected in CSF, brain MRI showed involvement of temporal lobe, patient presented generalized seizures (treatment included ceftriaxone, vancomycin, acyclovir, steroids, levetiracetam, favipinavir) | 1/2 samples on CSF positive for viral RNA at first (reanalysis showed 2/2 positive); differential diagnosis with hippocampal sclerosis accompanying post‐convulsive encephalopathy |
| Padroni [47] | PNS | Case report | 1 | Nasopharyngeal swab | Moderate symmetric distal limb weakness, loss of deep tendon reflexes, preserved sensation | High proteins (dissociations with no cellularity) | NA | Nerve conduction studies consistent with motor‐sensory demyelinating polyneuropathy | GBS developed 22 days after first COVID‐19 symptom | No viral RNA testing on CSF; no anti‐ganglioside antibody testing |
| Poyiadji [26] | Encephalitis | Case report | 1 | Nasopharyngeal swab | Fever and altered mental status (no neurological examination reported) | Negative bacterial culture, negative tests for HSV, Varicella‐Zoster, West Nile virus; testing for the presence of SARS‐CoV‐2 in the CSF was unable to be performed | MRI: hemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes and subinsular regions | No | Acute necrotizing encephalopathy associated with SARS‐CoV‐2 (treated with IVIG) | No viral RNA testing on CSF; necrotizing encephalopathy also attributable to cytokine storm (rather than SARS‐CoV‐2 direct neuroinvasion) |
| Sedaghat [48] | PNS | Case report | 1 | Nasopharyngeal swab | Acute progressive symmetric ascending quadriparesis | Not performed | MRI (brain and cervical): normal | Nerve conduction studies consistent with motor‐sensory axonal involvement | GBS (AMSAN) 2 weeks after COVID‐19 infection | No data on CSF; hypothesized a post‐infectious autoimmune sensitization (no supporting serology data) |
| Sharifi‐Razavi [33] | Stroke | Case report | 1 | Nasopharyngeal swab | Headache, haemoptysis, confusion | Not performed | CT: intracerebral hemorrhage | No | Intracerebral hemorrhage associated with SARS‐CoV‐2 | No data on CSF, no data on coagulation screening |
| Toscano [11] | PNS | Case series | 5 | Nasoharyngeal swab (positive) CSF (negative) | Flaccid areflexic tetraparesis (n = 3), paraparesis (n = 1), facial diplegia and limb paresthesia (n = 1) | High proteins (dissociation with no cellularity) (n = 3), normal proteins (n = 2); negative anti‐ganglioside antibodies (n = 3/3), negative SARS‐CoV‐2 RNA (n = 5/5) | MRI (brain and cervical): enhancement of nerve roots or facial nerve (n = 3/5) | Nerve conduction studies consistent with axonal variant (n = 3) and demyelinating variant (n = 2) of GBS | GBS developed 5–10 days after first symptoms of COVID‐19; response to IVIG was poor in 2 cases. SARS‐CoV‐2 IgG positive in 3/5 patients. | No data on other potential pathogens, anti‐ganglioside antibodies tested negative |
| Catello Vollono [49] | Seizure | Case report | 1 | Nasoharyngeal swab | NCSE in a patient with post‐HSV1 encephalitis structural epilepsy | Not performed | MRI: temporo‐parietal gliosis (previous encephalitis), no acute lesions | EEG: left centro‐temporal lateralized semi‐rhythmic delta activity | NCSE as sole manifestation of SARS‐CoV‐2 | No data on CSF, NCSE attributable to structural epilepsy and fever |
| Xiang [25] a | Encephalitis | Case report | 1 | Nasoharyngeal swab CSF (positive) | Seizures and persistent hiccups developed 96 h after starting mechanical ventilation; meningism, pyramidal signs | High opening pressure, colorless, clear, PCR positive for SARS‐CoV‐2 RNA | CT: normal | NA | Acute encephalitis associated with SARS‐CoV‐2 (treated with IVIG, steroids, antibiotics, antiseizure medications) | No brain MRI data, methods for PCR in CSF not available |
| Yan [13] | Smell/taste | Cross‐sectional | 262 | Nasopharyngeal swab | No neurological assessment; smell and taste loss assessed with a subjective olfaction test | Not performed | No | No | Smell and taste loss in 68% and 71% of SARS‐CoV‐2 positive vs. 16% and 17% in negative subjects | No data on CSF or brain imaging; only smell and taste tested; no consecutive enrollment; phone survey |
| Ye [22] | Encephalitis | Case report | 1 | Nasopharyngeal swab (negative) CSF (negative) | Meningism and pyramidal signs | Normal opening pressure, normal biochemistry, RT‐PCR negative for SARS‐CoV‐2 RNA, with IgM and IgG not detectable | CT: normal | No | Presumed encephalitis associated with SARS‐CoV‐2 infection | No data on brain imaging, negative nasopharyngeal and CSF testing |
| Yin [23] | Meningitis | Case report | 1 | Nasopharyngeal swab (negative) CSF (negative) | Meningismus and pyramidal signs | Normal opening pressure, high proteins, negative for SARS‐CoV‐2 RNA | CT: normal | No | Presumed SARS‐CoV‐2 nervous system invasion through meninges | No advanced neuroimaging performed, negative viral RNA testing on CSF |
| Zhang [31] | ADEM | Case report | 1 | Nasopharyngeal swab | Encephalopathy, dysphagia, dysarthria (ADEM) | Normal, no viral RNA testing | MRI: atypical ADEM | No | Atypical ADEM in a patient with SARS‐CoV‐2 | No CSF viral testing, no spinal cord MRI, brain MRI atypical for ADEM |
| Zhao [20] | PNS | Case report | 1 | Nasopharyngeal swab | Symmetric weakness, areflexia, distal decrease of thermo‐dolorific sensation | Normal cell count, increased proteins; viral testing not performed | No | Nerve conduction studies: demyelinating neuropathy | GBS 8 days before COVID‐19 onset | Swab test positive 8 days after onset of neuropathy No SARS‐CoV‐2 PCR in CSF No microbiological assessment at diagnosis |
| Zhao [30] | Myelitis | Case report | 1 | Nasopharyngeal swab | Flaccid paraparesis, urinary and bowel incontinence, sensory thoracic level, decreased tendon reflexes | Not performed | CT: no acute lesion | No | Presumed SARS‐CoV‐2 myelitis developing 7 days after fever onset | No data on spinal cord MRI, no CSF analysis, 4/6 swabs negative |
ADEM, acute disseminated encephalomyelitis; AMSAN, acute motor‐sensory axonal neuropathy; ARDS, acute respiratory distress syndrome; CNS, central nervous system; CSF, cerebrospinal fluid; CT, computerized tomography; DWI, diffusion‐weighted imaging; EEG, electroencephalography; FLAIR, fluid‐attenuated inversion recovery; GBS, Guillain‐Barré syndrome; HSV, Herpes simplex virus; ICU, intensive care unit; Ig, immunoglobulin; IVIG, intravenous immunoglobulin; MRI, magnetic resonance imaging; NA, not available; NCSE, non‐convulsive status epilepticus; PCR, polymerase chain reaction; PNS, peripheral nervous system; RNA, ribonucleic acid; RT‐PCR, real‐time polymerase chain reaction;
Publication is untraceable in ChinaXiv; data extracted from hospital site report, available at http://www.bjdth.com/html/1/151/163/3665.html
Figure 2.
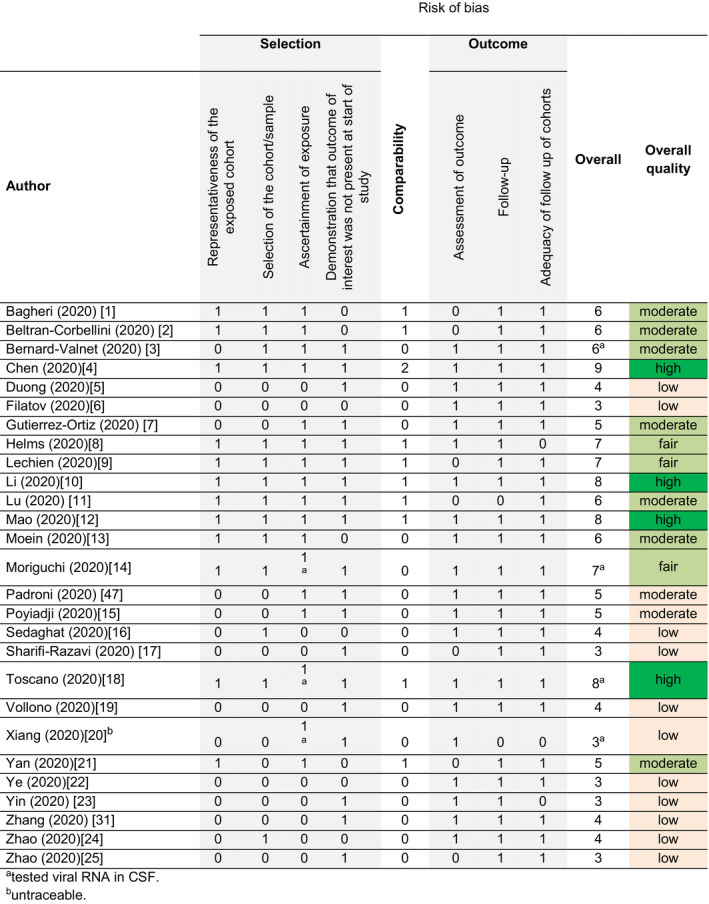
Assessment of study bias using the Ottawa–Newcastle scale. CSF, cerebrospinal fluid.
Five observational studies evaluated smell and taste dysfunction. The first study used an internet‐based platform in adults who underwent testing for COVID‐19 and found a higher rate of smell and taste impairment in SARS‐CoV‐2‐positive patients [13]. The second report was a European multicenter study of patients with mild‐to‐moderate COVID‐19, which used standardized questionnaires [14]. The authors found olfactory and gustatory dysfunction in 85.6% and 88.0% of patients, respectively. The third study used an online checklist for self‐reported anosmia/hyposmia [15]. In that cohort, 48% had hyposmia or anosmia and the onset was reported as sudden in 76%. The fourth report was a case‐control study of smell and taste disorders among patients positive for SARS‐CoV‐2 on nasopharyngeal swab examination and also included SARS‐CoV‐2‐negative patients as controls [16]. The authors found that patients with COVID‐19 were significantly younger (81%) and had a high rate of smell (45%) and taste (90%) disorders. The fifth study found smell dysfunction in 98% of the SARS‐CoV‐2 nasopharyngeal swab PCR‐positive patients and reported that this was evident for all 40 odorants studied [17]. The study also had an age‐ and sex‐matched control group. None of these five studies provided data on CSF analysis or brain imaging.
Five studies examined neurological disorders in cohorts. In the first study, hypoxic encephalopathy was the cause of death in 20% of patients who died from COVID‐19 [10]. The second report assessed neurological manifestations in a cohort hospitalized at three dedicated COVID‐19 inpatient centers [8]. They found that 36.4% of patients had various neurological manifestations that involved the CNS, peripheral nervous system and skeletal muscles. Brain imaging, CSF analysis and further work‐up were reported in neither study. The third study reported neurological features in 90.6% of consecutive patients admitted because of acute respiratory distress syndrome due to COVID‐19 and treated in the intensive care unit [18]. Confusion, agitation, pyramidal signs and dysexecutive syndrome were the most common clinical manifestations. Cerebral magnetic resonance imaging (MRI) was performed in 13/58 (22%) patients and there was evidence of leptomeningeal enhancement in 62% and ischaemic stroke in 23% of patients, respectively. Electroencephalography (8/58, 14%) and CSF (7/58, 12%) examinations were performed in some patients. None of the patients had a pleocytosis in CSF. A multicenter retrospective study evaluated the occurrence of seizures in patients with COVID‐19 [19]. There was not a single case of symptomatic seizures or status epilepticus among this cohort in which patients with epilepsy were excluded a priori. The fifth study has already been covered above and concerned the rate of acute cerebrovascular events (6%) in a cohort of patients with COVID‐19 [12]. The average time from symptoms of SARS‐CoV‐2 infection to clinical manifestation of cerebrovascular disease was 10 (interquartile range 1–29) days. The patients with cerebrovascular disease were significantly older, more likely to suffer from severe respiratory disease and more likely to have cardiovascular risk factors and medical history of cerebrovascular disease. They were also more likely to have an increased inflammatory response and hypercoagulable state.
Four publications reported eight cases of Guillain‐Barré syndrome (GBS) in patients with confirmed SARS‐CoV‐2 infection. Nerve conduction studies disclosed both demyelinating and axonal neuropathies (n = 4 and n = 4, respectively). All but one case occurred with a time lag from the respiratory symptoms (range 5–22 days). In the remaining case, the clinical manifestation of GBS preceded COVID‐19 symptoms by 8 days [20]. In the case series of five patients with GBS, three patients had high protein levels and all tested negative for SARS‐CoV‐2 in CSF, as well as for anti‐ganglioside antibodies [11]. The other reports did not perform PCR for SARS‐CoV‐2 or testing for immunoglobulin (Ig) levels and did not investigate anti‐ganglioside antibodies in CSF or serum. In addition to the GBS cases, a case of Miller‐Fisher syndrome with positive GD1b‐IgG and a case of multiple cranial neuropathies, both with negative SARS‐CoV‐2 PCR in CSF, were found [21].
Nine cases of encephalitis/meningitis and presumed association with COVID‐19 were reported in eight publications. Amongst these was a case of encephalitis in a patient with negative SARS‐CoV‐2 testing on both nasopharyngeal swab and CSF (normal cell count) in whom MRI was not performed [22]. Another patient with presumed encephalitis had normal cell count and negative SARS‐CoV‐2 PCR in CSF and MRI was not performed [23]. A similar constellation was reported for another case [24]. There is one patient with a diagnosis of COVID‐19‐related encephalitis for whom data could only be retrieved from the hospital report [25]. In that case, the neurological symptoms included seizures and hiccups and SARS‐CoV‐2 PCR in CSF was positive. For the case of acute necrotizing encephalitis, a SARS‐CoV‐2 PCR was not performed in CSF [26]. A pathogenesis triggered by a COVID‐19‐related cytokine storm was subsequently assumed. A positive SARS‐CoV‐2 PCR in CSF was present in a patient with right temporal lobe encephalitis and ventriculitis [27]. Two patients were classified as having meningoencephalitis in association with COVID‐19 [28]. Both had encephalitic symptoms, including non‐convulsive status epilepticus and mental changes, with normal MRI and negative SARS‐CoV‐2 PCR in CSF. A case of meningoencephalitis was described where the patient had meningism, headache, fever and seizures and was PCR negative for SARS‐CoV‐2 in CSF [29].
There were five further case reports, which were related to various aspects. There was one patient in whom the authors assumed myelitis as the final diagnosis [30]. In detail, the patient had a myelopathic syndrome 7 days after the onset of respiratory symptoms but was not evaluated with MRI or lumbar puncture. There was a case of presumed acute disseminated encephalomyelitis with only minimal contrast enhancement on brain MRI [31]. CSF examination was normal (cell count, protein, glucose) and SARS‐CoV‐2 PCR was only performed for nasopharyngeal swab, which was positive. Furthermore, there was a patient with pre‐existing epilepsy related to Herpes simplex virus encephalitis who presented with non‐convulsive status epilepticus in the context of COVID‐19 infection [32]. The authors discussed fever as the cause of lowering of the threshold for seizures in a brain with structural damage. The case of intracerebral hemorrhage that occurred 3 days after fever and respiratory symptoms did not have obvious coagulation disturbances [33]. Vascular imaging and CSF diagnostic were not performed. A patient with headache, altered mental status, fever and cough was classified as acute encephalopathy [34]. Electroencephalography ruled out status epilepticus, CSF showed normal results and SARS‐CoV‐2 PCR in CSF was not performed.
Overall, there were two patients positive for SARS‐CoV‐2 PCR in CSF among the four examined patients [25, 27].
Discussion
Our systematic search yielded only a limited number of studies and a significant reporting bias. This does not enable an in‐depth characterization of neuroinfectious diseases associated with COVID‐19. Indeed, the quality, design and sample size of the available studies prevent us from drawing conclusions on possible direct neuroinvasive disease caused by SARS‐CoV‐2. The available literature does, however, provide evidence for unspecific symptoms commonly seen in viral infections, including smell and taste disturbances and the chance of immune‐mediated peripheral nerve involvement. Our analysis also suggests that there is an overdiagnosis of neurological disorders due to the inappropriate use of case definitions and restricted exclusion of potential mimics.
Nervous system involvement has been reported during previous coronavirus epidemics. Interestingly, the analysis of the SARS‐CoV‐1 and Middle East Respiratory Syndrome epidemics identified only a few anecdotal case reports and could not provide comprehensive insights into the clinical and radiological picture of neurological disease [1]. Moreover, there are pre‐clinical studies reporting the neuroinvasive potential of coronaviruses and their immunogenicity [3]. The cases of GBS that we identified in our analysis are more consistent with a parainfectious disorder, i.e. a syndrome occurring during or soon after the viral syndrome, rather than a post‐infectious syndrome. The limited literature for the COVID‐19 outbreak could be seen in the restricted documentation due to the restriction on resources posed by the medical challenges. Indeed, it is conceivable that most emphasis was placed on the management of severe respiratory symptoms and restricted intensive care unit capacity. It is obvious that neurologists are required for the care of patients with COVID‐19 [35, 36]. Their active involvement is not only mandatory for the work‐up of presumed infectious and immune‐mediated conditions but also for patients with reduced consciousness and nervous system complications of cardiac, pulmonary and coagulation disturbances related to SARS‐CoV‐2 [36]. Moreover, hypoxic brain injury may be the reason for clinical deterioration in a subgroup of patients. The potential association of SARS‐CoV‐2 with cerebrovascular diseases needs to be assessed in more detail; prospective trials with systematic use of ancillary investigations to confirm direct and indirect mechanisms of action are mandatory in order to gain further insights. From a neuroinfectious viewpoint, the major limitation of the available reports was that precise case definitions were not used, CSF testing was performed in only a subgroup of patients and exclusion of other potential diagnoses was reported only on occasion. There were just two cases with positive SARS‐CoV‐2 PCR in CSF among 27 patients with potential neurological symptoms and proven COVID‐19. However, to date, nothing is known about the sensitivity of this detection method for the examination of CSF. Indeed, CSF examination for tick‐borne encephalitis virus with PCR is not standard due to the low sensitivity of the method and probably also the transient presence of the virus in CSF. The best diagnostic approach to diagnose CNS infection with SARS‐CoV‐2 or parainfectious immune reaction associated with SARS‐CoV‐2 remains to be elucidated. Until now, no reports about intrathecal SARS‐CoV‐2‐specific IgG synthesis in these cases is available but this could be key for diagnosis. In addition, a better understanding of the reported non‐specific symptoms including olfactory and gustatory disturbances, impaired consciousness and encephalopathy is needed. The systemic inflammatory response is a relevant feature of severe COVID‐19 and could explain some of these scenarios.
The current analysis tells us that we do need more careful clinical, diagnostic and epidemiological studies to define the manifestations and burden of neurological disease caused by SARS‐CoV‐2. In this regard, we see a clear need for the use of precise case definitions and focused diagnostic work‐up to distinguish non‐specific complications of severe disease and focused reporting of neurological involvement in association with SARS‐CoV‐2 infection. Moreover, appropriate investigations are required to rule out other established causes of brain infections and parainfectious disease before attributing a condition to SARS‐CoV‐2. It also needs to kept in mind that SARS‐CoV‐2 causes a large number of asymptomatic or mildly symptomatic infections. A coincidental infection may exacerbate a so‐far asymptomatic or known neurological disease of other causes. Here, we provide guidance for assembling key clinical and paraclinical data that are required to establish insights into the true spectrum of direct and indirect effects of SARS‐CoV‐2 infection on the nervous system (Table 2).
The timing and results from nasopharyngeal swab PCR need to be reported. Most important is the relation to the development of respiratory and neurological signs/symptoms. As soon as antibody testing becomes more widely available, this will also apply to this method. Both IgM and IgG need to be reported. For all detection methods, the testing kit and ideally the exact values need to be mentioned.
Potential differentials need to be ruled out. Frequent mimics in a report from Spain of patients evaluated for SARS‐CoV‐2 infection included hypercapnia, renal or liver failure and side‐effects of drug therapy [37]. Comorbidities are frequent in certain patients and risk factors for neurological complications need to be identified.
If neuroinvasion or immune‐mediated disease of the nervous system is suspected, it is mandatory to perform PCR testing for SARS‐CoV‐2 in CSF and anti‐SARS‐CoV‐2 IgM/IgG testing in serum and CSF to check for intrathecal humoral immune reaction. It will be of major importance to determine whether PCR in CSF specimen is sensitive enough and to define the time window of potential SARS‐CoV‐2 detection in relation to respiratory and neurological symptoms.
Table 2.
Recommendations for reporting of clinical features, ancillary examination in patients with severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection and nervous system involvement
| General | Current symptoms, medical history, comorbidities and concomitant medication | |
|---|---|---|
| Clinical features and neurophysiological studies | Focal neurological signs | Findings of neurological examination |
| CNS | Acute/subacute, standardized definition of encephalopathy and encephalitis [16] | |
| PNS | Neurological examination, laboratory results with full myopathy panel and nerve conduction studies/electromyography | |
| Seizures | Report semiology and seizure type according to ILAE guidelines [28]; EEG findings according to standardized reporting | |
| Neuroimaging | Head CT | Report abnormal findings, brain edema, focal contrast enhancement, vascular status |
| Brain MRI | Abnormal findings, focal parenchymal, leptomeningeal contrast enhancement or vasculitic changes | |
| Spine MRI | Abnormal findings, contrast‐enhancement (including cranial nerves or peripheral nerve roots in cases of suspected acute neuropathy), myelopathy/atrophy | |
| SARS‐CoV‐2 viral RNA testing | Nasopharyngeal swab | Results; if multiple test performed, report time point of positive results |
| Immunoassay | Antibodies assay (IgM/IgG titers) | |
| CSF | Results PCR (qualitative and quantitative, if available) and IgM/IgG antibodies in CSF and serum (intrathecal SARS‐CoV‐2‐specific antibody production) | |
| CSF analysis | Routine analysis | Opening pressure, erythrocyte and leukocyte count with differential, glucose, proteins, oligoclonal bands and IgG index |
| Differential for neuroinfections and autoimmune conditions | Gram stain, bacterial culture, PCR testing for and common neurotropic viruses (HSV, VZV, enterovirus), cryptococcal antigen testing, venereal diseases testing (if suspected); further testing for the following infectious agents according to medical history, immune status, age and travel history: CMV, Toxoplasma gondii, Mycobacterium tuberculosis, Treponema pallidum, Borrelia species, opportunistic fungal infections, tick‐borne encephalitis virus, Toscana virus and West Nile virus | |
| Laboratory serum testing | Routine studies | Cell blood count and leukocyte differentials, D‐dimer, electroytes, LDH, C‐reactive protein, kidney and liver function |
| Infectious and autoimmune disease | Routine blood cultures, HIV serology, treponemal testing (if suspected), autoimmune antibodies | |
| Treatment | Antivirals, steroids/ immunomodulatory treatments, convulsive medication, symptomatic therapy | Specific drug type, dosage, route of administration |
| Outcome | Short (7 days) and long‐term outcome (3–6 months) |
CMV, cytomegalovirus; CNS, central nervous system; CSF, cerebrospinal fluid; CT, computerized tomography; EEG, electroencephalography; HIV, human immunodeficiency virus; HSV, Herpes simplex virus; Ig, immunoglobulin; ILAE, International League Against Epilepsy; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; PCR, polymerase chain reaction; PNS, peripheral nervous system; RNA, ribonucleic acid; VZV, Varicella‐zoster virus.
The list of differential diagnoses for meningitis, encephalitis and myelitis is extensive [38, 39]. A guide is given in Table 2. We recently summarized potential indications for CSF examination [1]. Briefly, a permissive strategy for CSF testing should be exerted as follows.
-
On suspicion of encephalitis.
-
New focal neurological deficit of no plausible differential etiology or no better explanation.
-
Delirious condition of no plausible differential etiology or no better explanation.
-
Acute cerebrovascular disorders.
-
Convulsive or non‐convulsive seizures of no plausible differential etiology or no better explanation.
-
Patients in the intensive care unit with disorders of consciousness of no plausible differential etiology or no better explanation.
The distinction of encephalopathy and encephalitis needs to be performed according to standard criteria [40]. Brain MRI is critical and contrast‐enhanced sequences are mandatory [41]. Coagulation disorders are relatively frequently encountered among patients with COVID‐19 and need to be considered in the work‐up of cerebrovascular disorders [42, 43]. In cases of peripheral nervous system involvement, nerve conduction studies and electromyographic findings need to be reported and antibodies specific for immune‐mediated conditions are obligatory to evaluate for differentials of critical illness neuropathy, acute non‐inflammatory neuropathies and myopathy [44].
-
Reporting of timing and type of treatment. There is currently no study evidence for the efficacy of a specific treatment for SARS‐CoV‐2 [45]. Guidelines for the management of respiratory symptoms and systemic complications are outlined elsewhere, may be regionally distinct and are likely to be updated on a regular basis. With regard to neuroinfectious manifestations, one should adhere to guidance on the management of viral meningitis and encephalomyelitis [46]. The management of immune‐mediated conditions including GBS and Miller‐Fisher syndrome should follow standard guidelines, with intravenous Ig or plasma exchange as first‐line options [44]. Coagulation disorders and other systemic complications of SARS‐CoV‐2 are likely to be of relevance for neurological care and complications. Although an increased risk of seizures has not been reported so far, the potential interaction of antiepileptics and antiviral/antimicrobial therapy needs to be kept in mind.
-
We need information on critical care illness, prognostic factors and outcome.
-
The neuropathological evaluation of patients who died from SARS‐CoV‐2 infection and had neurological symptoms or atypical clinical courses could provide invaluable insights.
Conclusion
Appropriate data collection, use of precise case definitions and concerted action for larger clinical studies are required to establish a better understanding of neuroinvasive and immune‐mediated conditions in the context of SARS‐CoV‐2 infection. This is not only critical for improved diagnosis and management but also for the development of specific therapies in clinical treatment trials. We therefore propose a reassessment of the available literature on neurological complications in 3–6 months on the basis of a follow‐up analysis. It would be interesting to evaluate whether a change in the quality of the reports took place in the meantime.
Disclosure of conflicts of interest
All authors are members of the Infectious Disease Panel of the EAN. P.T. and J.S. are the co‐chairs. The Chair of the Scientific Committee of the EAN approved the addition of the phrase “for the Infectious Disease Panel of the EAN”.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
References
- 1. Akhvlediani T, Jelcic I, Taba P, Pfausler B, Steiner I, Sellner J. What did we learn from the previous coronavirus epidemics and what can we do better: a neuroinfectiological point of view. Eur J Neurol 2020. 10.1111/ene.14395. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS‐CoV‐2. Nat Med 2020; 26: 450–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Natoli S, Oliveira V, Calabresi V, Maia LF, Pisani A. Does SARD‐CoV‐2 invade the brain? Translational lessons from animal models. Eur J Neurol 2020; 27: 1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Das G, Mukherjee N, Ghosh S. Neurological insights of COVID‐19 pandemic. ACS Chem Neurosci 2020; 11: 1206–1209. [DOI] [PubMed] [Google Scholar]
- 5. Chen R, Wang K, Yu J, Chen Z, Wen C, Xu Z. The spatial and cell‐type distribution of SARS‐CoV‐2 receptor ACE2 in human and mouse brain. BioRxiv 2020. 10.1101/2020.04.07.030650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brann DH, Tsukahara T, Weinreb C et al. Non‐neuronal expression of SARS‐CoV‐2 entry genes in the olfactory system suggests mechanisms underlying COVID‐19‐associated anosmia. bioRxiv 2020. 10.1101/2020.03.25.009084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tseng CT, Huang C, Newman P, et al. Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human angiotensin‐converting enzyme 2 virus receptor. J Virol 2007; 81: 1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77: 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wells GA, Shea B, O'Connell D et al. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 25/04/2020)
- 10. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 368: m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Toscano G, Palmerini F, Ravaglia S, et al. Guillain‐Barre syndrome associated with SARS‐CoV‐2. N Engl J Med 2020; 382: 2574–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y, Wang M, Zhou Y, et al. Acute cerebrovascular disease following COVID‐19: a single center, retrospective, observational study. SSRN Electron J 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and Covid‐19 in patients presenting with influenza‐like symptoms. Int Forum Allergy Rhinol 2020. 10.1002/alr.22579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol 2020. 10.1007/s00405-020-05965-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bagheri SHR, Asghari AM, Farhadi M et al. Coincidence of COVID‐19 epidemic and olfactory dysfunction outbreak. medRxiv 2020. 10.1101/2020.03.23.20041889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beltran‐Corbellini A, Chico‐Garcia JL, Martinez‐Poles J, et al. Acute‐onset smell and taste disorders in the context of Covid‐19: a pilot multicenter PCR‐based case‐control study. Eur J Neurol 2020. 10.1111/ene.14273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moein ST, Hashemian SMR, Mansourafshar B, Khorram‐Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID‐19. Int Forum Allergy Rhinol 2020. 10.1002/alr.22587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med 2020; 382: 2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu L, Xiong W, Liu D, et al. New‐onset acute symptomatic seizure and risk factors in corona virus disease 2019: a retrospective multicenter study. Epilepsia 2020; 61: e49–e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain‐Barre syndrome associated with SARS‐CoV‐2 infection: causality or coincidence? Lancet Neurol 2020; 19: 383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gutierrez‐Ortiz C, Mendez A, Rodrigo‐Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID‐19. Neurology 2020. 10.1212/WNL.0000000000009619 [DOI] [PubMed] [Google Scholar]
- 22. Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID‐19. Brain Behav Immun 2020. 10.1016/j.bbi.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yin R, Feng W, Wang T, et al. Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019. J Med Virol 2020. 10.1002/jmv.25888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID‐19 and other coronaviruses. Brain Behav Immun 2020; 87: 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xiang P, Xu XM, Gao LL, Wang HZ, Xiong HF, Li RH. First case of 2019 novel coronavirus disease with encephalitis. ChinaXiv 2020. T202003:00015. [Google Scholar]
- 26. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID‐19‐associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 2020; 201187296: E119–E120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS‐Coronavirus‐2. Int J Infect Dis 2020; 94: 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bernard‐Valnet R, Pizzarotti B, Anichini A et al. Two patients with acute meningo‐encephalitis concomitant to SARS‐CoV‐2 infection. medRxiv 2020. 10.1101/2020.04.17.20060251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duong L, Xu P, Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID‐19 infection in downtown Los Angeles, early April 2020. Brain Behav Immun 2020. 10.1016/j.bbi.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Acute myelitis after SARS‐CoV‐2 infection: a case report. MedRxiv 2020. 10.1101/2020.03.16.20035105 [DOI] [Google Scholar]
- 31. Zhang T, Rodricks MB, Hirsh E. COVID‐19‐associated acute disseminated encephalomyelitis: a case report. medRxiv 2020. 10.1101/2020.04.16.20068148 [DOI] [Google Scholar]
- 32. Vollono C, Rollo E, Romozzi M, et al. Focal status epilepticus as unique clinical feature of COVID‐19: a case report. Seizure 2020; 78: 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sharifi‐Razavi A, Karimi N, Rouhani N. COVID‐19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect 2020; 35: 100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID‐19): encephalopathy. Cureus 2020; 12: e7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vonck K, Garrez I, De Herdt V, et al. Neurological manifestations and neuro‐invasive mechanisms of the severe acute respiratory syndrome coronavirus type 2. Eur J Neurol 2020. 10.1111/ene.14329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sellner J, Taba P, Öztürk S, Helbok R. The need for neurologists in the care of COVID‐19 patients. Eur J Neurol 2020. 10.1111/ene.14257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ezpeleta D, Garcia Azorin D eds. Manual COVI‐19 Para el Neurologo General. San Sebastian de los Reyes: Sociedad Espanola Neurologia, 2020. [Google Scholar]
- 38. Glaser CA, Bloch KC. Encephalitis: a global problem deserving of a global approach. Clin Infect Dis 2019. [DOI] [PubMed] [Google Scholar]
- 39. Bloch KC, Glaser C. Diagnostic approaches for patients with suspected encephalitis. Curr Infect Dis Rep 2007; 9: 315–322. [DOI] [PubMed] [Google Scholar]
- 40. Venkatesan A, Geocadin RG. Diagnosis and management of acute encephalitis: a practical approach. Neurol Clin Pract 2014; 4: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Venkatesan A, Jagdish B. Imaging in encephalitis. Semin Neurol 2019; 39: 312–321. [DOI] [PubMed] [Google Scholar]
- 42. Khalili N, Haseli S, Bahrami‐Motlagh H, et al. Neurologic involvement in COVID‐19: radiologists' perspective. Acad Radiol 2020; 27: 1051–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID‐19. Am J Hematol 2020; 95: 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain‐Barre syndrome in ten steps. Nat Rev Neurol 2019; 15: 671–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review. JAMA 2020; 323: 1824–1836. [DOI] [PubMed] [Google Scholar]
- 46. Tunkel AR, Glaser CA, Bloch KC, et al. The management of encephalitis: clinical practice guidelines by the infectious diseases society of America. Clin Infect Dis 2008; 47: 303–327. [DOI] [PubMed] [Google Scholar]
- 47. Padroni M, Mastrangelo V, Asioli GM, et al. Guillain‐Barre syndrome following COVID‐19: new infection, old complication? J Neurol 2020. 10.1007/s00415-020-09849-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID‐19 infection: a case report. J Clin Neurosci 2020; 76: 233–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Catello Vollono C, Rollo E, Romozzi M, et al. Focal status epilepticus as unique clinical feature of COVID‐19: a case report. Seizure 2020; 19: 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


