Abstract
Objectives
To investigate the incidence of clinical, ultrasonographic and biochemical findings related to pre‐eclampsia (PE) in pregnancies with COVID‐19, and to assess their accuracy to differentiate between PE and the PE‐like features associated with COVID‐19.
Design
A prospective, observational study.
Setting
Tertiary referral hospital.
Participants
Singleton pregnancies with COVID‐19 at >20+0 weeks.
Methods
Forty‐two consecutive pregnancies were recruited and classified into two groups: severe and non‐severe COVID‐19, according to the occurrence of severe pneumonia. Uterine artery pulsatility index (UtAPI) and angiogenic factors (soluble fms‐like tyrosine kinase‐1/placental growth factor [sFlt‐1/PlGF]) were assessed in women with suspected PE.
Main outcome measures
Incidence of signs and symptoms related to PE, such as hypertension, proteinuria, thrombocytopenia, elevated liver enzymes, abnormal UtAPI and increased sFlt‐1/PlGF.
Results
Thirty‐four cases were classified as non‐severe and 8 as severe COVID‐19. Five (11.9%) women presented signs and symptoms of PE, all five being among the severe COVID‐19 cases (62.5%). However, abnormal sFlt‐1/PlGF and UtAPI could only be demonstrated in one case. One case remained pregnant after recovery from severe pneumonia and had a spontaneous resolution of the PE‐like syndrome.
Conclusions
Pregnant women with severe COVID‐19 can develop a PE‐like syndrome that might be distinguished from actual PE by sFlt‐1/PlGF, LDH and UtAPI assessment. Healthcare providers should be aware of its existence and monitor pregnancies with suspected pre‐eclampsia with caution.
Tweetable abstract
This study shows that a pre‐eclampsia‐like syndrome could be present in some pregnancies with severe COVID‐19.
Keywords: Angiogenic factors, COVID‐19, PlGF, pre‐eclampsia, pre‐eclampsia‐like syndrome, pregnancy, SARS, SARS‐CoV‐2, sFlt‐1
Tweetable abstract
This study shows that a pre‐eclampsia‐like syndrome could be present in some pregnancies with severe COVID‐19.
Introduction
On 11 March 2020, the World Health Organization (WHO) declared the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) outbreak a pandemic disease, given its increasing number of cases worldwide. 1 Studies have shown that the disease caused by SARS‐CoV‐2, named as COVID‐19 (coronavirus disease 2019) typically presents with fever, dry cough and fatigue; nevertheless, up to 14% of the cases can evolve to severe pneumonia and 5% to severe acute respiratory syndrome (SARS), both requiring admission to intensive care for intensive respiratory support. 2 Whereas COVID‐19 is primarily a respiratory infection, it has important systemic effects including hypertension, kidney disease, thrombocytopenia and liver injury. 3 , 4 , 5 , 6 As SARS‐CoV‐2 is believed to invade the host through the cell entry receptor angiotensin‐converting enzyme 2 (ACE2), these signs and symptoms in SARS‐CoV‐2 infection are thought to be due to the vasoconstriction resulting from the dysfunction of the renin‐angiotensin system. 7 , 8 By contrast, clinical features of pre‐eclampsia (PE) are mainly a consequence of the endothelial damage originated by placental oxidative stress and antiangiogenic status, which leads to the appearance of hypertension and proteinuria, elevated liver enzymes, renal failure or thrombocytopenia, among others. 9 , 10 An increased incidence of PE has been reported among mothers infected with SARS‐CoV‐2 compared with the general population. 11 Misdiagnosis, however, might have occurred in some of these cases, as COVID‐19 and PE have overlapping clinical features. Therefore, differential diagnosis might be challenging in COVID‐19 pregnant women presenting with hypertension and proteinuria, thrombocytopenia or elevated liver enzymes. 10 Thus, the aim of this study was to investigate the prevalence of clinical, ultrasonographic and biochemical findings related to PE in women with SARS‐CoV‐2 infection and to assess their accuracy in differentiating between actual PE and PE‐like features associated with COVID‐19.
Methods
We carried out a prospective cohort study of all consecutive pregnant women at >20 weeks of gestation who presented to the emergency department of our tertiary care center for suspicion of COVID‐19 (dry cough and fever) and had laboratory‐confirmed SARS‐CoV‐2 infection, between 13 March and 10 April 2020. Patients were not actively involved in the research.
Patients were classified in two groups: severe and non‐severe COVID‐19, according to the occurrence of severe pneumonia. The laboratory and clinical data were prospectively recorded in a database. The recorded data included the following: platelet count (per microlitre), D‐dimer (microg/l), lactate dehydrogenase (U/l), aspartate aminotransferase (U/l), alanine amninotransferase (U/l), urine protein to creatinine ratio (mg/g), systolic blood pressure (mmHg), diastolic blood pressure (mmHg), mean arterial pressure (mmHg), creatinine (mg/dl) and gestational age (GA) in weeks. GA to describe particular cases was expressed in weeks+days. Mean arterial pressure was calculated as: 1/3 × (systolic blood pressure) + 2/3 × (diastolic blood pressure). Maternal baseline characteristics were compared between groups. In severe cases, data were analysed at three different time points during COVID‐19: before, during and after intensive care unit (ICU) admission for severe pneumonia.
According to the WHO guidance, laboratory confirmation for SARS‐CoV‐2 was defined as a positive result of real‐time reverse transcriptase‐polymerase chain reaction (RTPCR) assay of nasal and pharyngeal swabs. 12
PE was defined as new onset of high blood pressure (systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg) or worsening of previous high blood pressure in addition to new‐onset proteinuria (protein to creatinine ratio >300) or worsening of previous proteinuria, or to at least one of the following signs and symptoms of severe PE: cerebral or visual symptoms, elevation of liver enzymes to twice normal concentration, platelet count <100 000/µl, serum creatinine concentrations >1.1 mg/dl or pulmonary oedema. 10 The HELLP syndrome is frequently considered a variant of PE. Diagnostic criteria for HELLP syndrome are haemolysis with increased LDH (>600 U/l) and AST (≥70 U/l), and platelets <100 000/µl. 13
In women with new‐onset hypertension, the uterine artery pulsatility index (UtAPI) was assessed by transabdominal Doppler ultrasound and maternal serum levels of placental growth factor (PlGF) and soluble fms‐like tyrosine kinase‐1 (sFlt‐1) in pg/ml were determined by means of the fully automated Elecsys assays for sFlt‐1 and PlGF on an electrochemiluminescence immunoassay platform (cobas e analyzers; Roche® Diagnostics, Rotkreuz, Switzerland). 14 , 15 The sFlt‐1/PlGF was then calculated. UtAPI above the 95th centile for gestational age, and sFlt‐1/PlGF values ≥85 (at <34 weeks) or ≥110 (at ≥34 weeks) were considered highly suggestive of underlying placental disease. 16 , 17 , 18 , 19
Statistical analysis
The open‐source satistical software R Commander(R package version 2.3‐1), which is freely available on CRAN (https://cran.r‐project.org), was used for statistical analysis. Categorical data were reported as frequency and percentage, and comparisons between severity groups were estimated by Chi‐square or Fisher tests, as appropriate. Continuous variables were described as median and interquartile (IQR) range and Mann–Whitney U test was used to assess differences between severity groups. The statistical significance level was set at P < 0.05.
Results
During the study period (31 days), 42 cases of SARS‐CoV‐2‐infected women were identified at a median GA of 32.0 (IQR 26.0–37.5) weeks of gestation. Among them, eight (19.0%) cases developed severe pneumonia and required admission to the ICU. Median maternal age of cases with severe COVID‐19 was significantly greater than in the non‐severe cases (39.4 [34.2–44.5] versus 30.9 [25.0–41.8], P = 0.006). No other pregnancy baseline characteristics differed between severity groups. Among the eight severe cases, five (62.5%) developed PE features (new‐onset hypertension and proteinuria and/or thrombocytopenia and/or elevated liver enzymes), requiring antihypertensive drugs in all of them. No cases with diagnostic criteria for PE were found among the 34 non‐severe COVID‐19 women (Table 1).
Table 1.
Maternal characteristics in pregnant women with COVID‐19
| All patients (n = 42) | Nonsevere patients (n = 34) | Severe patients (n = 8) | P | |
|---|---|---|---|---|
| Maternal age (years) | 32.0 (26.0–37.5) | 30.9 (25.0–41.8) | 39.4 (34.2–44.5) | 0.006 |
| Pre‐pregnancy BMI (kg/m2) | 26.2 (23.5–29.3) | 26.1 (22.8–29.3) | 27.9 (25.4–30.6) | 0.378 |
| Gestational age (weeks) | 31.6 (25.9–36.1) | 32.8 (26.7–36.1) | 28.6 (22.3–32.4) | 0.211 |
| Ethnicity | ||||
| White | 22 (52.4%) | 19 (55.9%) | 3 (37.5%) | 0.304 |
| Latin American | 17 (40.5%) | 12 (35.3%) | 5 (62.5%) | |
| Others | 3 (7.1%) | 3 (8.8%) | 0 | |
| ART | 4 (9.5%) | 2 (5.9%) | 2 (25.0%) | 0.158 |
| Smoking | 2 (4.8%) | 1 (2.9%) | 1 (12.5%) | 0.348 |
| Nuliparous | 20 (47.6%) | 16 (47.1%) | 4 (50.0%) | 1.0 |
| History of PE | 0 | 0 | 0 | 1.0 |
| Pre‐pregnancy HTN | 0 | 0 | 0 | 1.0 |
| Pre‐pregnancy diabetes | 1 (2.4%) | 1 (2.4%) | 0 | 1.0 |
| Chronic kidney disease | 0 | 0 | 0 | 1.0 |
| PE diagnostic criteria during COVID‐19 | 5 (11.9%) | 0 | 5 (62.5%) | <0.001 |
ART, assisted reproductive technology; BMI, body mass index; HTN, hypertension; PE, pre‐eclampsia.
Continuous data are given as median and interquartile range. Categorical data as frequency and percentage. P‐values denoted the comparison between non‐severe and severe subgroups.
Evolution of clinical and laboratory findings in the severe cases of COVID‐19
Before severe pneumonia, all eight women were normotensive, had normal platelet count, liver enzymes, LDH and proteinuria, and only one case with UtAPI above the 95th centile was identified. During severe pneumonia, the most frequent findings were: elevated liver enzymes to twice normal concentrations (87.5%), proteinuria >300 mg/g (75.0%) and hypertension (62.5%) (Figure 1). No cases with creatinine >1.1 mg/dl or platelet count <100 000/µl were found; nevertheless, one case presented mild thrombocytopenia (platelet count <150 000/µl). sFlt‐1/PlGF ≥85/110 and UtAPI >95th centile were present in only one woman. Only one case with LDH >600 IU/l was identified. Based on these findings, five women (62.5%) had diagnostic criteria of PE and/or HELLP syndrome. Caesarean delivery was performed during ICU stay in four cases. HELLP syndrome was the indication for delivery in case 1 (at a GA of 30+1) and worsening of SARS in cases 3, 4 and 7 (at a GA of 37+6, 36+6 and 28+3, respectively). After recovery from severe pneumonia, hypertensive therapy was no longer required in all cases and only the woman who had presented with sFlt‐1/PlGF >110, LDH >600 and UtAPI above the 95th centile, still met PE diagnostic criteria (more details on clinical and laboratory findings and their evolution in severe cases can be seen in Table 2 and Figure 1).
Figure 1.
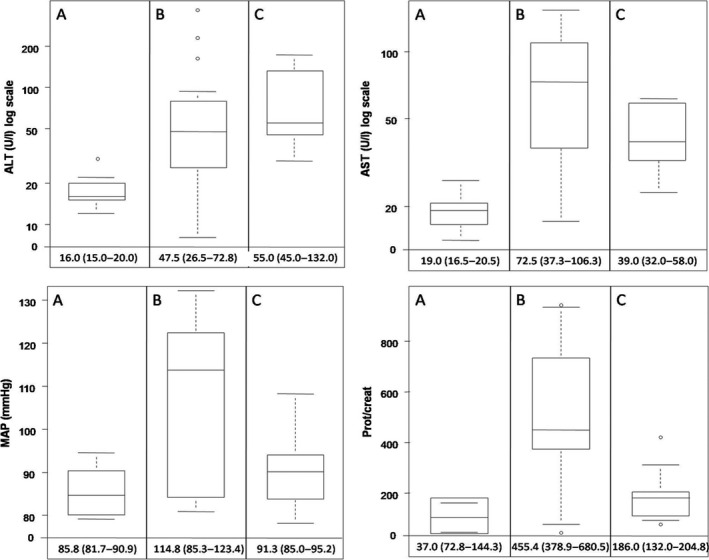
Evolution of ALT, AST, proteinuria and mean arterial blood pressure in pregnant women with COVID‐19 before (A), during (B) and after (C) severe pneumonia. The bottom and top edges of each box represent the first and third quartiles, respectively, the band within the box represents the median value and the whiskers represent values that are 1.5 times the interquartile range. Median values and interquartile range of each variable are displayed.
Table 2.
Clinical and biochemical pre‐eclampsia‐related findings in pregnant women with COVID‐19 before, during and after severe pneumonia
| Variables | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Before severe pneumonia | GA (weeks) | 30+0 | 22+6 | 37+5 | 36+4 | 32+0 | 20+3 | 27+4 | 20+1 |
| SBP (mmHg) | 130 | 120 | 123 | 117 | 104 | 135 | 107 | 116 | |
| DBP (mmHg) | 74 | 72 | 74 | 67 | 71 | 76 | 67 | 63 | |
| MAP (mmHg) | 92.7 | 88.0 | 90.3 | 83.7 | 82.0 | 95.7 | 80.3 | 80.7 | |
| Prot/creat (mg/g) | 37 | 180 | — | — | — | — | — | — | |
| AST (U/l) | 17 | 19 | 15 | 20 | 26 | 19 | 23 | 14 | |
| ALT (U/l) | 16 | 30 | 13 | 26 | 20 | 12 | 14 | 22 | |
| Platelets/µl | 158 000 | 242 000 | 402 000 | 210 000 | 275 000 | 234 000 | 319 000 | 242 000 | |
| LDH (U/l) | — | — | — | — | — | — | — | — | |
| D‐dimer (mg/ml) | — | — | — | — | — | — | — | — | |
| Creatinine (mg/dl) | 0.63 | 0.49 | — | 0.79 | 0.74 | 0.36 | 0.45 | 0.66 | |
| UtAPI >95th centile | No | No | Yes | No | No | No | No | No | |
| PE/HELLP diagnostic criteria | No | No | No | No | No | No | No | No | |
| During severe pneumonia | GA (weeks) | 30+1 | 24+4 | 37+6 | 36+5 | 32+1 | 20+4 | 28+3 | 20+2 |
| SBP (mmHg) | 145 | 168 | 156 | 155 | 116 | 115 | 140 | 108 | |
| DBP (mmHg) | 90 | 116 | 98 | 108 | 70 | 68 | 105 | 69 | |
| MAP (mmHg) | 108.3 | 133.3 | 117.3 | 123.7 | 85.3 | 83.7 | 116.7 | 82.0 | |
| Prot/creat (mg/g) | 855 | 622 | 378 | 514 | 396 | 49 | 948 | 130 | |
| AST (U/l) | 153 | 122 | 104 | 62 | 38 | 52 | 138 | 113 | |
| ALT (U/l) | 170 | 136 | 52 | 39 | 38 | 14 | 65 | 230 | |
| Platelets/µl | 324 000 | 160 000 | 279 000 | 231 000 | 336 000 | 243 000 | 108 000 | 505 000 | |
| LDH (U/l) | 482 | 370 | 672 | 555 | 517 | 176 | 463 | 312 | |
| D‐dimer (mg/ml) | 457 | 2129 | 5065 | 1800 | 326 | 119 | 514 | 376 | |
| Creatinine (mg/dl) | 0.34 | 0.42 | 0.85 | 0.88 | 0.42 | 0.20 | 0.39 | 0.26 | |
| Hidralazine | Yes | Yes | Yes | Yes | No | No | No | No | |
| Labetalol | Yes | Yes | Yes | Yes | No | No | Yes | No | |
| sFlt‐1/PlGF | 9.40 | 20.24 | 378.90 | 49.36 | 24.78 | 4.60 | 7.60 | 5.19 | |
| UtAPI >95th centile | No | No | Yes | No | No | No | No | No | |
| PE/HELLP diagnostic criteria | Yes | Yes | Yes | Yes | No | No | Yes | No | |
| After severe pneumonia | GA at delivery (weeks) | 30+1 | Not delivered | 37+6 | 36+6 | Not delivered | Not delivered | 28+3 | Not delivered |
| Reason for delivery | HELLP | — | SARS | SARS | — | — | SARS | — | |
| GA (weeks) | — | 25.5 | — | — | 33.2 | 21.5 | — | 21.3 | |
| SBP (mmHg) | 123 | 132 | 142 | 115 | 116 | 108 | 109 | 110 | |
| DBP (mmHg) | 83 | 75 | 93 | 68 | 79 | 62 | 75 | 64 | |
| MAP (mmHg) | 96.3 | 94.0 | 109.3 | 83.7 | 91.3 | 77.3 | 86.3 | 79.3 | |
| Prot/creat (mg/g) | 210 | 183 | 426 | 83 | 115 | 83 | 189 | 128 | |
| AST (U/l) | 39 | 32 | 56 | 43 | 58 | — | 23 | 61 | |
| ALT (U/l) | 45 | 132 | 41 | 29 | 55 | — | 29 | 172 | |
| Platelets/µl | 312 000 | 218 000 | 232 000 | 258 000 | 292 000 | 169 000 | 364 000 | 762 000 | |
| LDH (U/l) | 222 | 277 | 692 | 325 | 211 | — | 353 | 192 | |
| D‐dimer (mg/ml) | 617 | 1745 | 3258 | — | 454 | — | 347 | 470 | |
| Creatinine (mg/dl) | 0.25 | 0.3 | 0.58 | — | 0.41 | — | 0.42 | 0.65 | |
| UtAPI >95th centile | — | No | — | — | No | No | — | No | |
| PE/HELLP diagnostic criteria | No | No | Yes | No | No | No | No | No |
ALT, alanine amninotransferase; AST, aspartate aminotransferase; DBP, diastolic blood pressure; GA, gestational age; LDH, lactate dehydrogenase; MAP, mean arterial pressure; PE, pre‐eclampsia; PlGF, placental growth factor; prot/creat; urine protein to creatinine ratio; SARS, severe acute respiratory syndrome; sFlt‐1, soluble fms‐like tyrosine kinase‐1; UtAPI, uterine artery pulsatility index.
Discussion
Main findings
This study shows that 11.9% of COVID‐19 pregnant women develop PE features; however, they only appeared in COVID‐19 cases complicated by severe pneumonia. In this situation, PE/HELLP diagnostic criteria were found in five (62.5%) of the cases; nevertheless, abnormal angiogenic status, increased LDH and placental underperfusion could only be confirmed in one of them, which indicates that this case was probably an actual PE. These findings suggest that the signs and symptoms compatible with PE/HELLP present in four of these five cases, could be derived from the complex polypharmacy administrated or from the renal and cardiovascular dysfunction for severe SARS‐CoV‐2 infection. In our cohort, only one of these five cases remained pregnant after severe pneumonia recovery, and then all PE/HELLP features recovered spontaneously. PE and HELLP syndrome do not resolve spontaneously and delivery is the only definitive cure. For these reasons, we believe that the four women with PE/HELLP signs and symptoms, and normal sFlt‐1/PlGF, UtAPI and LDH <600, had developed a PE‐like syndrome.
Strengths and limitations
To our knowledge, this is the first study to describe the incidence of signs and symptoms of PE in a relatively large cohort of pregnancies with COVID‐19 and to show that a PE‐like syndrome could be induced by severe COVID‐19. Furthermore, our findings are of great value to improve maternal care of pregnancies with severe pneumonia due to COVID‐19.
This study has several limitations. First, this is a small series and the results should be considered with caution. Further research is needed to better understand the systemic consequences of COVID‐19 in pregnant women. Second, only four women with PE‐like syndrome are reported, which could mean that our findings are not applicable to all pregnancies with severe pneumonia due to COVID‐19. Third, only one of the four women who developed a PE‐like syndrome remained pregnant after severe pneumonia and despite the PE‐like syndrome recovering spontaneously, we cannot affirm that the three other cases did not improve due to delivery. Nevertheless, we believe that the PE‐like syndrome alone may not be an obstetric indication for delivery, as it seems that it might not be a placental complication itself, but one of the clinical manifestations of severe COVID‐19. Finally, although UtAPI and sFlt‐1/PlGF ratio have a high negative predictive value to predict the short‐term absence of PE, they are not diagnostic criteria of PE; 20 , 21 thus, we cannot categorically state that the case with PE features and elevated UtAPI and sFlt‐1/PlGF was an actual PE and not a PE‐like syndrome.
Interpretation
Several disorders have previously proved to imitate PE because they share some of the clinical and laboratory findings of patients with PE. The pathophysiologic causes of these conditions include vasospasm, platelet activation or destruction, microvascular thrombosis, endothelial cell dysfunction and reduced tissue perfusion. Some of these disorders include gestational hypertension, chronic kidney disease, acute fatty liver of pregnancy, thrombotic thrombocytopenic purpura, haemolytic uremic syndrome, acute exacerbation of systemic lupus erythematosus, severe hypothyroidism and sepsis. 17 , 22 , 23 Differential diagnosis may be a challenge to caregivers due to the overlap of diagnostic criteria among them. Additionally, some of them are potentially life‐threatening for both the mother and the fetus; thus, accurate diagnosis is important, as the management and prognosis of these conditions differ widely. Recent studies have shown that angiogenic factors support the differential diagnosis between PE and some of its imitators. 17 , 24 , 25 PlGF and sFlt‐1 are placenta‐related angiogenic factors that are highly specific to placental insufficiency. 26 In PE, the placenta fails properly to invade and remodel maternal uterine spiral arteries, leading to impaired perfusion and placental oxidative stress. 27 , 28 This condition leads to increased UtAPI and to an antiangiogenic status with increased s‐Flt‐1/PlGF ratio due to up‐regulation of sFlt‐1 and down‐regulation of PlGF. 9 , 20 The identification of an sFlt‐1/PlGF imbalance is detectable in the maternal circulation at least 5 weeks before the onset of clinical PE. 26 Thus, COVID‐19 patients with normal early phase of placental implantation should have normal values of sFlt‐1/PlGF and UtAPI in spite of proteinuria, thrombocytopenia, elevated liver enzymes or hypertension. This hypothesis, however, had not been previously investigated due to the very recent outbreak of the SARS‐CoV‐2 infection.
This study has important clinical implications, as we show that sFlt‐1/PlGF, UtAPI and LDH allow PE to be differentiated from the PE‐like syndrome present in some of the pregnant women with severe COVID‐19. This knowledge could improve management and reduce misdiagnosis in pregnancies with severe COVID‐19. In our cohort, case 1 was probably misdiagnosed as HELLP syndrome; this, in addition to the concurrence of SARS, influenced the indication of delivery. The fact that the sFlt‐1/PlGF results were not available at the time of worsening of the maternal condition, and the scarce evidence available at that time of the consequences of COVID‐19 during pregnancy, prompted the delivery indication at 30+1 weeks. After the experience with this first case, a more conservative management was adopted in the following cases that developed PE‐like syndrome. Fortunately, they completely recovered after severe pneumonia and became normotensive again without any antihypertensive drugs and without being delivered.
Conclusion
Pregnant women with severe COVID‐19 could develop a PE‐like syndrome, which might be distinguished from actual PE by sFlt‐1/PlGF, LDH and UtAPI assessment. Therefore, healthcare providers should be aware of its existence and monitor pregnancies with suspected PE with caution. PE‐like syndrome might not be an indication for earlier delivery in itself, as it might not be a placental complication and could resolve spontaneously after recovery from severe pneumonia.
Disclosure of interests
Manel Mendoza and Itziar Garcia‐Ruiz received lecture fees by Roche Diagnostics. The other authors have no conflicts of interest to declare. Completed disclosure of interests forms is available to view online as supporting information.
Contribution to authorship
AS and EC had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. EC, AS, MM and IG‐R conceived and designed the study. RML‐M, JB, NM and NF‐H contributed to the literature research. NM, CR, PG‐M and BS contributed to data collection and confirmation. MM, IG‐R and AS contributed to data analysis, and MM, IG‐R, AS, RMLM, JB, NF‐H and EC contributed to data interpretation. MM and IG‐R were in charge of writing the manuscript draft. AS and EC made substantial revisions to the manuscript. MM and IG‐R contributed equally to this article. AS and EC also contributed equally to this article.
Details of ethics approval
This study was approved by the Vall d'Hebron University Hospital Ethics Committee (PR[AMI]181/2020) on 13 March 2020. Written informed consent was waived due to the rapid emergence of this infectous disease. However, verbal informed consent was obtained from all patients, which was included in the patient's medical record.
Funding
None.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank all the physicians who facilitated the recruitment of individuals at the Hospital Universitari Vall d'Hebron and the participants who agreed to take part in the study.
Mendoza M, Garcia‐Ruiz I, Maiz N, Rodo C, Garcia‐Manau P, Serrano B, Lopez‐Martinez RM, Balcells J, Fernandez‐Hidalgo N, Carreras E, Suy A. Pre‐eclampsia‐like syndrome induced by severe COVID‐19: a prospective observational study. BJOG 2020; 10.1111/1471-0528.16339. 127:1374–1380.
References
- 1. WHO Director‐General's opening remarks at the media briefing on COVID‐19. 2020. [www.who.int/dg/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐media‐briefing‐on‐covid‐19‐‐‐11‐march‐2020]. Accessed 12 March 2020.
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; published online Feb 24. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 3. Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. Characteristics of liver tests in COVID‐19 patients. J Hepatol 2020; published online Apr 13. 10.1016/j.jhep.2020.04.006 [DOI] [Google Scholar]
- 4. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in‐hospital death of patients with COVID‐19. Kidney Int 2020; published online Mar 20. 10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kreutz R, Algharably EAE‐H, Azizi M, Dobrowolski P, Guzik T, Januszewicz A, et al. Hypertension, the renin‐angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID‐19. Cardiovasc Res 2020; published online Apr 15. 10.1093/cvr/cvaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clin Chim Acta 2020;506:145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong J‐C, Turner AJ, et al. Angiotensin converting enzyme 2: SARS‐CoV‐2 receptor and regulator of the renin‐angiotensin system. Circ Res 2020; published online Apr 8. 10.1161/CIRCRESAHA.120.317015 [DOI] [PubMed] [Google Scholar]
- 8. Liu PP, Blet A, Smyth D, Li H. The science underlying COVID‐19: implications for the cardiovascular system. Circulation 2020; published online Apr 15. 10.1161/CIRCULATIONAHA.120.047549 [DOI] [PubMed] [Google Scholar]
- 9. Turpin CA, Sakyi SA, Owiredu WKBA, Ephraim RKD, Anto EO. Association between adverse pregnancy outcome and imbalance in angiogenic regulators and oxidative stress biomarkers in gestational hypertension and preeclampsia. BMC Pregnancy Childbirth 2015;15:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy . Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' task force on hypertension in pregnancy. Obstet Gynecol 2013;122:1122–31. [DOI] [PubMed] [Google Scholar]
- 11. Di Mascio D, Khalil A, Saccone G, Rizzo G, Buca D, Liberati M, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID 1–19) during pregnancy: a systematic review and meta‐analysis. Am J Obstet Gynecol MFM 2020). 2, 100107 10.1016/j.ajogmf.2020.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. 2020. [https://apps.who.int/iris/handle/10665/330854]. Accessed 11 March 2020.
- 13. Haram K, Svendsen E, Abildgaard U. The HELLP syndrome: clinical issues and management. A review. BMC Pregnancy Childbirth 2009;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin AM, Bindra R, Curcio P, Cicero S, Nicolaides KH. Screening for pre‐eclampsia and fetal growth restriction by uterine artery Doppler at 11–14 weeks of gestation. Ultrasound Obstet Gynecol 2001;18:583–6. [DOI] [PubMed] [Google Scholar]
- 15. Schiettecatte J, Russcher H, Anckaert E, Mees M, Leeser B, Tirelli AS, et al. Multicenter evaluation of the first automated Elecsys sFlt‐1 and PlGF assays in normal pregnancies and preeclampsia. Clin Biochem 2010;43:768–70. [DOI] [PubMed] [Google Scholar]
- 16. Gómez O, Figueras F, Fernández S, Bennasar M, Martínez JM, Puerto B, et al. Reference ranges for uterine artery mean pulsatility index at 11–41 weeks of gestation. Ultrasound Obstet Gynecol 2008;32:128–32. [DOI] [PubMed] [Google Scholar]
- 17. Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H, et al. The sFlt‐1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e1–8. [DOI] [PubMed] [Google Scholar]
- 18. Stepan H, Herraiz I, Schlembach D, Verlohren S, Brennecke S, Chantraine F, et al. Implementation of the sFlt‐1/PlGF ratio for prediction and diagnosis of pre‐eclampsia in singleton pregnancy: implications for clinical practice. Ultrasound Obstet Gynecol 2015;45:241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paules C, Youssef L, Rovira C, Crovetto F, Nadal A, Peguero A, et al. Distinctive patterns of placental lesions in pre‐eclampsia vs small‐for‐gestational age and their association with fetoplacental Doppler. Ultrasound Obstet Gynecol 2019;54:609–16. [DOI] [PubMed] [Google Scholar]
- 20. Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, et al. Predictive value of the sFlt‐1:PlGF ratio in women with suspected preeclampsia. N Engl J Med 2016;374:13–22. [DOI] [PubMed] [Google Scholar]
- 21. 1 Recommendations | PlGF‐based testing to help diagnose suspected pre‐eclampsia (Triage PlGF test, Elecsys immunoassay sFlt‐1/PlGF ratio, DELFIA Xpress PlGF 1‐2‐3 test, and BRAHMS sFlt‐1 Kryptor/BRAHMS PlGF plus Kryptor PE ratio). NICE Guidance. [www.nice.org.uk/guidance/dg23/chapter/1‐Recommendations]. Accessed 28 March 2020.
- 22. Sibai BM. Imitators of severe pre‐eclampsia. Semin Perinatol 2009;33:196–205. [DOI] [PubMed] [Google Scholar]
- 23. Inversetti A, Serafini A, Manzoni MF, Dolcetta Capuzzo A, Valsecchi L, Candiani M. Severe hypothyroidism causing pre‐eclampsia‐like syndrome. Case Rep Endocrinol 2012;2012:586056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rolfo A, Attini R, Nuzzo AM, Piazzese A, Parisi S, Ferraresi M, et al. Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney Int 2013;83:177–81. [DOI] [PubMed] [Google Scholar]
- 25. Hirashima C, Ogoyama M, Abe M, Shiraishi S, Sugase T, Niki T, et al. Clinical usefulness of serum levels of soluble fms‐like tyrosine kinase 1/placental growth factor ratio to rule out preeclampsia in women with new‐onset lupus nephritis during pregnancy. CEN Case Rep 2019;8:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levine RJ, Maynard SE, Qian C, Lim K‐H, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672–83. [DOI] [PubMed] [Google Scholar]
- 27. Hung TH, Skepper JN, Burton GJ. In vitro ischemia‐reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am J Pathol 2001;159:1031–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pijnenborg R, Vercruysse L, Verbist L, Van Assche FA. Interaction of interstitial trophoblast with placental bed capillaries and venules of normotensive and pre‐eclamptic pregnancies. Placenta 1998;19:569–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material


